Pathogenesis and treatment of parenteral nutrition-associated liver disease
2012-07-10
Nanjing, China
Pathogenesis and treatment of parenteral nutrition-associated liver disease
Zi-Wei Xu and You-Sheng Li
Nanjing, China
BACKGROUND:Parenteral nutrition-associated liver disease (PNALD) has been common in patients who require long-term parenteral nutrition. PNALD develops in 40%-60% of infants on long-term parenteral nutrition compared with 15%-40% of adults on home parenteral nutrition for intestinal failure. The pathogenesis of PNALD is multifactorial and remains unclear. There is no specific treatment. Management strategies for its prevention and treatment depend on an understanding of many risk factors. This review aims to provide an update on the pathogenesis and treatment of this disease.
DATA SOURCES:A literature search was performed on the MEDLINE and Web of Science databases for articles published up to October 2011, using the keywords: parenteral nutrition associated liver disease, intestinal failure associated liver disease, lipid emulsions and fish oil. The available data reported in the relevant literatures were analyzed.
RESULTS:The literature search provided a huge amount of evidence about the pathogenesis and management strategies on PNALD. Currently, lack of enteral feeding, extended duration of parenteral nutrition, recurrent sepsis, and nutrient deficiency or excess may play important roles in the pathogenesis of PNALD. Recent studies found that phytosterols, present as contaminants in soy-based lipid emulsions, are also an important factor in the pathogenesis. Moreover, the treatment of PNALD is discussed.
CONCLUSIONS:The use of lipid emulsions, phytosterols in particular, is associated with PNALD. Management strategies for the prevention and treatment of PNALD include consideration of early enteral feeding, the use of specialized lipid emulsions such as fish oil emulsions, and isolated small bowel or combined liver and small bowel transplantation. A greater understanding of the pathogenesis of PNALD has ledto promising interventions to prevent and treat this condition. Future work should aim to better understand the mechanisms of PNALD and the long-term outcomes of its treatment.
(Hepatobiliary Pancreat Dis Int 2012;11:586-593)
parenteral nutrition-associated liver disease;phytosterol; farnesoid X receptor; fish oil
Introduction
Mainstreaming of long-term parenteral nutrition (PN) has profoundly impacted the prognosis and quality of lives of individuals with intestinal failure from congenital abnormalities or extensive gastrointestinal surgery. Although PN is lifesaving, its long-term use is associated with severe adverse effects, including septic infection, metabolic imbalance and hepatobiliary dysfunction. The hepatobiliary complications of PN are now well recognized as PN-associated liver disease (PNALD), or intestinal failureassociated liver disease (IFALD). PNALD has been common in both adults and infants who require longterm PN. The mechanisms underlying PNALD remain to be elucidated. Currently, lack of enteral feeding, extended duration of PN, recurrent sepsis, and nutrient deficiency or excess may play important roles in its pathogenesis. Recent studies[1-4]found that phytosterols, present as contaminants in soy-based lipid emulsions, may be an important factor in the pathogenesis of PNALD. Management strategies for its prevention and treatment depend on an understanding of many risk factors. Recently, lipid emulsions containing fish oil or glutamine were proposed to be protective. However, for patients who have established end-stage PNALD and cannot wean off total PN, the only option may be transplantation.
Methods
The relevant articles were identified by searching theMEDLINE and Web of Science databases for articles published up to October 2011 using the following key words: parenteral nutrition associated liver disease, intestinal failure associated liver disease, lipid emulsions, and fish oil. Additional papers were identified by a manual search of the references from key articles.
Results
Quantity of relevant research
The electronic literature search strategy identified 1139 potentially relevant articles. After screening titles and abstracts, 194 full papers were retrieved. Of these, 35 were identified as relevant for inclusion in this systematic review (Fig. 1).

Fig. 1.Summary of inclusion and exclusion criteria.
Definitions
The hepatobiliary complications of PN comprise PNALD or IFALD because they occur predominantly in parenterally-fed patients with intestinal failure. PNALD is a common complication in patients with intestinal failure and develops in 40%-60% of infants compared with 15%-40% of adults on long-term PN.[5]The clinical spectrum includes hepatic steatosis, cholestasis, cholelithiasis, and hepatic fibrosis.[6-10]PNALD is usually associated with prolonged PN administration (>2 weeks) and is characterized by cholestasis (commonly defined as a direct bilirubin ≥2 mg/dL).[11]As PNALD is a diagnosis of exclusion, in addition to customary hepatic work-up, a liver biopsy for histology may be required to provide additional information if there are doubts.[12]
Risk factors
The pathogenesis of PNALD appears to be multifactorial and remains unclear. Suggested theories include premature birth, disruption of the enterohepatic circulation of bile acids, intestinal stasis with subsequent bacterial overgrowth, bile sludging with subsequent bile duct obstruction,[13]early and/or recurrent central venous catheter-related sepsis, and nutrient deficiency or excess.[14]However, excess caloric intake of lipids and septic events may play key roles.[15,16]
Prematurity
PNALD is particularly common in small premature infants, and infants have a much higher incidence than adults (40%-60% vs 15%-40%).[17]In infants with a birth weight <1000 g, the incidence of cholestatic liver disease is as high as 50%, whereas in infants >1500 g it is only 10%.[18]Premature infants have an insufficient capacity for transsulfuration. Besides, animal and human data indicate that neonates have both decreased bile acid pools and low activity of other enzyme systems such as those involved in the synthesis, conjugation, hepatic uptake and secretion, and recirculation of bile acids.[19-21]
Nutrient excess
Reports in adults and children raise concerns about the possible relationship between long-term intravenous lipid emulsion (ILE) and PNALD. Clinical data show a positive relation of the dosage of soybean oil-based ILE with the development of cholestasis in children on long-term PN. Diamond et al[16]investigated the role of parenteral lipids in the development of a serumconjugated bilirubin >100 µmol/L (5.9 mg/dL; CB100) in infants. They reported that days of lipid >2.5 g/kg per day, and the number of septic episodes emerged as the strongest predictors for the development of CB100, while Cavicchi et al[15]showed that a lipid dose>1 g/kg per day is associated with the development of PNALD in adults. Colomb et al[22]reported that plasma bilirubin concentration decreases after stopping lipid administration. In most cases, the decrease of plasma bilirubin level is rapid within the first month, reaching a normal concentration by 3.2±2.0 months. Besides, Meisel et al[23]and Javid et al[24]investigated whether the route of lipid administration affects the development of PN-associated liver injury in a mouse model. They found that soybean-based lipid emulsions given intravenously cause liver injury, but little or none when given orally. This study provides evidence that artificial chylomicrons in ILE may not be metabolized in a physiological manner by the liver. The role of ILE in the development of PNALD is not clear, but at least two factors may contribute to theproblem. First, standard soybean oil-based ILE, which predominantly contains omega-6 polyunsaturated fatty acids (ω-6 PUFAs), has been shown to impair biliary secretion, generate a proinflammatory response, and impair immune function.[25]Furthermore, recent evidence suggests that one major contributing factor that predisposes patients to PNALD is hepatotoxic phytosterols. Conventional soybean-based ILE contains significant quantities of phytosterols (327-383 mg/L from manufacturers' data), and long-term use of conventional ILE leads to a progressive increase of phytosterol content in cell membranes and plasma lipoproteins.[1,4,26]The phytosterols, mainly campesterol, β-sitosterol and stigmasterol, are steroid compounds structurally similar to cholesterol. They carry out the same basic functions in plants as cholesterol does in animals. Epidemiologic and experimental studies suggest that dietary phytosterols may be used as a therapeutic option to lower plasma cholesterol and the risk of atherosclerotic disease. The cholesterol-lowering action of phytosterols is thought to occur, at least in part, through competitive replacement of dietary and biliary cholesterol in mixed micelles, which undermines the absorption of cholesterol.[27]It is possible that phytosterols are toxic to hepatocytes when administered intravenously but not when absorbed through the gastrointestinal tract.[24]Farnesoid X receptor (FXR) is a member of the ligand-activated nuclear receptor superfamily. FXR serves as a sensor for bile acids and promotes their enterohepatic clearance by controlling the expression of genes involved in their transport and metabolism.[3,28]A study[29]has demonstrated that FXR-knockout mice lack these hepatoprotective mechanisms and are ultrasensitive to bile acid-induced injury. Activation of FXRin vivois associated with increased hepatobiliary circulation of bile acids, inhibition of hepatic bile acid biosynthesis, and reduction in plasma triglycerides.[30]Phytosterols have antagonistic activity against FXR gene expression (i.e. BSEP, FGF-19, OSTα/β) and the antagonism of FXR significantly compromises the hepatoprotective mechanisms which normally act to attenuate cholestasis.[3]
ILE is an important source of energy and if inadvertently delivered in excess, it may promote phospholipidosis and hepatosteatosis.[31,32]However, excess carbohydrate is more likely to promote steatohepatitis, and adjusting the relative amount of carbohydrate and fat, so that more fat calories are provided, was shown to reduce hepatosteatosis.[33]Generally, high glucose infusion rates result in high plasma insulin concentrations, which subsequently inhibit mitochondrial fatty oxidation. This process results in the accumulation of fatty acids within hepatocytes.[6,34]At present when hepatosteatosis occurs, the management strategy is to reduce the total calories delivered.
In addition, some studies[35,36]found that high levels of amino-acids may be directly hepatotoxic because they affect the cannalicular membrane of the hepatocyte. Studies[37,38]also demonstrated an association between the onset of cholestatic liver disease and intravenous methionine intake.
Nutrient deficiency
Deficiency of some micronutrients such as choline and taurine may be involved in the pathogenesis of PNALD. Choline is not essential because of its endogenous synthesis from methionine (Fig. 2), but is low in >90% of the patients who require long-term total PN.[39]The biosynthesis of choline may be inadequate when methionine is given parenterally than when it enters the liver via the portal vein.[40]Fortunately, choline deficiency results in hepatic steatosis that may be reversed by the addition of choline to PN solutions.[41,42]Deficiency of taurine, another product of the hepatic transsulfuration pathway, could play a role in the development of PNALD in neonates. In older infants and adults, taurine is synthesized from methionine, but is diminished owing to cystathionase deficiency in premature infants.[19]Cystathionase is the rate-limiting enzyme in the formation of cysteine from cystathionine, an intermediate in the metabolism from methionine to taurine (Fig. 2).
Sepsis
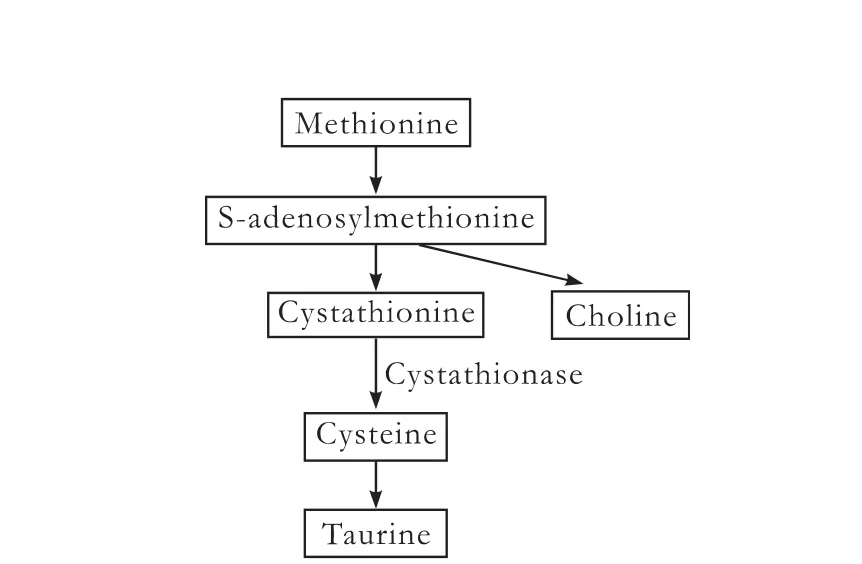
Fig. 2.A schematic diagram of the hepatic transsulfuration pathway from methionine to taurine (some intermediates are omitted for clarity).
Septic events are a major risk factor in the development of IFALD in patients with intestinal failure. Diamond et al[16]found that each septic episode is associated with a 3.2-fold increase in the risk of developing PNALD. The risk of sepsis is largely attributedto the risk of catheter-related bloodstream infection and bacterial translocation resulting from enteral bacterial overgrowth and underlying intestinal barrier damage. First and foremost, the administration of PN requires an intravenous line to be in place. This line, along with conditions associated with PN, increases the risk of recurrent sepsis.[5]Moreover, in this group of patients, there is mounting evidence to suggest that bacterial translocation from the gut may also be an important source of pathogens.[43,44]O'Brien et al[45]showed that translocation of Gram-negative bacteria to the mesenteric lymph nodes and liver is more frequent after massive small bowel resection in mice.
Sepsis has been strongly associated with the development of cholestasis in the setting of PN.[46]Evidence supports the proposal that these effects are mediated through endotoxin and other pro-inflammatory molecules. Sepsis likely causes a systemic inflammation in the liver because of the release of pro-inflammatory cytokines that are activated by endotoxins.[7]Proinflammatory cytokines such as TNF and IL-1β have been shown to down-regulate the transcription of crucial bile acid transporters.[47,48]In addition, bacterial lipopolysaccharide acts directly through toll-like receptor 4 to induce proinflammatory chemokines and adhesion molecules in activated human hepatic stellate cells, promoting on-going inflammation and fibrosis.[49]
Lack of enteral intake
Lack of enteral feeding has several metabolic and endocrine consequences for intestinal and liver function. Experimental studies[50-52]have shown that the fasted state reduces the secretion of several gastrointestinal hormones, such as cholecystokinin, gastrin, and peptide YY. These hormones are instrumental in stimulating bile flow and gallbladder contraction, and maintaining intestinal motility. Therefore, lack of enteral feeding can result in reduced bile acid secretion and reduced gallbladder contractility. The lower levels of these hormones may also lead to intestinal stasis and consequent bacterial overgrowth. Moreover, the fasted state and sepsis are associated with atrophy of the intestinal mucosa and impairment of the intestinal barrier.[53]These in turn promote bacterial translocation and increase the risk of sepsis.
Prevention and treatment
General therapy
It is possible to recommend the prevention and treatment of PNALD on the basis of the above risk factors, though not all can be altered. Conventional management includes treatment of sepsis, decreased PN administration and gradual achievement of full enteral tolerance. With our current understanding of the pathogenesis of PNALD, infections, especially central line-associated sepsis, should be prevented and treated in patients on PN. Aseptic technique should be observed and strict protocols should be followed with catheter insertion and care. Management of central line-associated sepsis varies according to the infective organism and how sick the patient is at the time. After appropriate cultures of blood and catheter samples, empirical antimicrobial therapy should be initiated on the basis of clinical clues, the severity of the patient's acute illness and the underlying disease. Catheter removal may be necessary for successful eradication of infection, and antimicrobial therapy for 7-10 days is imperative for patients with a tunnel infection or port abscess.[54]When a catheter-related infection is documented and a specific pathogen is identified, systemic antimicrobial therapy should be narrowed.
With regard to strategies to prevent intestinal bacterial overgrowth and translocation of bacteria from the gut, antibiotic agents such as metronidazole and norfloxacin appear to play an important role in preventing and managing PNALD.[55,56]Recent studies[57,58]showed that supplementation with probiotics promotes partial restoration of intestinal microflora, reduces bacterial translocation and improves intestinal barrier function. The addition of fiber to enteral feeds if tolerated prevents the loss of intestinal barrier function against luminal bacteria that occurs in mice fed an oral PN solution.[59]A cycle of oral antibiotics is often used after confirmation of small bowel bacterial overgrowth with fecal and/ or duodenal fluid flora.[7]Despite this, we still need more evidence on their effects from carefully designed randomized controlled trials.
Ursodeoxycholic acid (ursodiol), which is a naturally-occurring hydrophilic bile acid formed in the liver and intestine, is used periodically with questionable benefit. This drug may be helpful in reducing the duration of PN-induced cholestasis and improving liver function;[60,61]however, another study[62]in infants given ursodiol prophylactically to prevent PNALD found no benefit. Moreover, recent guidelines[63,64]on ursodiol for primary biliary cirrhosis suggest it does nothing to delay the time to liver transplant and as such may be of no benefit in PNALD.
Despite the benefits of PN, achievement of full enteral tolerance is the key point in the nutritional management of patients with PNALD. Early enteral intake may be beneficial in promoting the enterohepatic circulation of bile acids and improving bile flow, whichmay prevent or improve cholestasis.[7,65]All measures to optimize the enteral route for feeding should be taken.
In patients with intestinal failure who are intolerant of enteral feeding, where complete weaning from PN is impossible, avoidance of excessive PN energy and minimizing ILE may be helpful in preventing cholestasis.[66]There is evidence that a lipid dose >1 g/kg per day is associated with the development of PNALD in adults.[15]Therefore, receiving ILE in quantities <1 g/kg per day is reasonable in adult patients. However, it is not clear whether the decrease in lipid is applicable to children, who need to grow and whose liver disease progresses rapidly.
PN supplementation
Recently, evidence has suggested that ILE containing fish oil as a novel strategy may be useful in reversing PNALD. Research on animals has shown that fish oilderived ILE prevents hepatic steatosis, when compared to conventional soybean oil-based products.[67]In infants, Gura et al[68]first reported that two infants with PNALD had significant improvement of liver function and complete resolution of cholestasis after the use of fish oil-based ILE. Subsequently, the same group has published a number of small studies demonstrating the beneficial use of fish oil lipid emulsions in the prevention or treatment of cholestasis.[69,70]More recently, in a larger, open-label trial, they showed that infants who received fish oil as the sole lipid source experienced reversal of cholestasis more frequently (19/42 vs 2/49 receiving soybean oil) and many came off PN.[71]Similarly, Diamond and his colleagues[72]found that a mixture of fish oil and soybean oil ILE also restored liver function significantly and achieved sustained resolution of cholestasis with no adverse sideeffects in 9 of 12 patients. However, some patients did worse and had to be rescued with monotherapy (fish oil) and three required a liver-intestine transplant during the study. In adults with PNALD, Pironi et al[73]and Jurewitsch et al[74]reported cases of improvement of liver function tests during the use of a fish oil-based ILE. In our experience, we performed an open-label study of a fish oil-based ILE in 15 adults with short bowel syndrome, who developed cholestasis while receiving soybean oil-based ILE. Our data demonstrated that 12 of the 15 had their direct bilirubin normalized within 4 weeks and serial liver biopsy specimens showed progressive histological improvement.[75]In addition to cholestatic reversal, a fish oil-based lipid emulsion is associated with significant improvement in all major lipid panels such as total serum cholesterol, triglyceride and high-density lipoprotein.[76,77]It should also be noted that more researach into the long-term outcomes of this novel therapy for PNALD in adults is required.
The components of fish oil eicosapentaenoic acid and docosahexaenoic acid are thought to confer benefits on the liver. One important mechanism for these changes is the ability of ω-3 PUFAs to buffer against the proinflammatory effects of ω-6 PUFA intake. Lowered ω-6 PUFA intake is likely to reduce hepatic inflammation. In addition to this, fish oil does not contain phytosterols, which have antagonistic activity against FXR gene expression which significantly compromises the hepatoprotective mechanisms that normally act to attenuate cholestasis.[3]Moreover, Zhao et al[78]demonstrated that eicosapentaenoic acid has more affinity to enhance FXR agonist-induced bile salt export protein expression, which mediates bile acid excretion into bile.
Glutamine is a primary fuel for enterocytes and for gut-associated lymphoid tissue. Addition of glutamine to PN solutions may lead to a significant improvement in intestinal epithelium barrier function, by up-regulating the expression of tight junction proteins and reducing the translocation of bacteria from the gut lumen to the systemic circulation.[79]Wang et al[80]and Wu et al[81]reported their experience of parenteral glutamine improving outcomes in very low birth-weight infants and infant rabbits. They reported that the serum levels of aspartate aminotransferase and total bilirubin decreased after PN in the glutamine-supplemented group. However, intravenous forms of glutamine are not readily available in some countries, since the benefits of treatment remain controversial[82]and guidelines for their use are needed.
Transplantation
First of all, liver or combined liver-intestinal transplants should be used cautiously, because survival on intestinal and liver transplant is less than that on long-term total PN and the advent of fish oil as an emerging treatment. For patients who have established end-stage PNALD and cannot wean off total PN, fish oil may not improve established fibrosis, and certainly cirrhosis,[83]so the only option may be transplantation; this has been demonstrated to normalize liver blood test abnormalities[84]in patients receiving an isolated intestinal transplant. Over the last decade, there have been significant improvements in surgical techniques, organ allocation, immunosuppression and post-transplant care. With the use of antilymphoid preparations and minimal post-transplant immunosuppression, the University of Pittsburgh has recently reported that the 1- and 5-year survival rates of intestine-liver allografts were 92% and 70%, respectively. Of the current 272 survivors, 245 (90%) were completelyoff PN with full nutritional autonomy.[85]Therefore, though it is still associated with significant morbidity and mortality such as infection and chronic rejection, transplantation remains a promising option for patients with end-stage liver disease.
Conclusions
PNALD is a common and potentially life-threatening problem for patients receiving long-term PN. The use of emulsions, particularly those containing phytosterols, is associated with PNALD. Management strategies for its prevention and treatment include consideration of early enteral feeding, reduction of total calories and amounts of ω-6 PUFAs and removal of phytosterols, the use of specialized lipid emulsions such as fish oil emulsions, and isolated small bowel or combined liver and small bowel transplantation. A fish oil supplement is effective in infants, but in adults, fish oil is a useful salvage maneuver requiring more study. Whether there is a time-window during which the liver can be salvaged, but after which it cannot, needs to be determined. A greater understanding of the pathogenesis of PNALD has led to targeted interventions to prevent and treat the condition. Further study should aim to better understand the mechanisms of PNALD and the long-term outcomes of its therapies.
Contributors:XZW wrote the main body of the article under the supervision of LYS. LYS is the guaranter.
Funding:None
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Llop JM, Virgili N, Moreno-Villares JM, García-Peris P, Serrano T, Forga M, et al. Phytosterolemia in parenteral nutrition patients: implications for liver disease development. Nutrition 2008;24:1145-1152.
2 Hallikainen M, Huikko L, Kontra K, Nissinen M, Piironen V, Miettinen T, et al. Effect of parenteral serum plant sterols on liver enzymes and cholesterol metabolism in a patient with short bowel syndrome. Nutr Clin Pract 2008;23:429-435.
3 Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, et al. Stigmasterol, a soy lipidderived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res 2007;62:301-306.
4 Ellegård L, Sunesson A, Bosaeus I. High serum phytosterol levels in short bowel patients on parenteral nutrition support. Clin Nutr 2005;24:415-420.
5 Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol 2007;4:277-287.
6 Buchman AL, Iyer K, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/ liver transplantation. Hepatology 2006;43:9-19.
7 Kumpf VJ. Parenteral nutrition-associated liver disease in adult and pediatric patients. Nutr Clin Pract 2006;21:279-290.
8 Goulet O, Joly F, Corriol O, Colomb-Jung V. Some new insights in intestinal failure-associated liver disease. Curr Opin Organ Transplant 2009;14:256-261.
9 Wiles A, Woodward JM. Recent advances in the management of intestinal failure-associated liver disease. Curr Opin Clin Nutr Metab Care 2009;12:265-272.
10 Nehra D, Fallon EM, Puder M. The prevention and treatment of intestinal failure-associated liver disease in neonates and children. Surg Clin North Am 2011;91:543-563.
11 Slicker J, Vermilyea S. Pediatric parenteral nutrition: putting the microscope on macronutrients and micronutrients. Nutr Clin Pract 2009;24:481-486.
12 Naini BV, Lassman CR. Total parenteral nutrition therapy and liver injury: a histopathologic study with clinical correlation. Hum Pathol 2012;43:826-833.
13 Messing B, Bories C, Kunstlinger F, Bernier JJ. Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology 1983;84:1012-1019.
14 Duro D, Mitchell PD, Kalish LA, Martin C, McCarthy M, Jaksic T, et al. Risk factors for parenteral nutrition—associated liver disease following surgical therapy for necrotizing enterocolitis: A Glaser Pediatric Research Network Study. J Pediatr Gastroenterol Nutr 2011;52:595-600.
15 Cavicchi M, Beau P, Crenn P, Degott C, Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med 2000;132:525-532.
16 Diamond IR, de Silva NT, Tomlinson GA, Pencharz PB, Feldman BM, Moore AM, et al. The role of parenteral lipids in the development of advanced intestinal failure-associated liver disease in infants: a multiple-variable analysis. JPEN J Parenter Enteral Nutr 2011;35:596-602.
17 Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology 2006;130:S70-77.
18 Beale EF, Nelson RM, Bucciarelli RL, Donnelly WH, Eitzman DV. Intrahepatic cholestasis associated with parenteral nutrition in premature infants. Pediatrics 1979;64:342-347.
19 Viña J, Vento M, García-Sala F, Puertes IR, Gascó E, Sastre J, et al. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am J Clin Nutr 1995;61:1067-1069.
20 Lester R, St Pyrek J, Little JM, Adcock EW. Diversity of bile acids in the fetus and newborn infant. J Pediatr Gastroenterol Nutr 1983;2:355-364.
21 Balistreri WF, Heubi JE, Suchy FJ. Immaturity of the enterohepatic circulation in early life: factors predisposing to "physiologic" maldigestion and cholestasis. J Pediatr Gastroenterol Nutr 1983;2:346-354.
22 Colomb V, Jobert-Giraud A, Lacaille F, Goulet O, Fournet JC, Ricour C. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN J Parenter Enteral Nutr 2000;24:345-350.
23 Meisel JA, Le HD, de Meijer VE, Nose V, Gura KM, Mulkern RV, et al. Comparison of 5 intravenous lipid emulsions andtheir effects on hepatic steatosis in a murine model. J Pediatr Surg 2011;46:666-673.
24 Javid PJ, Greene AK, Garza J, Gura K, Alwayn IP, Voss S, et al. The route of lipid administration affects parenteral nutritioninduced hepatic steatosis in a mouse model. J Pediatr Surg 2005;40:1446-1453.
25 Wanten GJ, Calder PC. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr 2007;85:1171-1184.
26 Clayton PT, Bowron A, Mills KA, Massoud A, Casteels M, Milla PJ. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology 1993;105:1806-1813.
27 Rocha M, Banuls C, Bellod L, Jover A, Victor VM, Hernandez-Mijares A. A review on the role of phytosterols: new insights into cardiovascular risk. Curr Pharm Des 2011;17:4061-4075.
28 Fiorucci S, Baldelli F. Farnesoid X receptor agonists in biliary tract disease. Curr Opin Gastroenterol 2009;25:252-259.
29 Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000; 102:731-744.
30 Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys 2005;433:397-412.
31 Degott C, Messing B, Moreau D, Chazouillères O, Paris R, Colombel JF, et al. Liver phospholipidosis induced by parenteral nutrition: histologic, histochemical, and ultrastructural investigations. Gastroenterology 1988;95:183-191.
32 Richardson TJ, Sgoutas D. Essential fatty acid deficiency in four adult patients during total parenteral nutrition. Am J Clin Nutr 1975;28:258-263.
33 Zagara G, Locati L. Role of total parenteral nutrition in determining liver insufficiency in patients with cranial injuries. Glucose vs glucose + lipids. Minerva Anestesiol 1989;55:509-512.
34 Kaminski DL, Adams A, Jellinek M. The effect of hyperalimentation on hepatic lipid content and lipogenic enzyme activity in rats and man. Surgery 1980;88:93-100.
35 Black DD, Suttle EA, Whitington PF, Whitington GL, Korones SD. The effect of short-term total parenteral nutrition on hepatic function in the human neonate: a prospective randomized study demonstrating alteration of hepatic canalicular function. J Pediatr 1981;99:445-449.
36 Vileisis RA, Inwood RJ, Hunt CE. Prospective controlled study of parenteral nutrition-associated cholestatic jaundice: effect of protein intake. J Pediatr 1980;96:893-897.
37 Mosharov E, Cranford MR, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 2000;39: 13005-13011.
38 Moss RL, Haynes AL, Pastuszyn A, Glew RH. Methionine infusion reproduces liver injury of parenteral nutrition cholestasis. Pediatr Res 1999;45:664-668.
39 Buchman AL, Moukarzel A, Jenden DJ, Roch M, Rice K, Ament ME. Low plasma free choline is prevalent in patients receiving long term parenteral nutrition and is associated with hepatic aminotransferase abnormalities. Clin Nutr 1993;12:33-37.
40 Chawla RK, Berry CJ, Kutner MH, Rudman D. Plasma concentrations of transsulfuration pathway products during nasoenteral and intravenous hyperalimentation of malnourished patients. Am J Clin Nutr 1985;42:577-584.
41 Buchman AL, Ament ME, Sohel M, Dubin M, Jenden DJ, Roch M, et al. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. JPEN J Parenter Enteral Nutr 2001;25:260-268.
42 Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology 1995; 22:1399-1403.
43 Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 1988; 104:185-190.
44 Verbeke L, Nevens F, Laleman W. Bench-to-beside review: acute-on-chronic liver failure - linking the gut, liver and systemic circulation. Crit Care 2011;15:233.
45 O'Brien DP, Nelson LA, Kemp CJ, Williams JL, Wang Q, Erwin CR, et al. Intestinal permeability and bacterial translocation are uncoupled after small bowel resection. J Pediatr Surg 2002;37:390-394.
46 Moss RL, Amii LA. New approaches to understanding the etiology and treatment of total parenteral nutritionassociated cholestasis. Semin Pediatr Surg 1999;8:140-147.
47 Trauner M, Arrese M, Lee H, Boyer JL, Karpen SJ. Endotoxin downregulates rat hepatic ntcp gene expression via decreased activity of critical transcription factors. J Clin Invest 1998; 101:2092-2100.
48 Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept 2004;2:4.
49 Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 2003;37:1043-1055.
50 Lucas A, Bloom SR, Aynsley-Green A. Metabolic and endocrine consequences of depriving preterm infants of enteral nutrition. Acta Paediatr Scand 1983;72:245-249.
51 Reinshagen K, Adams R, Trunk M, Wessel LM. The chronic liver disease in patients with short bowel syndrome: etiology and treatment. Minerva Pediatr 2009;61:273-281.
52 Greenberg GR, Wolman SL, Christofides ND, Bloom SR, Jeejeebhoy KN. Effect of total parenteral nutrition on gut hormone release in humans. Gastroenterology 1981;80:988-993.
53 Ding LA, Li JS, Li YS, Zhu NT, Liu FN, Tan L. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J Gastroenterol 2004;10:2373-2378.
54 Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis 2001;32:1249-1272.
55 Lambert JR, Thomas SM. Metronidazole prevention of serum liver enzyme abnormalities during total parenteral nutrition. JPEN J Parenter Enteral Nutr 1985;9:501-503.
56 Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, et al. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut 2005;54:718-725.
57 Ren ZG, Liu H, Jiang JW, Jiang L, Chen H, Xie HY, et al. Protective effect of probiotics on intestinal barrierfunction in malnourished rats after liver transplantation. Hepatobiliary Pancreat Dis Int 2011;10:489-496.
58 Besselink MG, van Santvoort HC, Renooij W, de Smet MB, Boermeester MA, Fischer K, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg 2009;250:712- 719.
59 Spaeth G, Specian RD, Berg RD, Deitch EA. Bulk prevents bacterial translocation induced by the oral administration of total parenteral nutrition solution. JPEN J Parenter Enteral Nutr 1990;14:442-447.
60 Al-Hathlol K, Al-Madani A, Al-Saif S, Abulaimoun B, Al-Tawil K, El-Demerdash A. Ursodeoxycholic acid therapy for intractable total parenteral nutrition-associated cholestasis in surgical very low birth weight infants. Singapore Med J 2006;47:147-151.
61 Spagnuolo MI, Iorio R, Vegnente A, Guarino A. Ursodeoxycholic acid for treatment of cholestasis in children on long-term total parenteral nutrition: a pilot study. Gastroenterology 1996;111:716-719.
62 Heubi JE, Wiechmann DA, Creutzinger V, Setchell KD, Squires R Jr, Couser R, et al. Tauroursodeoxycholic acid (TUDCA) in the prevention of total parenteral nutritionassociated liver disease. J Pediatr 2002;141:237-242.
63 Gong Y, Huang ZB, Christensen E, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev 2008:CD000551.
64 Gong Y, Huang Z, Christensen E, Gluud C. Ursodeoxycholic acid for patients with primary biliary cirrhosis: an updated systematic review and meta-analysis of randomized clinical trials using Bayesian approach as sensitivity analyses. Am J Gastroenterol 2007;102:1799-1807.
65 Kelly DA. Preventing parenteral nutrition liver disease. Early Hum Dev 2010;86:683-687.
66 Cober MP, Teitelbaum DH. Prevention of parenteral nutrition-associated liver disease: lipid minimization. Curr Opin Organ Transplant 2010;15:330-333.
67 Alwayn IP, Gura K, Nosé V, Zausche B, Javid P, Garza J, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res 2005;57:445-452.
68 Gura KM, Duggan CP, Collier SB, Jennings RW, Folkman J, Bistrian BR, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics 2006;118:e197-e201.
69 Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 2008;121:e678-686.
70 Lee SI, Valim C, Johnston P, Le HD, Meisel J, Arsenault DA, et al. Impact of fish oil-based lipid emulsion on serum triglyceride, bilirubin, and albumin levels in children with parenteral nutrition-associated liver disease. Pediatr Res 2009;66:698-703.
71 Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg 2009;250:395-402.
72 Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr 2009;48:209-215.
73 Pironi L, Colecchia A, Guidetti M, Belluzzi A, D'Errico A. Fish oil-based emulsion for the treatment of parenteral nutrition associated liver disease in an adult patient. e-SPEN Eur E J Clin Nutr Metab 2010;5:e243-e246.
74 Jurewitsch B, Gardiner G, Naccarato M, Jeejeebhoy KN. Omega-3-enriched lipid emulsion for liver salvage in parenteral nutrition-induced cholestasis in the adult patient. JPEN J Parenter Enteral Nutr 2011;35:386-390.
75 Xu Z, Li Y, Wang J, Wu B, Li J. Effect of omega-3 polyunsaturated fatty acids to reverse biopsy-proven parenteral nutrition-associated liver disease in adults. Clin Nutr 2012;31:217-223.
76 Le HD, de Meijer VE, Zurakowski D, Meisel JA, Gura KM, Puder M. Parenteral fish oil as monotherapy improves lipid profiles in children with parenteral nutrition-associated liver disease. JPEN J Parenter Enteral Nutr 2010;34:477-484.
77 Le HD, de Meijer VE, Robinson EM, Zurakowski D, Potemkin AK, Arsenault DA, et al. Parenteral fish-oil-based lipid emulsion improves fatty acid profiles and lipids in parenteral nutrition-dependent children. Am J Clin Nutr 2011;94:749-758.
78 Zhao A, Yu J, Lew JL, Huang L, Wright SD, Cui J. Polyunsaturated fatty acids are FXR ligands and differentially regulate expression of FXR targets. DNA Cell Biol 2004;23 (8):519-526.
79 Nose K, Yang H, Sun X, Nose S, Koga H, Feng Y, et al. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res 2010; 30:67-80.
80 Wang Y, Tao YX, Cai W, Tang QY, Feng Y, Wu J. Protective effect of parenteral glutamine supplementation on hepatic function in very low birth weight infants. Clin Nutr 2010;29:307-311.
81 Wu J, Hong L, Cai W, Tang Q, Shi C. Glutamine attenuates TPN-associated liver injury in infant rabbits. Eur J Pediatr 2007;166:601-606.
82 Albers MJ, Steyerberg EW, Hazebroek FW, Mourik M, Borsboom GJ, Rietveld T, et al. Glutamine supplementation of parenteral nutrition does not improve intestinal permeability, nitrogen balance, or outcome in newborns and infants undergoing digestive-tract surgery: results from a double-blind, randomized, controlled trial. Ann Surg 2005; 241:599-606.
83 Fitzgibbons SC, Jones BA, Hull MA, Zurakowski D, Duro D, Duggan C, et al. Relationship between biopsy-proven parenteralnutrition-associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J Pediatr Surg 2010;45:95-99.
84 Lauro A, Zanfi C, Ercolani G, Dazzi A, Golfieri L, Amaduzzi A, et al. Recovery from liver dysfunction after adult isolated intestinal transplantation without liver grafting. Transplant Proc 2006;38:3620-3624.
85 Abu-Elmagd KM, Costa G, Bond GJ, Soltys K, Sindhi R, Wu T, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg 2009;250:567-581.
November 8, 2011
Accepted after revision April 3, 2012
Author Affiliations: Department of Surgery, Jinling Hospital, Nanjing University School of Medicine, Nanjing 210002, China (Xu ZW and Li YS)
You-Sheng Li, MD, Department of Surgery, Jinling Hospital, Nanjing University School of Medicine, 305 East Zhongshan Road, Nanjing 210002, China (Tel: 86-25-80860137; Fax: 86-25-8480396; Email: liys@medmail.com.cn)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60229-X
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer
- Risk factors and incidence of acute pyogenic cholangitis
- Endoscopic sphincterotomy associated cholangitis in patients receiving proximal biliary self-expanding metal stents
- Biliary drainage for obstructive jaundice caused by unresectable hepatocellular carcinoma: the endoscopic versus percutaneous approach
- Gallstone-related complications after Roux-en-Y gastric bypass: a prospective study
- Inhibiting the expression of hepatocyte nuclear factor 4 alpha attenuates lipopolysaccharide/ D-galactosamine-induced fulminant hepatic failure in mice
