Gastric- and intestinal-type marker expression in invasive ductal adenocarcinoma of the pancreas
2012-07-07YuichiTakanoNobuyukiOhikeTakumaTajiriKunioAsonumaKenjiHaradaHiroshiTakahashiandToshioMorohoshi
Yuichi Takano, Nobuyuki Ohike, Takuma Tajiri, Kunio Asonuma, Kenji Harada, Hiroshi Takahashi and Toshio Morohoshi
Tokyo, Japan
Original Article / Pancreas
Gastric- and intestinal-type marker expression in invasive ductal adenocarcinoma of the pancreas
Yuichi Takano, Nobuyuki Ohike, Takuma Tajiri, Kunio Asonuma, Kenji Harada, Hiroshi Takahashi and Toshio Morohoshi
Tokyo, Japan
BACKGROUND:Although invasive ductal adenocarcinoma of the pancreas (PDAC) manifests as a relatively uniform histomorphological feature of the pancreatobiliary type, it may be complicated by metaplastic changes and heterogeneous gastric and intestinal elements. This study aimed to investigate the complication rate and clinicopathological significance of such heterogeneous elements.
METHODS:Fifty-nine patients who underwent resection of PDAC were examined in this study. Immunohistochemically, tumors showing high expression (>25%) of the intestinal-type (INT) marker CDX2 were classified as PDAC with INT. Those with high expression (>25%) of the gastric-type (GAS) marker MUC5AC were classified as PDAC with GAS, while those with high expression of both markers were classified as PDAC with INT/GAS. These patients were compared with those with PDAC of the negative group in which neither markers was highly expressed to examine their clinicopathological significance.
RESULTS:In the 59 patients, 31 (52.5%) showed high CDX2 or MUC5AC expression. Twenty-eight patients (47.5%) belonged to a negative group, 11 (18.6%) to a PDAC with INT group, 15 (25.4%) to a PDAC with GAS group, and 5 (8.5%) to a PDAC with INT/GAS group. No significant differences were observed for age, gender, size, localization, Tclassification, or prognosis among the four groups. Although the PDAC with GAS group had well differentiated types significantly more than the other groups, the rate of lymph node metastasis in this group was significantly higher (PDAC with GAS: 73%; other groups: 36%).
CONCLUSION:Complications with heterogeneous elements are not uncommon in PDAC, and this should be considered during the diagnosis and treatment of PDAC along with histogenesis of the disease.
(Hepatobiliary Pancreat Dis Int 2012;11:424-428)
ductal adenocarcinoma; pancreatic neoplasms; gastric-type; intestinal-type; immunohistochemistry
Introduction
Although numerous diagnostic methods are being investigated and developed for the early detection and treatment of highly malignant invasive ductal adenocarcinoma of the pancreas (PDAC), most of this cancer still remains discovered at an advanced inoperable stage. Even when curative resection is performed, the 5-year survival rate is 15%-25%, and prognosis is extremely unfavorable.[1,2]Most patients are treated with chemotherapy, and the present standard chemotherapy includes gemcitabine hydrochloride. However, the benefits of chemotherapy for PDAC are limited.[3,4]Chemotherapy is considered not beneficial because PDAC is hypovascular and characterized by marked fibrosis. In addition, the mutation of cell membrane transporters in pancreatic cancer cells is involved in gemcitabine resistance.[5]Furthermore, although PDAC generally lacks histological variety and shows an exclusive expression of MUC1, a pancreatobiliary-type marker, the expression of other phenotypic markers such as MUC2 and CDX2 (intestinal-type) or MUC5AC and MUC6 (gastric-type) is considered exceptional, a range of phenotypes may exist. Thus, absence of accurate and appropriate chemotherapy strategies in each case, taking into account differences between phenotypes, may be another cause. This diversity of phenotypes in PDAC has also been indicatedby various cell phenotypes and established histological classification systems (gastric, pancreatobiliary, intestinal, and oncocytic types) for intraductal papillary mucinous neoplasm, another ductal tumor of the pancreas.[6]
The purpose of this study was to investigate the frequency of expression and clinicopathological significance of heterogeneous gastric- and intestinaltype markers in PDAC.
Methods
Patients
The study included 59 patients who had undergone pancreatic resection at a facility affiliated with our laboratory from 2005 to 2009. The patients were histopathologically diagnosed with PDAC (pancreatoduodenectomy in 45 patients, distal pancreatectomy in 13, and total pancreatectomy in 1). The male to female ratio was 1:0.97 and the median age of the patients was 69.9 years (range 46-90). The tumor, node, metastasis (TNM) stage was classified according to the criteria issued by the International Union Against Cancer. Stageitumors were found in 2 patients, stage II in 7, stage III in 32, and stage IV in 18. Postoperative chemotherapy was given to 48 patients, most of whom were treated with gemcitabine hydrochloride (alone or by a combination with S-1). A prognostic survey was conducted using clinical records of 46 patients who were followed up for 2 years after surgery. Two of the 46 patients died 2 months after surgery: 1 died after occurrence of a pancreatic leak and the other was due to complications from failed biliaryjejunal anastomosis. These 2 patients were excluded from the prognostic study.
Immunohistochemistry
Immunohistochemical staining was performed using the avidin-biotin complex detection system with the BENCHMARK (Ventana Medical Systems, Inc. Tucson, AZ, USA) automated immunostaining device. For each patient, a 3-µm thick section that included the central area and margins of the tumor was cut from a formalin-fixed paraffin-embedded block and used for immunostaining. The markers used were MUC5AC (CLH2, Novocastra, Newcastle upon Tyne, UK; diluted 100-times), CDX2 (AMT28, Novocastra; diluted 50-times), and MUC1 (Ma695, Novocastra; diluted 100-times). MUC5AC was treated as a gastric-type marker, CDX2 as an intestinal-type marker, and MUC1 as a pancreatobiliary-type marker. Intestinal-type marker MUC2 was not used because its expression was very limited (unpublished data), and gastric-type marker MUC6 was also not used because MUC6 is known to be not so useful for differentiating gastric phenotype from other phenotypes. For each marker, a positive staining of 25% or more of the area of tumor involvement was arbitrarily considered to represent high expression. The ratio of positive cells was evaluated by three researchers (TY, ON and TT) of our team, and agreed upon by them. Based on the marker expression, PDAC was classified into the following four groups:
i) PDAC of negative group: low MUC5AC and CDX2 expression;
ii) PDAC with intestinal phenotype (PDAC with INT): low MUC5AC expression and high CDX2 expression;
iii) PDAC with gastric phenotype (PDAC with GAS): high MUC5AC expression and low CDX2 expression;
iv) PDAC with intestinal and gastric phenotype (PDAC with INT/GAS): high MUC5AC expression and high CDX2 expression.
Clinicopathological investigation and statistical analysis
Clinicopathological findings were compared between the groups. Student'sttest and the Chi-square test were used for statistical analysis. Overall survival was analyzed using the Kaplan-Meier method and differences among the groups were assessed by the log-rank test. Results were considered to be significant ifP<0.05.
Results
MUC1 expression was found in all patients. High MUC1 expression was observed in 55 (93.2%) of the 59 patients. High CDX2 or MUC5AC expression was observed in 31 (52.5%) of the 59 patients. Furthermore, 28 patients (47.5%) belonged to a PDAC of the negative group, 11 (18.6%) to PDAC with INT, 15 (25.4%) to PDAC with GAS, and 5 (8.5%) to PDAC with INT/GAS.

Fig. 1. CDX2 expression in tumorous glands exhibiting pseudostratified spindle-shaped nuclei and chromophilic cytoplasm, similar to those in colonic adenoma or colon cancer.
CDX2 expression appeared most frequently in tumorous glands exhibiting intestinal-type morphology (Fig. 1), which was similar to that observed in colonicadenoma or colon cancer, i.e., exhibiting pseudostratified spindle-shaped nuclei and chromophilic cytoplasm. However, CDX2 expression was also observed in tumorous glands lacking these intestinal-type characteristics (Fig. 2). Similarly, MUC5AC expression was most frequently observed in columnar tumorous glands with mucinous cells, similar to the gastric foveolar epithelium (Fig. 3) but also in cuboidal epithelial tumorous glands with little mucus (Fig. 4).

Fig. 2. CDX2 expression in tumorous glands lacking intestinaltype characteristics.

Fig. 3. MUC5AC expression in columnar tumorous glands with mucinous cells, similar to the gastric foveolar epithelium.
Clinicopathological findings in the four groups are displayed in Table 1. No significant differences were observed for age, gender, tumor size, localization, or T classification among the four groups. However, the number of females was larger in the three groups of PDAC with INT and/or GAS, with a tendency toward tumor localization in the pancreatic head. When only PDAC in the pancreatic head was considered, the number of patients in the three groups of PDAC with INT and/or GAS exceeded that in the negative group, with a particularly high frequency of PDAC with GAS (Table 2). Although patients in the PDAC with GAS group had well differentiated types significantly more than those in the other three groups (P=0.047), a significantly higher rate of lymph node metastasis wasobserved in this group (P=0.013; Table 1). While the postoperative survival rate and curve for the three groups of PDAC with INT and/or GAS was lower than that for the negative group, the difference was not statistically significant (P=0.55) (Fig. 5).

Fig. 4. MUC5AC expression in tumorous glands in which mucinous cytoplasm is not so prominent.

Fig. 5. Kaplan-Meier survival curves for the four groups (P=0.55).
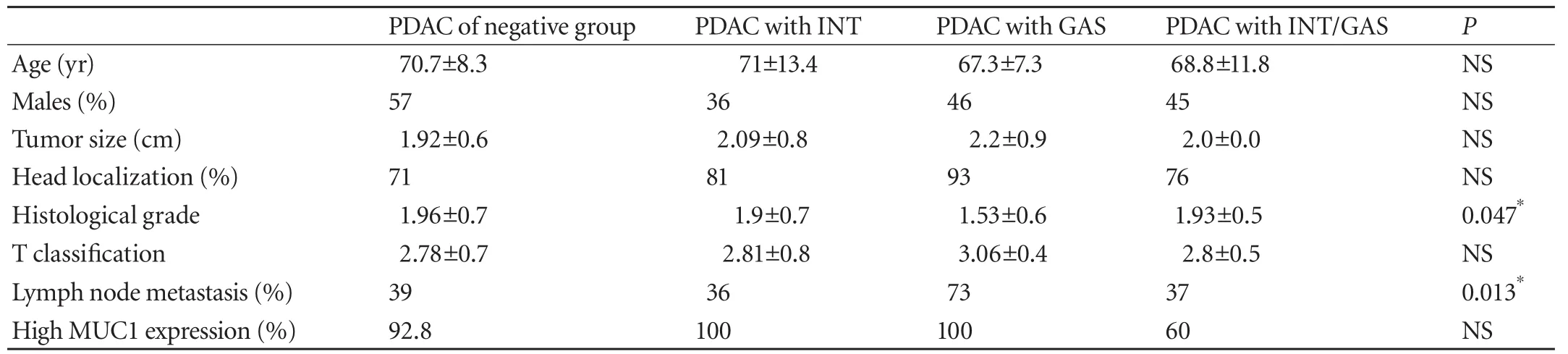
Table 1. Clinicopathological findings for the four groups

Table 2. The four groups according to pancreatic location (n, %)
Discussion
In this study significant expression of CDX2 and MUC5AC, which are heterogeneous to PDAC, was observed in approximately half of all PDAC patients. Thus, the study confirmed that similar to other carcinomas, PDAC also has heterogeneous aspects. Indeed, PDAC is relatively uniform histomorphologically and has a high expression rate of MUC1 (93.2%), indicating that the pancreatobiliary phenotype is essential so that the expression of heterogeneous markers is no more than a secondary phenomenon. However, when discussing histogenesis, diagnosis, treatment, and particularly the selection of chemotherapy drugs for PDAC, the diversity of these cell phenotypes should be considered.
In the present study, the patients in the PDAC with GAS group, who exhibited more significant expression of MUC5AC, had well differentiated types significantly more than those in the other three groups. This may correlate to pancreatic intraepithelial neoplasia (Pan-IN),[7]which is hypothesized to be a precursor lesion of PDAC, and is known for its high expression rate of MUC5AC.[8]
Nevertheless, a significantly higher rate of lymph node metastasis was observed in patients in the PDAC with GAS group than in the other groups. Although this result may be affected by the fact that tumors in this group were slightly larger than those in the other groups, it is still a result worth noting.
Scientists have not yet established a view regarding MUC5AC expression and its biological activity in PDAC.[9-13]Jinfeng et al[9]reported that MUC5AC expression indicated a good prognosis in their study of 33 PDAC cases, in which frequency of lymph node metastasis was low when MUC5AC was positive. Other reports[10,11]suggest that MUC5AC suppression due to RNA interference reduces the adhesive and invasive capacity of human pancreatic cancer cells or that tumorassociated MUC5AC stimulatesin vivotumorigenicity of human pancreatic cancer. A recent large-scale study[12]investigated 126 cases of periampullary cancer (of which 44 were PDAC) and found that MUC5AC overexpression correlated with perineural invasion, tumor recurrence, and reduced survival rates. Park et al[13]reported that MUC5AC was more frequently expressed in advanced tumors in their study on 85 cases of cholangiocarcinoma. There was no significant difference in prognosis in the present study, partly because of our small sample population. However, our results suggest that MUC5AC expression is related to metastasis and progression of PDAC and that it is an adverse prognostic factor.
In the present study, CDX2 expression was of little clinicopathological significance. Matsumoto et al[14]reported that positive CDX2 expression was a favorable prognostic factor based on their study of 41 PDAC cases, while Takahashi et al[15]reported that CDX2 inhibits human pancreatic cancer cell proliferation by downregulating cyclinD1 transcriptional activity. Albores-Saavedra et al[16]advocated the concept of intestinal type adenocarcinoma of the pancreas characterized by intestinal-type morphology and MUC2, CDX2, cytokeratin-7, and CEA expression, but reported that the clinical features were not different from those of ordinary pancreatic cancer. Our results are consistent with those of their study.
Finally, a high incidence of heterogeneous CDX2 and MUC5AC expression was observed in PDAC cases, especially PDAC in the pancreatic head, in the present study. Thus, there is a possible correlation between this high incidence and frequent exposure of the pancreatic head to physical stimulation, such as gastric juice and bile reflux, in the ductal epithelium from which PDAC originates.
In conclusion, this study investigated the frequency of expression and clinicopathological significance of gastric- and intestinal-type markers in PDAC. Significant (>25%) complications with heterogeneous elements were observed in approximately half of the PDAC cases, a phenomenon that should be considered during the diagnosis and treatment of PDAC along with histogenesis of the disease.
Contributors:TY and ON proposed the study, performed the research and wrote the first draft. TY, ON and TT collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ON is the guarantor.Funding:None.
Ethical approval:This study was approved by patients' and the ethics committee of our institute.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol 2005;23:7529-7535.
2 House MG, Gönen M, Jarnagin WR, D'Angelica M, DeMatteoRP, Fong Y, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg 2007;11:1549-1555.
3 Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-1585.
4 Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-277.
5 Zalatnai A, Molnár J. Molecular background of chemoresistance in pancreatic cancer. In Vivo 2007;21:339- 347.
6 Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 2005;447:794-799.
7 Sipos B, Frank S, Gress T, Hahn S, Klöppel G. Pancreatic intraepithelial neoplasia revisited and updated. Pancreatology 2009;9:45-54.
8 Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci 2010;17:108-124.
9 Jinfeng M, Kimura W, Hirai I, Sakurai F, Moriya T, Mizutani M. Expression of MUC5AC and MUC6 in invasive ductal carcinoma of the pancreas and relationship with prognosis. Int J Gastrointest Cancer 2003;34:9-18.
10 Yamazoe S, Tanaka H, Sawada T, Amano R, Yamada N, Ohira M, et al. RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells. J Exp Clin Cancer Res 2010; 29:53.
11 Hoshi H, Sawada T, Uchida M, Saito H, Iijima H, Toda-Agetsuma M, et al. Tumor-associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int J Oncol 2011;38:619-627.
12 Aloysius MM, Zaitoun AM, Awad S, Ilyas M, Rowlands BJ, Lobo DN. Mucins and CD56 as markers of tumour invasion and prognosis in periampullary cancer. Br J Surg 2010;97: 1269-1278.
13 Park SY, Roh SJ, Kim YN, Kim SZ, Park HS, Jang KY, et al. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact. Oncol Rep 2009; 22:649-657.
14 Matsumoto K, Mizoshita T, Tsukamoto T, Ogasawara N, Hirata A, Shimizu Y, et al. Cdx2 expression in pancreatic tumors: Relationship with prognosis of invasive ductal carcinomas. Oncol Rep 2004;12:1239-1243.
15 Takahashi K, Hirano F, Matsumoto K, Aso K, Haneda M. Homeobox gene CDX2 inhibits human pancreatic cancer cell proliferation by down-regulating cyclin D1 transcriptional activity. Pancreas 2009;38:49-57.
16 Albores-Saavedra J, Simpson K, Dancer YJ, Hruban R. Intestinal type adenocarcinoma: a previously unrecognized histologic variant of ductal carcinoma of the pancreas. Ann Diagn Pathol 2007;11:3-9.
February 7, 2012
Accepted after revision June 12, 2012
Author Affiliations: First Department of Pathology, Showa University School of Medicine, Tokyo 142-8555, Japan (Takano Y, Ohike N, Asonuma K, Harada K and Morohoshi T); Division of Gastroenterology (Takano Y, Asonuma K and Takahashi H) and Department of Diagnostic Pathology (Tajiri T), Kanagawa, Japan
Yuichi Takano, MD, First Department of Pathology, Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan (Tel: 81-3-3784-8119; Fax: 81-3-3784-8249; Email: yuichitakano1028@yahoo.co.jp)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60202-1
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells
- Expression of SOCS3 throughout liver regeneration is not regulated by DNA methylation
- Early changes of hepatic hemodynamics measured by functional CT perfusion in a rabbit model of liver tumor
- A common variant in the precursor miR-146a sequence does not predispose to cholangiocarcinoma in a large European cohort
- Hepatocyte differentiation of mesenchymal stem cells
- Early control of short hepatic portal veins in isolated or combined hepatic caudate lobectomy
