Synthesis and Characterization of Novel Fluoroalkyl Unsaturated Multi-carboxylic Acid Esters
2012-02-07YEHaohua叶皓华LIZhanxiong李战雄FANDanCHENGuoqiang陈国强
YE Hao-hua(叶皓华),LI Zhan-xiong(李战雄),FAN Dan(樊 丹),CHEN Guo-qiang(陈国强)*
1 National Engineering Laboratory for Modern Silk,Soochow University,Suzhou 215123,China
2 College of Textile and Clothing Engineering,Soochow University,Suzhou 215006,China
Introduction
Fluoride compounds are extensively used in fabrics finishing industry, electronic industry, and national defense[1-4].Owing to the acidity of fluorinated alcohols,direct esterification of such alcohols with carboxylic acid is difficult.Acryl chloride is usually used to react with fluorinated alcohols to prepare fluorinated acrylic esters.In the 1950s,1,1-heptafluorobutyl acrylate had been obtained in America and the Soviet Union by reacting CF3CF2CF2CH2OH with CH2=CHCOCl.Fluorinated acrylic ester could also be obtained by transesterification or reaction of methacrylate and fluorinated alcohols with strong acids as dehydrant[5].
Poly(fluoroalkyl acrylate)s with long fluoroalkyl(Rf)groups have excellentwaterand oilrepellentproperty.However,the degradation of them into perfluorinated carboxylic acids(PFCA)with long carbon chain may cause a series of problems such asbioaccumulating throughoutthe globe,particularly in the arctic[6,7].Research effort is now under way to develop environment-friendly materials such as polymers with short perfluorocarbon chains to replace the traditional reagents[8].Closely packed clusters of 2 or more Rf groups could lower the critical free surface energy and crystallinity,while they increase the water and thermal stability[9,10].Maleic acid,itaconic acid,and trans-aconitic acid are unsaturated carboxylic acids which can be polymerized and have been widely used in chemical industries.Among plenty of esterification methods reported[11,12],methods forpreparing unsaturated multicaboxylic acid esters with perfluoroalkyl groups were only reported in some patents.In the 1970s,maleic,itaconic esters of α,α,-dihydroperfluoroalcoholshad been prepared by reacting fluorinated alcohol with oleum,sulfur trioxide or chlorosulfonic acid,and then esterifying the resulting sulfate using corresponding acid[13].Several years later,fluorinated α,β-unsaturated esters were obtained by transesterification procedures[14].However,the previous method is not feasible and transesterifacation is neither economical nor practical.The current research is directed to partial filling of this gap.In this paper,a series of environment-friendly monomers derived from unsaturated carboxylic acid have been synthesized by directly reacting unsaturated multi-carboxylic acids and alcohols containing short fluorocarbon chains.The objective monomers were obtained by using p-toluene sulfonic acid as catalyst,hydroquinone as the anti-polymerizing agent,toluene as solvent and water carrying agent.The products were characterized by Fourier transform infrared spectroscopy(FT-IR),hydrogen-1 nuclear magnetic resonance(1H NMR),fluorine-19 nuclear magnetic resonance(19F NMR),and high resolution mass spectrometry(HRMS).
1 Experimental
1.1 Materials and instrumentation
FT-IR spectra were recorded on Thermo Electron Corporation Nicolet 5700 FT-IR spectrometer,using KBr pellets and films.1H NMR spectra were recorded on a Bruker AV 400(400 MHz)spectrometer and Bruker AV 300(300 MHz)spectrometer,respectively,using deuterated chloroform as solvent,Me4Si as internal standard.19F NMR spectra were obtained on a Bruker AV 300(300 MHz)spectrometer.HRMS spectra were obtained on a time-of-flight mass spectrometer(TOF-MS)instrument with electronimpact ionization at 70 eV and liquid chromatograph massspectrometer(LC-MS)instrument with electronimpact ionization at 175 V,respectively.
1.2 Synthesis of trans-aconitic acid
Trans-aconitic acid was synthesized according to Ref.[15].The reaction is shown in Scheme 1.

Scheme 1 Synthesis of trans-actonitic acid
The structure of the product was in well agreement with the result reported in Ref.[15].White small needles,mp:190℃,FT-IR(KBr)ν:2 983.7,1 731.1,1 700.4,1 421.6,1 292.0,1 224.1,912.0,853.2.
1.3 Typical procedure for the synthesis of fluorinated unsaturated ester
The synthetic routes for the preparation of the fluorinated monomers were outlined in Scheme 2.

To a 250 mL round bottomed flask equipped with thermometer,magnetic stirrer and Dean-Stark tube joined with a reflux condenser,maleic anhydride(9.8 g,0.1 mol)(or equivalent maleic acid),1,1,2,2-tetrahydroperfluoro-1-octanol(72.82 g,0.2 mol),hydroquinone(0.96 g),ptoluene sulfonic acid(1.52 g),toluene(150 mL)were added.Under a nitrogen atmosphere,the reaction vessel was heated in an oil bath,and the temperature was maintained at 114-118℃.Readings of temperature and the amount of water collected in the Dean-Stark tube were taken every 10 min from the time that the water was removed by azeotropic distillation with toluene.The reaction was kept for about 6 h until no water yielded and the water collected in the Dean-Stark tube was about 1.8 mL.After cooling to room temperature,the salt precipitated from the liquid was filtered to get red filtrate.And then the solvent was removed underreduced pressureto yield crudeproduct.Following column chromatography isolating,the pure2-butenedioic acid(Z)-,bis(1,1,2,2-perfluorooctyl)ester(1a)was obtained. Oil,yield 82%;FT-IR(KBr)v:3 001.5,1 741.4,1 647.3,1 467.7,1 389.4,1 334.0,1 289.9,1 226.3,1 158.1,1 110.7,1 095.5,1 047.8,735.4.1H NMR(CDCl3,300 MHz)δ(ppm):6.306(m,2H),4.478(t,J=7.5 Hz,4H),2.595(t,J=7.8 Hz,4H).19F NMR(CDCl3,300 MHz)δ(ppm):-64.822(m,12F),-81.161(t,J=14.4 Hz,6F),-108.536(m,4F),-124.090(m,J=11.7 Hz,4F).HRMS(ESI,70 eV)m/z(%):99.008 0(100),195.004 5(23.16),445.007 2(26.07).HRMS Calc.for C20H10O4F26:808.016 4,found:808.014 6.
Compound 2a,3a,1b,2b,and 3b were prepared under the same condition as that of 1a by the reaction of corresponding acid with 1,1,2,2-tetrahydroperfluorooctanol and 1,1,5-octafluoropentanol,respectively.
2-Butenedioic acid (Z)-, bis (1, 1, 5-octafluoropentanol)ester(1b)Oil,yield 58%;FT-IR(KBr)v:1 754.5,1 645.6,1 410.5,1 290.8,1 172.3,1 132.1,1 085.6,979.9,903.8,808.5.1H NMR(CDCl3,300 MHz)δ:6.417(s,2H),6.045(tt,J=5.2 Hz,and 52 Hz,2H),4.678(t,J=14 Hz,4H).19F NMR(CDCl3,300 MHz)δ:- 120.491(m,4F),- 126.062(m,4F),-130.691(m,4F),-138.104(d,J=55.2 Hz,4F).HRMS(ESI,175 V)m/z(%):562.050 7([M+NH4]+,100),567.006 9([M+Na]+,40).
Bis(1,1,2,2-perfluorooctyl)methylenesuccinate(2a)Oil,yield 85%;FT-IR(KBr)v:3 001.9,1 750.7,1 726.8,1 642.2,1 330.3,1 317.9,1 269.7,1 217.2,1 157.8,1 096.2,1 048.6,734.8.1H NMR(CDCl3,400 MHz)δ:6.377(m,1H),5.804(m,1H),4.449(t,J=7.8 Hz,2H),4.377(t,J=7.8 Hz,2H),3.359(m,2H),2.559(t,J=7.5 Hz,2H),2.521(t,J=8.1 Hz,2H).19F NMR(CDCl3,300 MHz)δ:-64.786(m,12F),-81.134(m,6F),-108.508(m,4F),-124.117(m,4F).HRMS(ESI,175 V)m/z(%):840.065 6([M+NH4]+,100),1 667.055 2([2M+NH4]+,10).
Bis(1,1,5-octafluoropentyl)methylenesuccinate(2b)Oil,yield 73%;FT-IR(KBr)v:2 977.8,1 762.2,1 740.8,1 644.7,1 403.3,1 322.7,1 291.0,1 171.1,1 129.7,1 084.7,1 059.0,975.0,903.2,809.4.1H NMR(CDCl3,400 MHz)δ:6.497(m,1H),6.054(tt,J=5.2 Hz,and 51.6 Hz,1H),6.045(tt,J=5.2 Hz,and 52 Hz,1H),5.908(m,1H),4.665(t,J=13.6 Hz,2H),4.602(t,J=13.6 Hz,2H),3.483(s,2H).19F NMR(CDCl3,300 MHz)δ:- 120.414(m,4F),- 125.996(m,2F),-126.089(m,2F),- 130.715- - 130.768(m,4F),-137.963(t,J=3 Hz,2F),-138.147(t,J=3 Hz,2F).HRMS(ESI,175 V)m/z(%):576.066 1([M+NH4]+,100).
Tri(1,1,2,2-tetrahydroperfluorooctanol)transaconitate(3a)Oil,yield 55%;FT-IR(KBr)v:3 001.5,1 781.2,1 738.1,1 653.4,1 468.1,1 416.6,1 330.7,1 272.8,1 223.5,1 159.8,1 110.8,1 094.0,1 048.7,961.9,828.7,735.0.1H NMR(CDCl3,400 MHz)δ:6.959(m,1H),4.510(t,J=7.6 Hz,2H),4.449(t,J=7.6 Hz,2H),4.387(t,J=7.6 Hz,2H),3.968(m,2H),2.513-2.603(m,6H).19F NMR(CDCl3,300 MHz)δ:-64.969(m,18F),-81.323(m,9F),-108.608(m,6F),-124.232(m,6F).HRMS(ESI,175 V)m/z(%):1 230.058 4([M+NH4]+,100),2 447.047 9([2M+NH4]+,10).
Tri(1,1,5-octafluoropentyl)trans-aconitate(3b)Oil,yield 30%;FT-IR(KBr)v:1 747.1,1 655.6,1 278.1,1 172.8,1 055.1.1H NMR(CDCl3,400 MHz)δ:7.122(d,J=12.9 Hz,1H),6.021(dtt,J=5.1 Hz,and 4.8 Hz,and 51.9 Hz,3H),4.752-4.534(m,6H),4.048(d,J=10.2 Hz,2H).19F NMR(CDCl3,300 MHz)δ:- 120.451(m,6F),-125.988(m,4F),-126.134(m,2F),-130.642(m,4F),-130.842(m,2F),-138.087(d,J=55.2Hz,6F).HRMS(ESI,175 V)m/z(%):834.058 7([M+NH4]+,100).
2 Results and Discussion
Plausible mechanism for the esterification of carboxylic acid is outlined in Scheme 3.The reaction between bicarboxylic anhydride and alcohol is usually carried by two steps:first,the generation of mono-ester can be proceeded fast even without the addition of catalyst.In the second step,generation of the bi-ester from mono-ester is slow,and the catalyst is favorable[16].The reaction of maleic acid,itaconic acid,transaconitic acid with alcohol into mono-ester could also be divided into two steps:dehydrating into anhydride and then addition reacting with alcohol.Once the temperature rises to a certain degree,the dehydration of these multi-carboxylic acids will begin.At the beginning of reaction,when temperature of mixed material raised to 110℃,equivalent amount of water could be isolated in 0.5-1.0 h,after then the generation of water was relatively slow.For trans-aconitic acid,two residual carboxylic acid groups in the monoester may locate in trans-positions and can not dehydrate into anhydride.The esterification of transcarboxylic acid groups could only carry on by the catalysis of acid,thus more time was required for the preparation of transaconitate.This phenomenon is in agreement with the mechanism mentioned above.Maleic anhydride is cheaper and easier to get,and it is more reasonable to use maleic anhydride as raw material.p-Toluene sulfonic acid was used as catalyst and toluene was used as water-carrying agent to separate the water from reactants and accelerate the reaction.

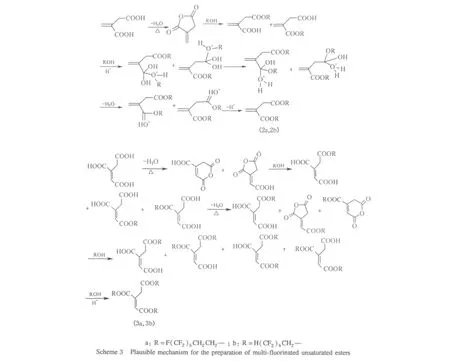
Esterification of 1,1,5-octafluoropentanol with certain carboxyl acid is more difficult than that of 1,1,2,2-tetrahydroperfluorooctanol.Under the same condition,the time needed for esterification of 1,1,2,2-tetrahydroperfluorooctanol with trans-aconitic acid was 12 h,and the volume of the collected water was equal to theoretical value,in contrast,it took 24 h to prepare tri(1,1,5-octafluoropentyl)trans-aconitate,and the water separated was only 3/4 of theoretical value.It may result from withdrawing electron effect of CF groups on—OH groups in fluorinated alcohols.The effect is more obvious in 1,1,5-octafluoropentanol.
Lower concentration NaOH solution is favorable for the neutralization of production.The addition of high concentration alkaline solution will generate too much p-toluene sulfonate,sodium hydroquinone,and sodium carboxylic acid,and may produce numerous foams during wash process,thus make it difficult to separate the product from water.
FT-IR spectra of the monomers show intense absorption bands at 1 730-1 760 cm-1arising from C=O stretch modes,C=C stretching vibrations around 1 640 cm-1and strong C—F absorption bands at 1 100-1 300 cm-1(Fig.1).The absence of wide absorption bands at about 3 400 cm-1shows no—OH group in the product.
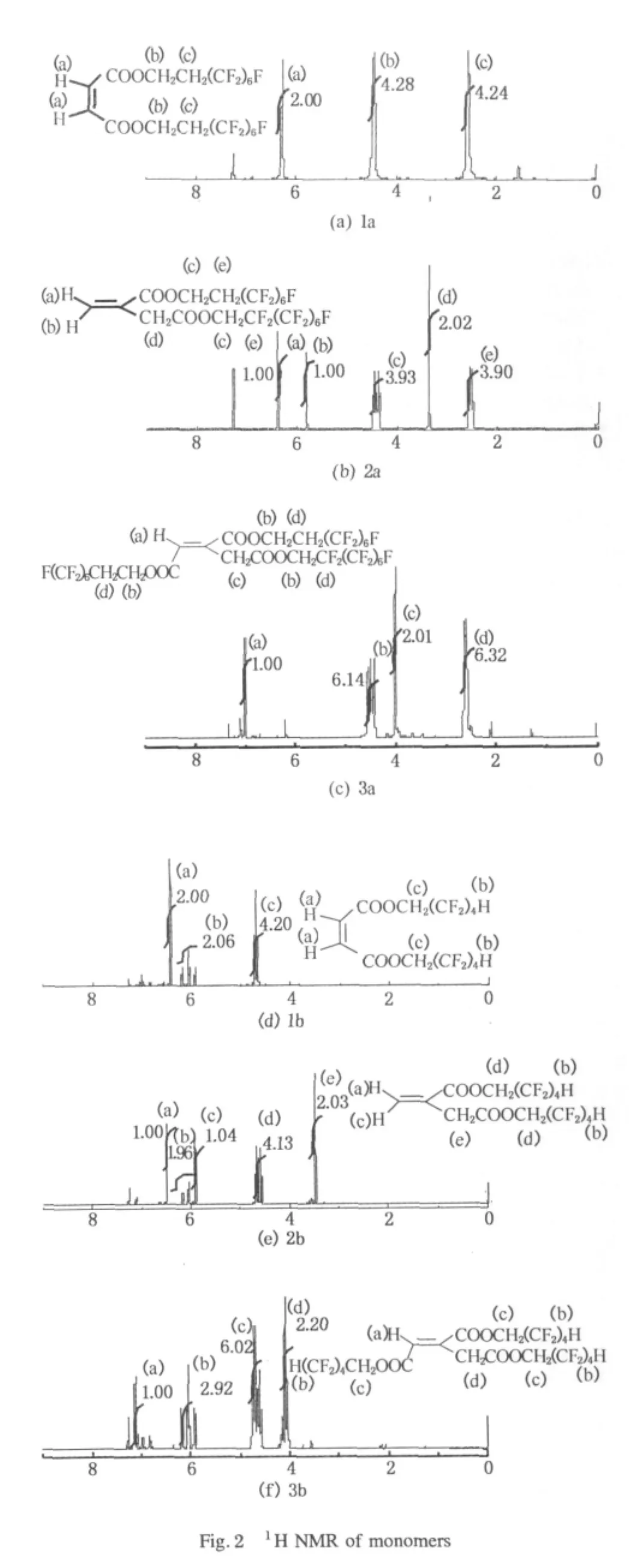
Because of the high boiling point of the products,it is difficult to purify them by distillation.Here the objective products were isolated by flash column chromatography.The1H NMR results of obtained monomers are shown in Fig.2.The1H NMR spectra indicate the products are relatively pure with only a few smallimpurity peaksin the spectra.The high electronegativity of C=O and CF2has lowered the electronic density of adjacent CH2,thus it causes lower field migration of1H NMR for all the objective fluorinated unsaturated esters.Due to the existence of carboxylic groups in the molecule of the product,the molecules may combine with Na+in electronic spray ionization(ESI)mode.[2M+Na]+peak had appeared in the HRMS data of 1b.
For the obvious steric hindrance of COOR groups in the monomer molecular,maleates,itaconates,and aconitates show lower tendency to homopolymerization than acrylate.However,they show a great tendency to form alternating copolymers with certain types of comonomers such as vinyl monomers[13].These fluorinated copolymers can impart oil,water,and soil repellent properties to substrates,and they are especially useful reagent for water and oil proofing of textiles and metal.More details aboutthepolymerization and application ofthese unsaturated multi-carboxylic esters will be reported in our future work.
3 Conclusions
A series of environment-friendly monomers derived from unsaturated carboxylic acid have been synthesized by direct esterification of unsaturated multi-carboxylic acid and alcohols containing short fluorocarbon chain.Structures of the objective products have been verified by FT-IR,1H NMR,19F NMR,and HRMS.The high electronegativity of C=O and CF2has lowered the electronic density of adjacent CH2,and caused lower field migration of1H NMR forallthe objective fluorinated unsaturated esters.
[1]Yu M H,Gu G T,Meng W D,et al.Superhydrophobic Cotton Fabric Coating Based on a Complex Layer of Silica Nanoparticles and Perfluorooctylated Quaternary Ammonium Silane Coupling Agent[J].Applied Surface Science,2007,253(7):3669-3673.
[2]Kessman A J,Huckaby D K P,Snyder C R,et al.Tribology of Water and Oil Repellent Sol-Gel Coatings for Optical Applications[J].Wear,2009,267(1/2/3/4):614-618.
[3]Ye H H,Li Z X,Chen G Q,et al.Research Progress of Fluoride-Containing Acrylate Derivatives and Their Application on Textile[J].Advanced Materials Research,2011,331:229-236.
[4]Tang C C,Bando Y,Huang Y,et al.Fluorination and Electrical Conductivity of BN Nanotubes[J].Journal of the American Chemical Society,2005,127(18):6552-6553.
[5]Zhou Y M,Huang J Y.Synthesis of Perfluoroalkylethylacrylate with p-Toluene Sulphonic Acid as Catalyst[J].Chemical World,2003,44(5):263-265.(in Chinese)
[6]Giesy J P,Kannan K.Global Distribution of Perfluorooctane Sulfonate in Wildlife[J].Environmental Science &Technology,2001,35(7):1339-1342.
[7]Ellis D A,Martin J W,de Silva A O,et al.Degradation of Fluorotelomer Alcohols:a Likely Atmospheric Source of Perfluorinated Carboxylic Acids[J].Environmental Science &Technology,2004,38(12):3316-3321.
[8]Huang J Q,Meng W D,Qing F L.Synthesis and Repellent Properties of Vinylidene Fluoride-Containing Polyacrylates[J].Journal of Fluorine Chemistry,2007,128(12):1469-1477.
[9]Reddy V S,Weikel W J,Arbaugh J,et al.Synthesis and Characterization of New Fluorinated Polyacrylates[J].Polymer,1996,37(20):4653-4656.
[10]Cassidy P E,Aminabhavi T M,Farley J M.Polymers Derived from Hexafluoroacetone[J].Journal of Macromolecular Science,Part C:Polymer Reviews,1989,29(2/3):365-429.
[11]Hamcerencu M,Desbrieres J,Khoukh A,et al.Synthesis and Characterization of New Unsaturated Esters of Gellan Gum[J].Carbohydrate Polymers,2008,71(1):92-100.
[12]William P S,Pascale C,Corinne L D,et al.Effect of Catalytic Conditions on the Synthesis of New Aconitate Esters[J].Industrial Crops and Products,2012,35(1):203-210.
[13]Holland D G,BeitchmanB D. EthylenicallyUnsaturated Dicarboxylic Acid Esters of α,α-dihydroperfluoro Alcohols:US,3868408[P].1975.
[14]Kleiner E K,Dear R E A.Polymers of Unsaturated Esters of Polyfluoroalkylthio-Alcohols:US,4171415[P].1979.
[15]Bruce W F.Aconitic Acid[J].Organic Synthesis,1943,2:12-14.
[16]Hao X R,Yu Z Q,Dong L Y,et al.Kinetic Study of Synthesis of Dimethyl Maleate[J].Chemical Reaction Engineering and Technology,2002,18(2):104-108.(in Chinese)
杂志排行
Journal of Donghua University(English Edition)的其它文章
- A Novel Preparation of Artificial Bile Ducts for Clinical Application of Biliary Diseases
- Service Robot Localization Based on Global Vision and Stereo Vision
- Efficient Rate Control Algorithm for Hierarchical Video Coding
- Effects of Pre-oxidation Conditions on Adsorption Performance of Activated Carbon Fibers
- A Modified Differential Evolution for Uniform Parallel Machines Scheduling Problem with Minimizing Makespan
- Sorption Kinetics and Capacity of Composite Materials Made up of Polymeric Fabric and Expanded Perlite for Oil in Water
