High-energy Metal Fuels for Rocket Propulsion:Characterization and Performance
2012-01-29LuigiDELUCAFilippoMAGGIStefanoDOSSIVolkerWEISERAndreaFRANZINVolkerGETTWERTThomasHEINTZ
Luigi T.DELUCA,Filippo MAGGI,Stefano DOSSI,Volker WEISER,Andrea FRANZIN,,Volker GETTWERT,Thomas HEINTZ
(1.SPLab,Dipartimento di Scienze e Tecnologie Aerospaziali,Politecnico di Milano,I-20156 Milan,Italy;2.Fraunhofer-Institut für Chemische Technologie(ICT),Joseph-von-Fraunhofer-Str.7,Pfintzal,D-76327,Germany)
Introduction
Solid rocket propulsion,offering a large thrust in compact and relatively simple propulsive systems,remains one of the most attractive options for low-cost access to space.Yet,its limited performance in terms of gravimetric specific impulse demands improvements.In general,the endless quest for more and more performing propulsive systems prompts for higher ballistic levels while maintaining acceptable mechanical properties,aging characteristics,cost,processability,and environmental respect.Due to their intrinsic way of working,performance of solid rocket propellants can mainly be augmented-in terms of gravimetric(Is)as well as volumetric specific impulse(Iv)and any other figure of merit-by resorting to more advanced propellant formulations and ingredients.In particular,highenergy metal fuels allow increasing energy content and compound density,but also require a suitable oxidizer/binder matrix providing enough oxygen and adequate mechanical properties.
In this respect,a variety of new ingredients is under examination in world-wide laboratories as possible candidates for industrial applications.Based on the work under progress for a cooperative European project[1]and propulsive industries,the ingredients taken into consideration in this paper are the following:
(1)AP(Ammonium Perchlorate)NH4ClO4,AN(Ammonium Nitrate)NH4NO3,and ADN(Ammonium Dinitramide)NH4N(NO2)2as solid inorganic oxidizers.
(2)Al and AlH3(of different size and variants by proprietary chemical/physical treatments)as solid metallic fuels.
(3)HTPB(Hydroxyl-Terminated PolyButadiene)HO-[(CH2=CH-CH=CH2)]n-OH and GAP(Glycidyl Azide Polymer)C3H5N3O as respectively inert and active polymeric binder of the propellant solid fractions.
Only laboratory level results are reported in this paper,being motor applications still under development[2].Two main families of metallized composite solid rocket propellants are discussed:APbased metallized formulations were mainly tested at the Space Propulsion Laboratory(SPLab)of Politecnico di Milano[3],while ADNbased metallized formulations were mainly tested at the ICT Fraunhofer-Institut für Chemische Technologie[4].The list of metals under examination includes on one side micrometric propulsion grade Al(μAl),activated Al(actAl),amorphous Al(amAl),aluminum hydride(AlH3)and on the other side nanometric Al(nAl).While AlH3is known to increase the ideal specific impulse due to its favorable thermochemical features,other additives(nAl and actAl)are expected to increase the delivered specific impulse by improving its efficiency through the formation of smaller size agglomerates.The standard sphericalμAl(30μm average grain size)is taken as reference and its performance can only be improved by changing the oxidizer/binder matrix.Within this broad framework,several high-energy density fuels suitable for solid rocket propulsion are fully being characterized and tested under a variety of burning conditions[3-5].
In principle,SPLab activity focuses on both solid propellants and solid fuels(HTPB-or wax-based)typically used in rocket propulsion.Numerous formulations were experimentally analyzed at SPLab and contrasted to the corresponding formulations using a conventional AP/HTPB-based propellant embedding standard spherical μAl taken as reference.
For a matter of space,in this paper only solid rocket propulsion is considered and only Al-based metallic fuels are examined.Several Al-based fuels(includingμAl,nAl,and actAl)and AlH3-based fuels are characterized and their burning properties assessed.Attention is also given to possible fuel interactions with curing and aggregation/agglomeration phenomena,both facts being important for practical applications.In fact,depending on the examined formulation,coated or high specific surface ingredients may affect significant details of both processes.Due to the conflicting properties manifested even by the same metallic fuel when used under different sizes or variants,it is recommended to implement dual fuel compositions,blending for example micro and nano Al particles,to optimize all together various aspects such as active metal content,propellant density and performance,agglomeration losses mitigation.
After presenting the solid oxidizers under consideration,a detailed discussion of the tested Al-based high-energy metallic fuels is offered.The two families of metallized composite solid rocket propellants under test are quickly described.Results so far obtained in terms of compound density,ideal thermochemistry,steady combustion rates,and aggregation/agglomeration phenomena are illustrated.Concluding remarks and hints about work either in progress or recommended will complete the paper.The final objective is to identify a viable High Specific Impulse Propellant(HISP)for space exploration.
1 Solid Oxidizers Survey
After decades of intense studies,possible candidates for rocket solid propulsion are AN,AP,the two well-known explosives RDX and HMX,ADN(originated in Russia),HNF(originated in USA[6]),and HNIW(commonly known as CL-20,originated in USA).For safety and performance reasons use of RDX,HMX,and HNF as main oxidizers is not recommended;likewise,HNIW still appears too expensive for large-scale industrial applications.The remaining candidate oxidizers are the ever green AN,the ubiquitous AP,and the promising ADN.A detailed and comparative experimental analysis of ballistic properties of the mentioned solid oxidizers(except AN)-as monopropellants -was performed under strand burner conditions by Atwood et al[7-8].AN is a low-cost green oxidizer much used for a wide-range variety of reasons;AP is considered a“miracle”of nature by propellant chemists[9]and still today is by far the most used solid oxidizer for space exploration missions.Main features[10-11]of oxidizers as monopropellants are listed in Table 1 ;Tfis the self-deflagration adiabatic temperature at 68bar.A note of caution:some data are affected by appreciable uncertainty and may be reported with disparate values in the literature;when so,a specific data source is pointed out.To assure consistency,when possible,one common reference was selected for the same property(for example,Tf).
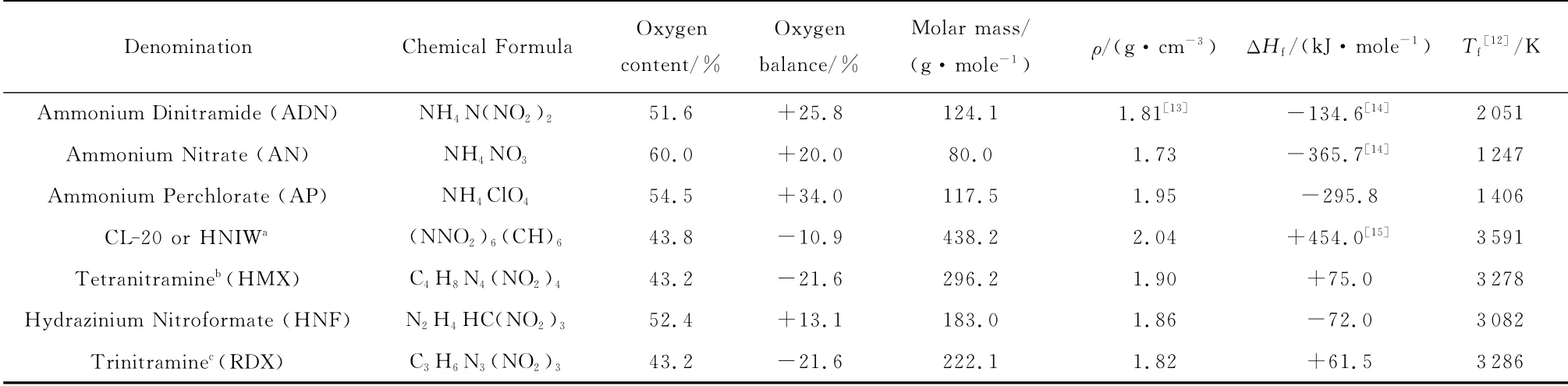
Table 1 Current solid propellant oxidizers from various sources
While AN and AP are both well-known and have been used at all levels for several decades,ADN is a relatively new product,first synthesized at the Zelinsky Institute of Organic Chemistry in Moscow,Russia,1971(and much later at SRI,USA,1988)and quickly diffused around the world after the cold war years.Main advantages of ADN as a monopropellant are superior ballistic properties(high burning rates and low pressure sensitivity),low signature,and full environmental respect;however,strong sensitivity to humidity(like AN)and initial temperature(like AN)as well as low melting[7-8](92-94°C or 365-367K)and decomposition[10](starting at 135℃)temperatures make its field use problematic.ADN also possesses a number of unique physical and chemical properties as compared to other solid oxidizers,such as high condensed phase heat capacity of 0.59cal/g/K,low surface temperatures,and a PDL around 2atm.However,the needle shaped ADN crystals hinder manufacture of propellant samples combining high ballistic performance and good mechanical properties.According to Academician Pak[16],general director of Soyuz Design Bureau,“Only a mass crystallization technology capable of turning flat,elongated crystal needles into roundish,nearly spherical particles will make it possible to raise the composition solid loading fraction up to 90%and take full advantage of ADN-based compositions”.At any rate,“the most beneficial feature of ADN salts,is that in burning they generate nothing else but air ingredients,i.e.nitrogen,oxygen,and water.And this implies environ-mental safety.What is more,there is enough oxygen to secure oxidation of both metallic and nonmetallic propellant components”.According to Pak[16],a composite propellant using ADN(instead of AP),AlH3(instead of Al),and nitrate ester binder offers the highest ideal specific impulse among all of the currently viable solid rocket propellants.
ADN burning as a monopropellant is a quite complex process whose characterization is hindered by a conspicuous data scattering reported over the years by many investigators[7-8,17-24].This is due to not only multiple ADN manufacture &supply sources and limited thermal stability,but also to a full series of reasons including unstable burning;crystals cracking at ignition or deconsolidation due to thermal stresses during burning(as for large unconfined single crystals of HMX);the influence on ballistic data of the kind of tested sample,such as crystal or pressed plate,or details of the tested strand,such as its cross-section size;the remarkable effects that minor amounts of organic and inorganic admixtures have on ADN burning;the role played by dispersion of the molten layer surface consisting of the initial ADN plus AN,the only condensed decomposition product;and so on.On the other hand,reducing agitation of the molten surface layer,for example by increasing its viscosity,reduces surface oscillations and data scatter;in turn this stabilizes burning and lowers PDL down to 0.2bar as well as burning rates up to 100 bar,when gas-phase heat release becomes dominant.
In spite of process complexity and data scattering,ADN self-deflagration rate is the highest among the considered solid oxidizers,as shown in Figure 1(AN,not reported,would be the slowest),while its initial temperature sensitivity is higher than that of AP for most of the commonly used pressure range(say,1-70bar for space launchers),as shown in Figure 2.For all tests,samples consisted of ADN powder dry pressed at 621MPa and held for 10min,resulting in rectangular pellets with 97%-98% TMD.In all experimental investigations,starting with the pioneering dataset collected on single crystals by Fogelzang et al.[17],a considerable scatter of results was observed,in particular over the pressure range 20to 100bar.
According to Pak[16],“burning rated of ADN-based propellants are characterized by an inverse,as compared to the AP-based ones,relationship with oxidizer dispersity”.This remark was generically confirmed by Manelis[18].A detailed experimental investigation on the combustion wave structure of ADN pressed pellets -by microthermocouples and up to 80bar -was offered by Zenin et al.[19],showing that the controlling heat release zone is located near the burning surface in the condensed-phase rather than gas-phase and that intrinsically unstable burning may occur in the gas-phase.Overall,a flame structure was suggested somehow recalling the classical multistage structure of DB propellant burning.
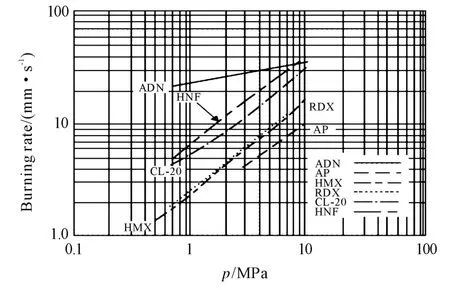
Fig.1 Steady burning rates of different monopropellants at ambient initial temperature,showing large rates and low pressure sensitivity of ADN[7]
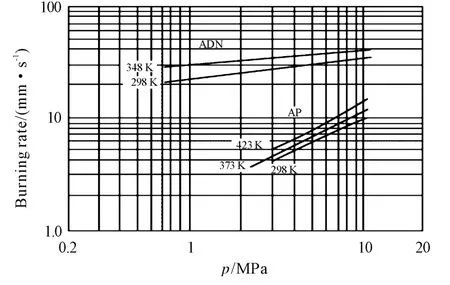
Fig.2 Steady burning rates of ADN and AP monopropellants at several initial temperatures,confirming large rates and low pressure sensitivity of ADN[7]
Experimental investigations carried out at GATech[20-21]on combustion of ADN pressed pellets,ADN sandwiches with binder,and ADN composite propellants underlined a large data scattering and reported self-deflagration rates with modest pressure sensitivity,as already indicated in Figure 1and Figure 2.Prills with different ADN size were tested as well,with unclear trends(except larger rates than single AP crystals).Further analyses conducted by Gross et al.[22]offered a multiphase combustion modelling of ADN monopropellant using a detailed chemical kinetics schemes based on an extensive liter-ature review,but with only partial success.
A comprehensive analysis of ADN flame structure was finally proposed by Sinditskii et al[23-24],who showed a three-zone flame structure(like DB propellants)with the controlling mechanism located in the condensed-phase at low pressures(below 10bar),gasphase at high pressures(above 100bar),and unstable burning in the intermediate pressure range.Pressure exponent is different in the low-and high-pressure regions.The ADN surface temperature corresponds to the dissociation temperature of AN,a decomposition product of ADN in melt.Understanding of the burning mechanism allowed the authors to understand and reduce data scattering.
Among the different attempts carried out worldwide,a method of direct nitration of salts of sulfamic acid using only standard industrial ingredients,followed by neutralization and separation of ADN,was patented by FOI in the mid-1990s[25].The purity of the produced ADN is above 99%and,having a high thermal stability,requires no stabilizer.This method was licensed to Eurenco Bofors in Sweden in 1996.To obtain a castable propellant formulation with reasonable viscosity and high solid fraction loading,particles with minimum spatial extension are required.A low aspect ratio and preferably a spherical shape are sought for.A method to produce ADN prills from the needle shaped crystals provided by Eurenco was further developed at FOI[26].Prills are produced by spraying molten ADN through a nozzle,thus forming droplets from the atomized molten ADN which later solidify to prills.Recently,an alternative technique for ADN prilling was set up at ICT by an emulsion method[27].
As an example of application,ADN-based minimum smoke propellants were developed at ICT[28]and FOI[29]to replace lead containing DB propellants for tactical missiles.In particular,test firing at FOI[29]of a rocket motor containing casted case bonded grains up to 3kg 70% ADN+30% GAP composite propellant was successfully carried out.Under standard operating conditions,Is=260s was obtained with 80% ADN+20% GAP much above the about 240s achievable by the best conventional minimum smoke DB propellants.During the years,more applications for military and civilian purposes have emerged,such as gas generators for automotive airbags[29].
2 Metal Burning
Metals are excellent fuels in terms of gravimetric or volumetric enthalpy release and density.As such,they are frequently used to augment performance of solid and hybrid rockets,for different reasons and with different features.However,metals in general burn with marked difficulties due to resistance to ignition,uncertain fuel and oxidizer feeding of the combustion process in both homogeneous and heterogeneous phase,and unlikely completion of the underlying oxidation reactions.Even larger difficulties are faced by the twophase mixtures in the gasdynamic nozzle expansion due to the presence of Condensed Combustion Products(CCP).
Among the several metals considered for rocket propulsion,Al(most abundant metal,2.699g/cm3density,933Kmelting point and 2791Kboiling point at 1bar)is by far the most used in operational propulsive systems.Al has been used for decades as high-energy metallic fuel in solid rocket propulsion thanks to its energy density(~30 kJ/g)and effective damping of high-frequency combustion instability.Owing to its good overall performance,low-cost,and wide availability,for a long time Al de facto plays the role of reference metal in motor applications.With regard to the composite solid formulations commonly used for rocket propulsion,the state-of-the-art practice is represented by the addition of micrometric propulsion-grade Al;this is a low-risk and widely experienced ingredient,easily compounded with other basic components during batch preparation.
In general,metals are spontaneously protected to external oxidation by a surface oxide coating hindering ignition and inhibiting combustion of the inner elemental metals.In some cases,particles are on purpose covered by specific coatings to mitigate these somewhat negative effects or promote other suitable effects(extended ageing,better dispersion,easier ignition,etc.).Ignition temperatures vary according to the surrounding atmosphere,operating conditions,and powder type.For Al,a strong dependence of ignition temperature on particle size has well been ascertained,spanning from about 2300K,the melting temperature of alumina(micrometric powders),down to 900K(typical of nanometric powders)[30-31].Burning of Al in solid propellants takes place through a simultaneous homogeneous diffusion flame in the gas-phase and heterogeneous attack of liquid Al,yielding a wide distribution of CCP whose size ranges from submicrometric to hundreds ofμm;the Al fraction participating in aggregation/agglomeration processes depends on the propellant properties and operating conditions.
The combustion of metals,and specifically Al,in the shape of micrometric powders is known to generate CCP leading to specific impulse losses due to several negative effects connected with the resulting 2Pflow in the nozzle.These losses are aggravated by the formation of particle agglomerates at or near the burning surface.Initially,metal particles tend to stick together building up a choral-like irregular heap,eventually collapsing into a drop after inflammation,mostly made by a mixture of fused metal and its oxide.Finally,this agglomerate starts burning,leaves the surface having an initial size around 150-200μm in many industrial applications,and enters the rocket core flow where metal combustion progresses.If residence time is long enough,such as in large space launchers,the final size is less than 10μm and only a negligible fraction of unreacted metal leaves the rocket vehicle.In general,agglomeration processes are mitigated by increasing pressure,lowering the metal ignition temperature or increasing particle reactivity,favoring CCP detachment from the burning surface,promoting prompt metal oxidation in the subsurface layer,using nanoscale metals;in all cases the aggregation mass(aprecursor step of agglomeration)is minimized.
3 Metallic Fuels Tested
The metallic fuels tested in this investigation are summarized in Table 2 and described hereunder.

Table 2 Metallic fuels tested in this investigation
Detailed information concerning the metal powders composition and morphology were collected at ENI Donegani Institute by running a series of advanced diagnostic techniques,including BET(Brunauer-Emmett-Teller),XPS(X-ray Photoelectron Spectroscopy),XRD(X-Ray Diffraction),SEM(Scanning Electron Microscopy),and TEM(Transmission Electron Microscopy).Particle size measurements were performed at SPLab by agranulometer through a laser light scattering technique,using a Malvern Mastersizer 2000 equipped with a Scirocco unit for dry analyses and Hydro unit for wet dispersion(nanometals only).
Ignition characterization of the tested metal powders was carried out in order to obtain clues about propellant ignitability as well as aggregation/agglomeration phenomena during burning.Ignition is also important for possible interactions of the powders with the propellant binder during curing.Ignition temperatures were measured by means of a proprietary facility consisting of a microthermocouple dipped in a heap of powder heated by a hot wire.This allows fast heating rates,up to 300K/s.Tests were carried out at 1bar in air.
The active metal content was systematically measured by a standard volumetric procedure in a solution of NaOH(10%concentration by mass)needed to dissolve the external oxide shell.Assuming a stoichiometric reaction allows determining the moles of reacted metal from the evolved hydrogen,once the process is over.Results indicate that both nAl and actAl loose an appreciable fraction of active Al,thus reducing the ideal specific impulse;this effect is negligible forμAl.
The properties just mentioned affect each other,since a higher powder reactivity may involve lower ignition temperatures and thus interact adversely with the binder curing process.Nanosized metals not only reduce agglomeration losses,but also the active metal content and propellant castability.Likewise,chemical activation of Al powders-altering the standard oxide layer at the particle surfacegrants lower ignition temperatures and faster propellant burning rates but depletes a fraction of the active metal content.As a rule of thumb,specific surface area and ignition temperatures are in general identified as crucial parameters for the characterization of reactivity.
3.1 Characterization of Monomodal Fuel Powders
Following the detailed work already performed at SPLab for the HISP program,three families of high-energy Al-based metallic fuels are discussed.For details,see references[3,32-33].
3.1.1 μAl
Relevant data for standard micron-sized Aluminum(μAl)are reported in Table 3 ;see also Figure 3a.PowderμAl-05ais a propulsion-grade product,mostly spherical,featuring a nominal size of 30 μm;powderμAl-05bis also a propulsion-grade product of spherical shape but with a nominal diameter of 15μm;powderμAl-06is a commercial-grade product of irregular shape(50μm flake).These powders feature pretty much similar properties,having all a BET lower than 1m2/g and an active aluminum content above 99%.As expected for micrometric powders,ignition in air at ambient pressure could not be detected because it is outside the current capabilities of the experimental rig(heating rate 300K/s,estimated maximum temperature around 1 200-1 300K).
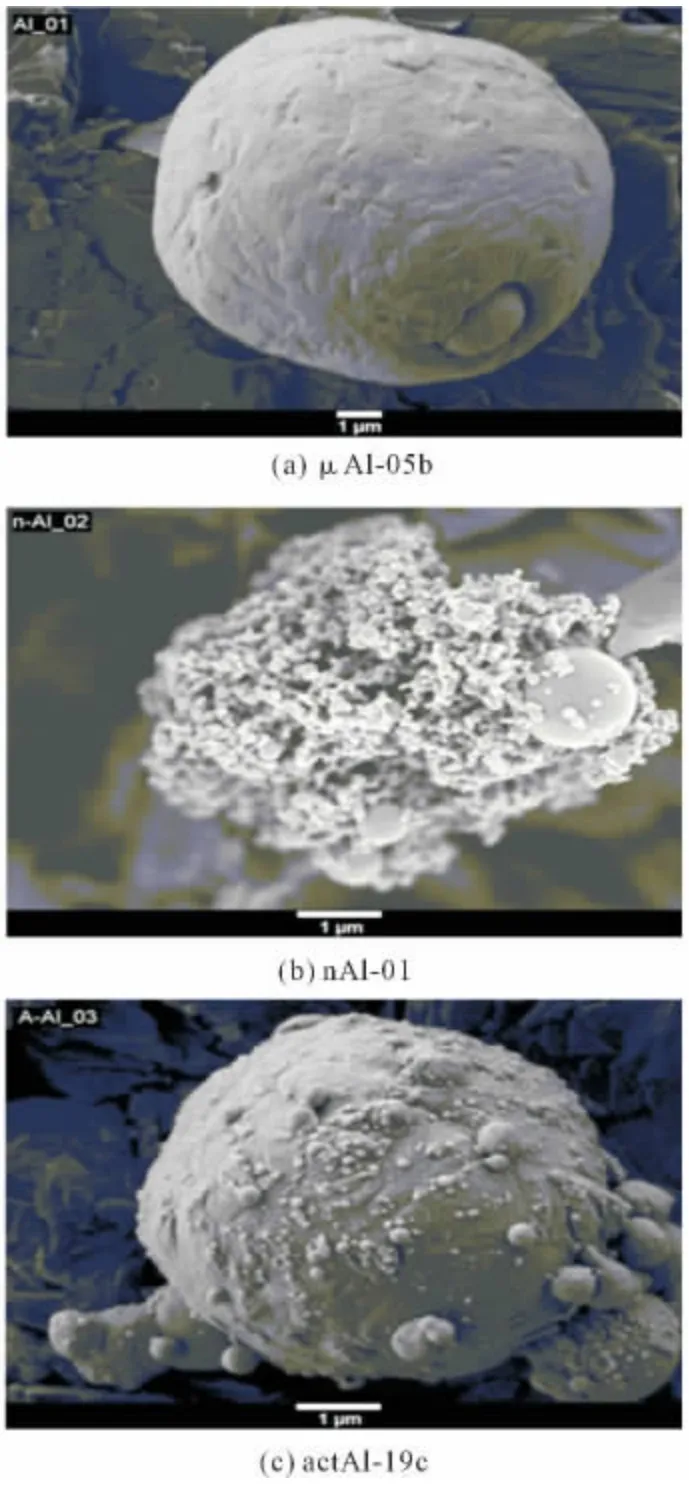
Fig.3 Representative SEM images for each family of metal fuel(a)μAl-05b,(b)nAl-01i,(c)actAl-19c.
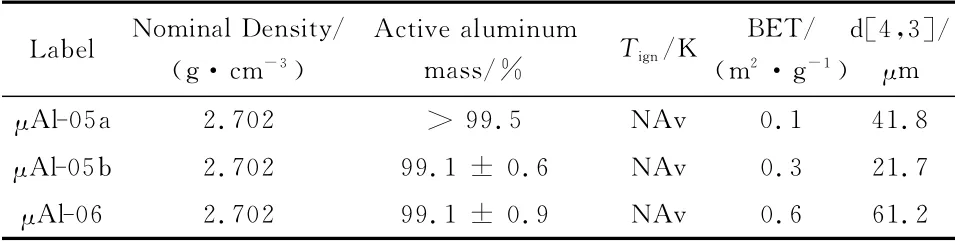
Table 3 Physical and chemical features of tested standardμAl particles.Uncertainty computed on the basis of t-student distribution and a 95%level of confidence.Nominal values from literature.
3.1.2 nAl
Several types of nano-sized Aluminum(nAl)are being tested at SPLab;most of them were produced by Explosion of Electric Wire(EEW)technique and properly coated.SEM and TEM images show almost spherical particles;see Figure 3b.The flake-shaped structures recognizable in some images may indicate some original aggregation processes(cohesion)between particles,probably due to the mechanisms of metal explosion and aerodynamics of particles formed in the EEW machine.According to XRD analyses,for all samples Al peaks are the only recognizable phase of the diffraction pattern,due to the very thin coating layer surrounding the particles,irrespective of whether coated or uncoated nAl particles are examined.
Relevant data for nAl powders are reported in Table 4.The active metal content is heavily affected by the reduced size of the powders.Both the naturally coated nAl-01hand nAl-01icontain less than 90% Al,this value being a critical issue from the performance point of view.About 14% by mass of nAl-01hresults to be already oxidized by passivation whereas nAl-07epowder,thanks to the presence of the organic protective coating,maintains almost 92%Al.As far as reactivity is concerned,under fast heating rates ignition temperatures settle between 771and 820K,with the coated powder granting the lowest ignition temperature.It is emphasized that ignition temperatures do not correlate with BET grading.In fact,coating acts as a protection and limits Al interactions with the environment.Organic coatings are soon decomposed and during heating do not interfere with high temperature reactions,leaving the underlying metal ready for prompt reactions.Datad[4,3],being misled by incomplete dispersion in measurement medium,are complemented byd[1,0].
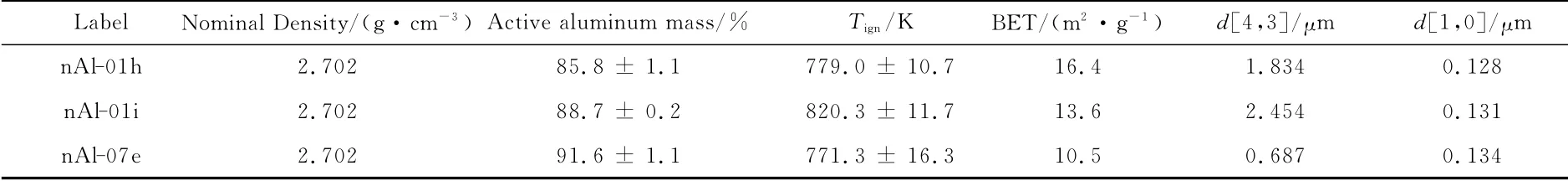
Table 4 Physical and chemical features of tested nAl particles(Lot HISP Jul 11).Uncertainty computed on the basis of t-student distribution and a 95%level of confidence.Nominal values assumed.
3.1.3 actAl
Several activated Al(actAl),of micrometric size,were produced at FOI starting from theμAl-18batch.Particle surfaces were treated with an activating solution containing a complex fluoride in different concentrations[34-35].The particle surface layer so modified has a much lower melting point than the remaining untreated Al2O3surface layer,thus allowing O2to diffuse toward the particle Al core at substantially lower temperatures.This increases particle reactivity and burning rates.SEM images,see Figure 3c,show rounded items on the external surface protruding from particles which are not visible in the raw batch.According to the manufacturers,activation intensity is highest for actAl-19cand lowest for actAl-19b.Particle size do not appear altered by the activation process,since grain size distributions are basically all the same,with a mass-mean size of about 5μm.XRD analyses revealed only the crystalline domain of metallic aluminum A10,without traces for other chemical structures,and thus suggest that the particle bulk remains untouched.
Relevant data for actAl powders are reported in Table 5.The active metal content is depleted in the amount of about 5%-8%,depending on activation intensity,with respect toμAl-18.However,at the same time BET and especially ignition temperatures improve with respect toμAl-18.Powder actAl-19c,subjected to the strongest activation,features the highest specific surface area and the lowest ignition temperature within this group,somewhat approaching the nAl values.
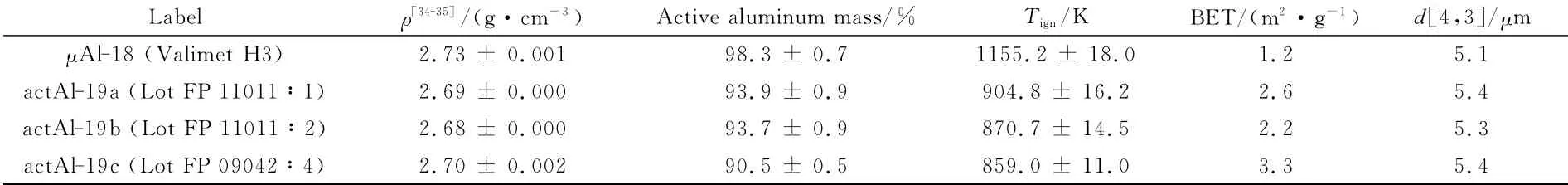
Table 5 Physical and chemical features of tested actAl particles.Uncertainty computed on the basis of t-student distribution and a 95%level of confidence.
3.2 Comparative Remarks
Powder nAl-07eappears the most suitable high-energy fuel among the several Al variants tested,since it combines the lowest ignition temperature with a satisfactory active metal content;its BET is second only to the other tested nAl.Ignition temperature of actAl-19bis just 100Khigher than nAl.Notwithstanding its still being a micrometric ingredient,as proved by particle size and specific surface,when compared to the unprocessedμAl-18reactivity improvements due to activation are obvious in terms of(strongly)reducedTignand(slightly)increased BET.The flight certified baselineμAl-05arepresents the least reactive fuel and does not ignite under the current capabilities of the experimental rig.
Due to the conflicting properties manifested even by the tested metallic fuels,dual fuel compositions are recommended to optimize all together the high-energy fuel contribution to propellant performance.An example is illustrated hereunder.
3.2.1 Ignition Temperatures of Mono-and Multimodal Fuel Powders
A comparative plot of ignition temperatures for some of the tested mono-and multimodal fuel powders is shown in Figure 4.
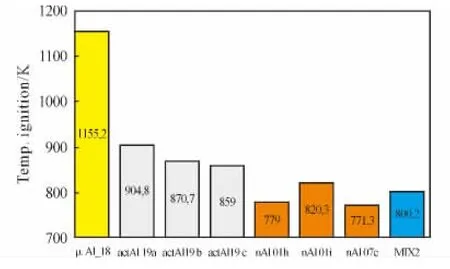
Fig.4 Comparing fuel ignition temperatures in air at ambient conditions.PowdersμAl-05a,μAl-05b andμAl-06 do not ignite under tested operating conditions.A blend can effectively reduceμAl ignition temperature.
In order to achieve good ignitability without loosing active metal content,a blend of 15% μAl-05aand 3% nAl-07e(MIX-2)is suggested.Experimental results point out that the a small fraction of nAl(1/6)acts as a driver for the ignition of the whole blend,as shown by MIX-02.For details,see reference[3].
3.2.2 Interaction with binder curing(HTPB)
Further chemical characterizations were carried out under lowheating rates in order to ascertain possible interactions of the metal powders with the propellant binder during curing.Experimental results indicate that both nAl and actAl tend to anticipate the curing onset of the HTPB-based polymer.Exothermic curing peaks are similar in shape with the sole exception of the coated nAl-07ewhich seems to speed up curing.Additional viscosimetry tests concurred with the calorimetric findings.For details,see reference[33].
4 Tested Solid Rocket Propellant Formulations
The solid propellant formulations tested in this investigation are briefly described.Different solid oxidizers(see Table 1 ),metal fuels(see Table 2 ),and binders(see Table 6 )were used.Except where otherwise stated,all AP/HTPB-based propellants were manufactured,processed,and tested at SPLab of Politecnico di Milano under identical conditions and using identical procedures.Except where otherwise stated,all ADN/GAP-based propellants were manufactured,processed,and tested at Fraunhofer-Institute for Chemical Technology(ICT)under identical conditions and using identical procedures.
4.1 Reference Solid Propellant Composition
The composite solid rocket propellant taken as baseline is a standard AP/HTPB/Metal formulation in the mass ratios 68/14/18,where Metal is the metallic fuel.This composition was taken as a convenient benchmark for the current state-of-the-art of metallized solid propellants for space propulsion applications.A laboratory reproduction of a flight certified Al-based formulation,containing as metal only propulsion-grade Al(30 μm average grain size),was taken as a suitable reference.As seen respectively in Table 7 and Table 8 ,the oxidizer is a bimodal AP blend while the binder is a standard HTPB R-45Mpolybutadiene prepolymer cured with IPDI(as for all HTPB propellants mentioned in this paper).
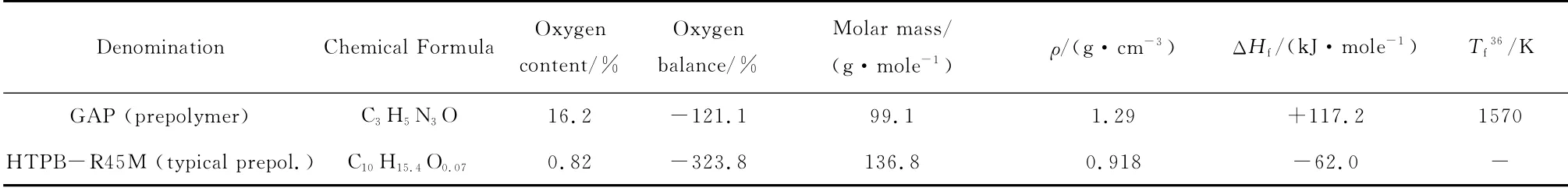
Table 6 Prepolymers for binders tested in this investigation
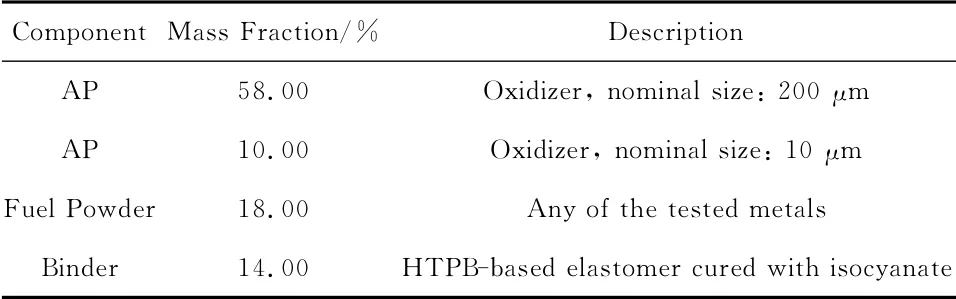
Table 7 Reference solid rocket propellant composition

Table 8 Inert binder composition
4.2 AP/HTPB-Based Propellants Tested
Different formulations were tested using different metal powders and variants/combinations of the metal powders.All of the AP/HTPB formulations keep the standard composition AP/HTPB/Metal= 68/14/18(mass%).In general,the fine fraction of AP was produced at SPLab through a milling process,then selected by sifting and quality-checked by laser granulometry.The reference baseline contains only micrometric aluminum of 30μm nominal size.Several Al variants were used to replace either totally or partly the referenceμAl fuel.In particular,in an attempt to optimize metal fuel reactivity,active metal content,and propellant processability,a set of multimodal powder blends was also examined.These fuel mixtures were produced by dry mixing and shaking of two or more of the monomodal ingredients using a ResodynTMresonant mixer.When dual fuels are used,the overall fuel fraction is split by mass into 15parts(in principle,the standardμAl-05a)+3parts of relevant novel metal fuel(s).All tested AP/HTPB/Al propellants are listed in Table 9.Stabilized alane AlH3was also tested,in view of its highly attractive,propulsive features(used indeed in the SS-24third stage).
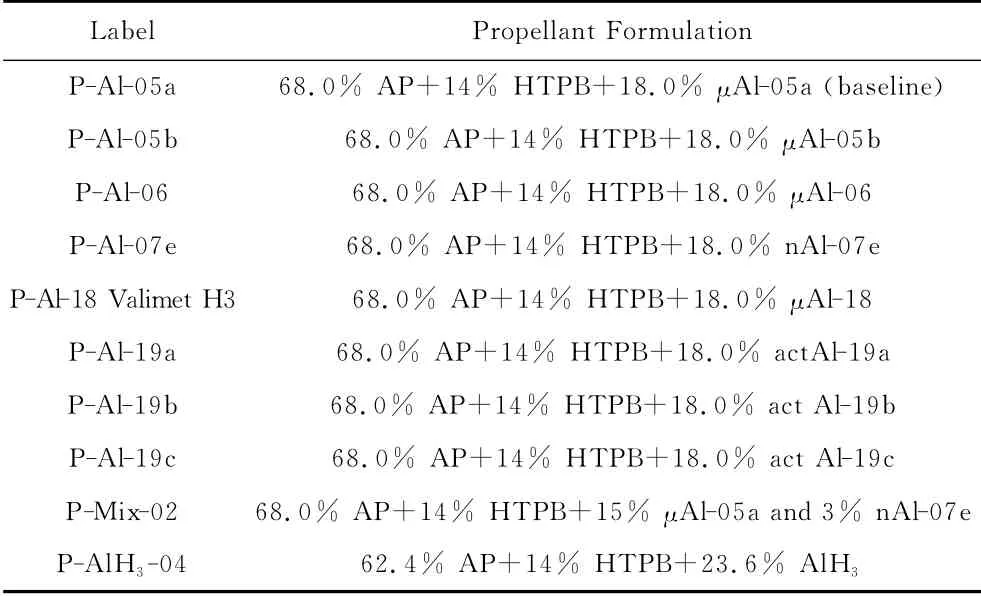
Table 9 AP/HTPB Propellants with Monomodal and Bimodal Fuels
4.3 ADN/GAP-Based Propellants Tested
Four propellant formulations were tested having the same nominal composition ADN/GAP/Metal= 60/24/16(mass%).Prilled ADN was produced by the ICT emulsion method using neat ADN delivered by Eurenco Bofors with 208μm average particle size.All tested ADN/GAP/Al propellants are listed in Table 10 ;relevant details are reported in references[4][27].Preliminary tests with the stabilized alane AlH3as high-energy fuel were also accomplished.
The amount of 16% Al was chosen as typical for metallized formulations in space launchers.This choice worked well withμAl(samples D and G);see Table 10.To provide as much as possible comparable combustion tests,it was chosen to use the same amount of nAl(samples E and F);see Table 10.The mixing and casting of propellant sheets using 18μm and 5μm aluminum proceeded without any problems.But for propellants containing nAl(100nm)it was impossible to prepare sheets of good enough quality because of the high viscosity of the resulting propellant slurry.Propellant sheets were prepared like rolling apaste and not by casting.From the sheets,propellant strands of 4mm×4mm×35mm were cut for combustion tests.
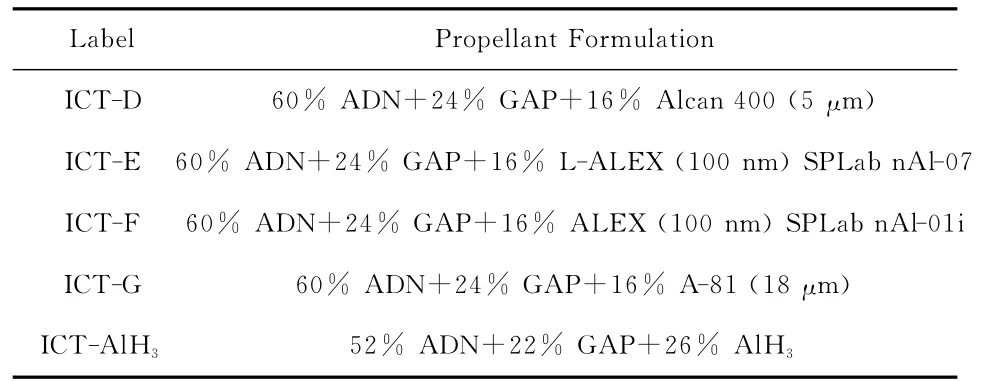
Table 10 ADN/GAP Propellants with Monomodal Fuels
4.4 ADN Prilling by Fraunhofer-ICT
The ADN-prilling Process,which is using an emulsion crystallization technology,was developed at Fraunhofer-ICT at the end of in the 1990s(European patent EP 0 953 555B1).The goal of the ADNPrilling process is the reshaping of irregular ADN crystals,as obtained from synthesis,into spherical particles(ADN-prills),as they are needed for further processing,e.g.in solid rocket formulations.The prilling process comprises principally three steps:
(1)Suspension of raw ADN in a nonpolar liquid,which is an antisolvent for ADN.
(2)Heating up above the melting point of ADN and emulsifying by stirring.
(3)Cooling down and recrystallize the molten ADN-droplets into the desired spherical particles(ADN-Prills).
The challenges are to avoid clustering and deformation of the prills and to overcome the inhibited recrystallization behavior of mol-ten ADN.This process is determined by several parameters(stirrer speed,viscosity,temperatures,etc.),which were investigated closely to improve the product quality.Currently the achievable particle size range could be enlarged down to 35μm and up to 320μm.Typical particle sizes for reproducible kg-scale productions are:Fine ADN-Prills:50μm;Coarse ADN-Prills:200μm.
Figure 5shows ADN-Prills with spherical shape and a mean particle size of 208μm.The particle size distribution is quite narrow(SPAN 90/10=1 093).
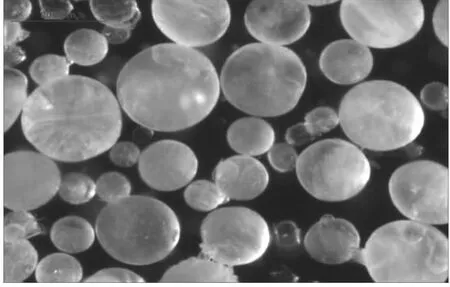
Fig.5 Micrograph(reflected light)of prilled ADN,mean particle size 208μm
Currently,two different ADN-prilling processes are established in Europe.The emulsion crystallization process of Fraunhofer-ICT in Germany and the spray prilling process developed at FOI in Sweden.Both processes are operating with molten ADN droplets that are urgently needed to form the spherical prills after recrystallization.The main difference of the two processes is the way to generate these molten droplets.At ICT the complete process of creating prills is running in a process liquid(Antisolvent).The generation of the droplets is achieved in a hot emulsion.During the cooling the nucleation starts gradually and the recrystallization occurs.Then the prills are separated,washed and dried.
In FOI spray prilling process,an ADN melting is prepared first.This is pumped to a spray nozzle,where the molten droplets are generated by pressure atomization.The inhibited recrystallization behavior of ADN,which is a very typical material property,makes it necessary to collect the molten droplets also in an antisolvent for recrystallization.Afterwards the prills are separated and dried.
The quality of prilled ADN is controlled by particle size measurement,e.g.laser light diffraction.The morphology may be checked visually by different kinds of microscopes.An important aspect is the suitability for the further use in solid propellant formulations.Here stability,compatibility and good mixabilty with binders are required.
Currently ADN is an upcoming material with a high potential for industrial applications.This will lead to the demand for scaling-up the prilling processes,which seems possible for both processes,whereas in both cases continuously running processes are to prefer due to safety and processing reasons.At ICT further investigations for a follow-up process were conducted to coat(encapsulate)the single ADN-Prills by fluidized bed technology,with a protection or functional polymer layer.
5 Density
The actual density as measured for several of the manufactured compositions is reported in Table 11.Theoretical Maximum Density(TMD)and the actual density ratio to TMD(due to porosity,voids,and other reasons)were also calculated.While TMD values depend only on the accuracy of the implemented dataset,the density ratio values are affected by the specific manufacturing technique followed,which may not be always exactly the same for safety or practical reasons.
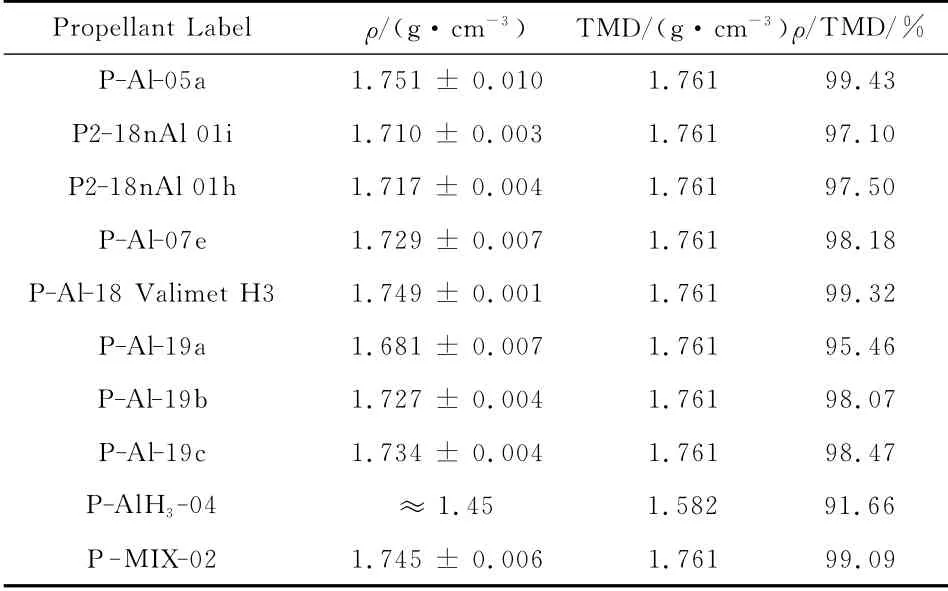
Table 11 Density of selected tested metallized solid propellants
While GAP(ρ=1.29g/cm3)increases density with respect to HTPB(ρ=0.918g/cm3measured at SPLab,but values up to 0.985g/cm3are also reported),ADN(ρ=1.812g/cm3)decreases density with respect to AP(ρ=1.949g/cm3);likewise,AlH3(ρ=1.477 g/cm3)decreases density with respect to Al(ρ=2.699g/cm3).Overall ADN/GAP results denser than AP/HTPB,as shown also by the computed comparative values in Table 12 and Table 13.
6 Ideal Thermochemistry
Ideal thermochemical features of the examined metallized propellants were systematically analyzed,by means of both the NASA CEA and ICT thermodynamic codes,under the following operating conditions:pressure in combustion chamber:70bar;nozzle expansion to vacuum withε=40or optimum to sea level(1bar)exit pressure;shifting equilibrium model.
All relevant propellant formulations were studied,starting from the reference AP/HTPB/Al(first column),replacing only AP by ADN(second column),replacing only Al by AlH3(third column),replacing only HTPB by GAP(fourth column),and finally replacing the whole matrix AP/HTPB by ADN/GAP(fifth column with Al and sixth column with AlH3).See Table 12 (ICT)and Table 13 (NASA CEA).In comparing performance one should bear in mind that GAP,while it enjoys a positive heat of formation(ΔHf=+117.2kJ/mole)and better OB(-121.2%)with respect to HTPB(ΔHf=-62kJ/mole and OB=-323.8%),is more expensive,not easily compatible with plasticizers,and does not lead to the best mechanical properties.Self-deflagration temperature of GAP reaches 1455Kat 68bar.
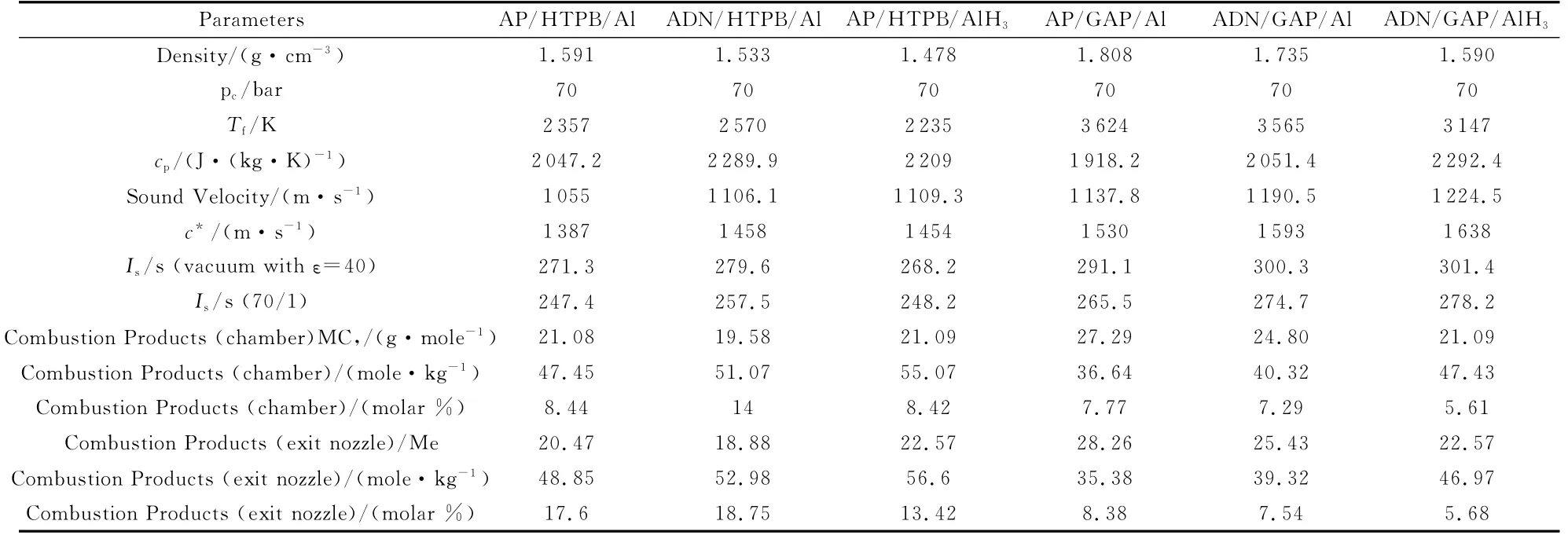
Table 12 AP/HTPB/Al vs.ADN/GAP/Al thermochemical calculations for ICT60/24/16 under shifting equilibrium
Table 12 lists the results of thermodynamic calculations for the ICT propellant formulations using the ICT-Thermodynamic code.For all propellants the enforced formulation is 60% oxidizer(AP or ADN),24%binder(HTPB or GAP),16%fuel(Al or AlH3).Under the explored operating conditions,everything else being the same,one can see that,in terms of idealIs:
(1)ADN/Al is more performing than AP/Al by about 9-10s for HTPB or GAP under both vacuum and sea level expansions(due toTf/Mcincrease);
(2)GAP/Al is more performing than HTPB/Al by almost 20s for AP and 10sfor ADN under both vacuum and sea level expansions(Tfincreases by about 1 300Kfor AP and 1 000Kfor ADN);
(3)In addition,GAP allows a significant reduction of CCP products at the exit nozzle,thus promising a further favorable effect on delivered specific impulse;
(4)The highest performance is offered by ADN/GAP/AlH3,although only slightly superior to ADN/GAP/Al.
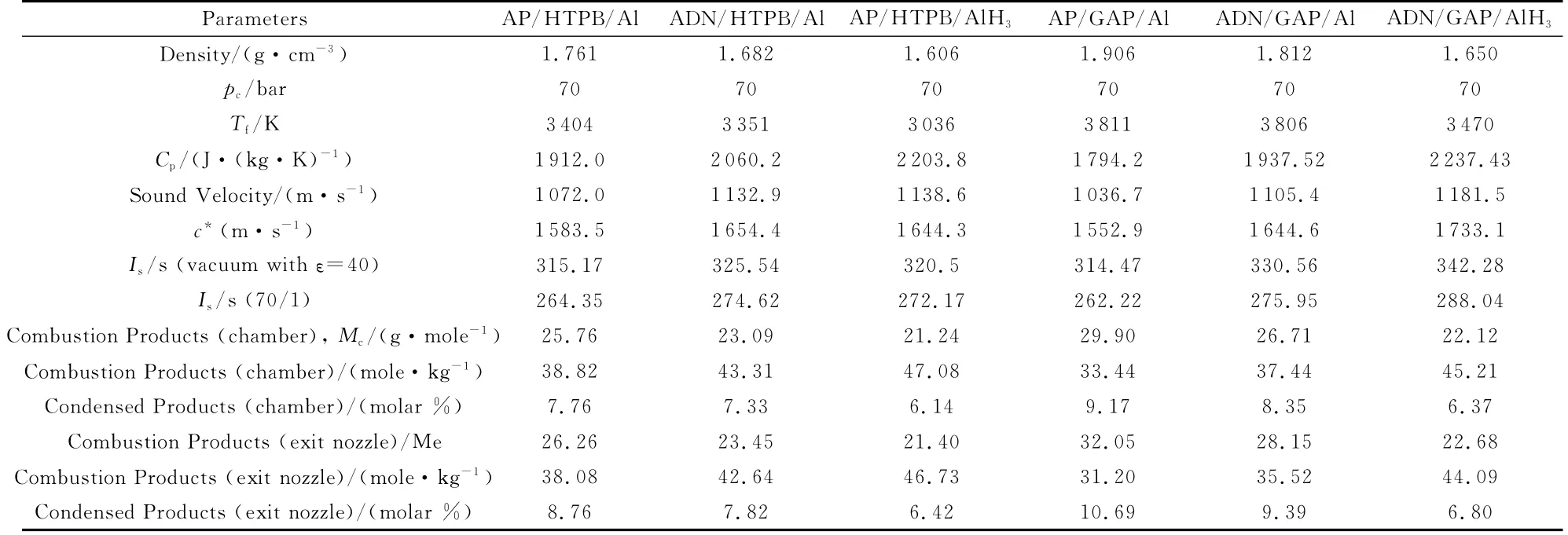
Table 13 AP/HTPB/Al vs.ADN/GAP/Al thermochemical calculations for SPLab 68/14/18 under shifting equilibrium
Table 13 lists the results of thermodynamic calculations for the SPLab formulations using the NASA CEA code.For all propellants the formulation is 68% oxidizer(AP or ADN),14% binder(HTPB or GAP),18%fuel(Al or AlH3).Under the imposed operating conditions,everything else being the same,one can see that,in terms of idealIs:
(1)ADN/Al is more performing than AP/Al by about 10sfor HTPB and 15sfor GAP(in spite of minor decreases ofTf)under both vacuum and sea level expansions;
(2)Under both vacuum and sea level expansions,GAP/Al is essentially ininfluential for AP(due to simultaneous strongTfandMcincrease)while it is more performing by about 5sfor ADN(Tfincrease more effective thanMcincrease);
(3)GAP also implies a somewhat increase of CCP products at the exit nozzle,thus penalizing the actually deliveredIs;
(4)by far the highest performance is offered by ADN/GAP/AlH3.
Overall,the ADN/GAP/Al formulation compared to AP/HTPB/Al allows an increase for idealIsof about 30sin the mass%composition 60/24/16and about 15sin the mass%composition 68/14/18.At the same time,Tfdramatically increases to respectively 3 565K(instead of 2 357K)and 3 806K(instead of 3 404K),implying more severe thermal loads.A contradictory effect is observed for the CCP molar fraction at the exit nozzle:it decreases for 60/24/16 but increases for 68/14/18.A further positive effect for ADN/GAP/Al formulation compared to AP/HTPB/Al is the nominal propellant density increase(1.812g/cm3in Table 13 )andc*increase(1 644.6 m/s in Table 13 ).
Systematic results collected by Calabro[2]by a third thermochemical code suggest for space applications(small throat motors)an optimum formulation around 16%-20%Al and 16%GAP;for large upper stages/apogee motor around 16%-20% Al and 20%-24%GAP.By doing so,an ideal Is larger than 330scan be obtained(say,ADN/GAP/Al=60%/20%/20%),above the about 315smax value achievable by AP/HTPB/Al under the same operating conditions.A still much higher value,close to 350s,could be obtained with AlH3(say,ADN/GAP/AlH3=58%/14%/28%).These findings are in broad agreement with the above results at ICT and SPLab.
7 Combustion Experimental Results
The combustion features of the tested metallized solid propellants are compared to the standard Al-based ones in terms of steady burning rates,ignition,and agglomeration phenomena.All burning rates were obtained in proprietary strand burners and data processed in terms of Vieille law(a and n being the only relevant ballistic parameters),with pressure in bar and burning rate in mm/s.
7.1 teady State Burning Rate of AP/HTPB/Al and AP/HTPB/AlH3
The experimental rig used at SPLab to measure steady state burning rates is a windowed strand burner.This is a nitrogen pressurized vessel in which a sample of propellant(4mm×4mm×30mm)is ignited by a hot NichromeTM wire.Lateral strand surfaces are inhibited to combustion with a Paraloid B-72 surface coating.The chamber pressure is kept constant by an analog pressure controller through a couple of electrovalves.The combustion is recorded using a video camera to obtain the video for post-processing analysis.Videos are successively unpacked and analyzed with a consolidated digital technique that traces the regression of combustion surface in time.The standard SPLab procedure requires three valid videos for each pressure in order to obtain three burning rate values to be correlated with the Vieille′s law for comparison purposes.
A direct comparison of the main families of tested Al fuels is illustrated in Figure 6.Under the explored operating conditions,experimental results show that nAl is way more effective than actAl in increasing the steady burning rate with respect to theμAl-baseline(the slowest of the crop).Differences in the coating and size of the tested nAl powders(not shown in the plot)do not lead to significant changes.The burning rate pressure sensitivity is little affected by the several Al variants under examination.A more general result is illustrated in Figure 7,showing the normalized effects of the Al particle specific surface area(BET).
All of the steady state burning rates were deduced by analyzing the recorded videos with a dedicated software package and then fitted by a standard Vieille law.Most tests were made in a N2atmosphere at 5,10,20,30and 40bar.A summary of the fitted steady state burning rates in N2,for many of the propellants studied in this work,is reported in Table 14.Burning rates at 60bar fall in the range 7to 13mm/s,except for the stabilized alane formulation close to 23mm/s.Pressure sensitivity of all tested AP/HTPB/Al formulations is only slightly affected with respect to baseline(n=0.46),while is appreciably mitigated by the alane formulation with an even bettern=0.33.
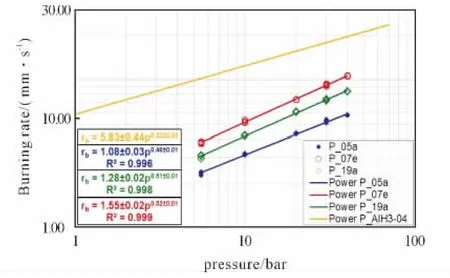
Fig.6 Comparing steady burning rates of AP/HTPB/Al formulations:nAl is more effective than actAl in increasing rates with respect to the implementedμAl-baseline,while slope is little affected.AP/HTPB/AlH3increases rates but decreases slope
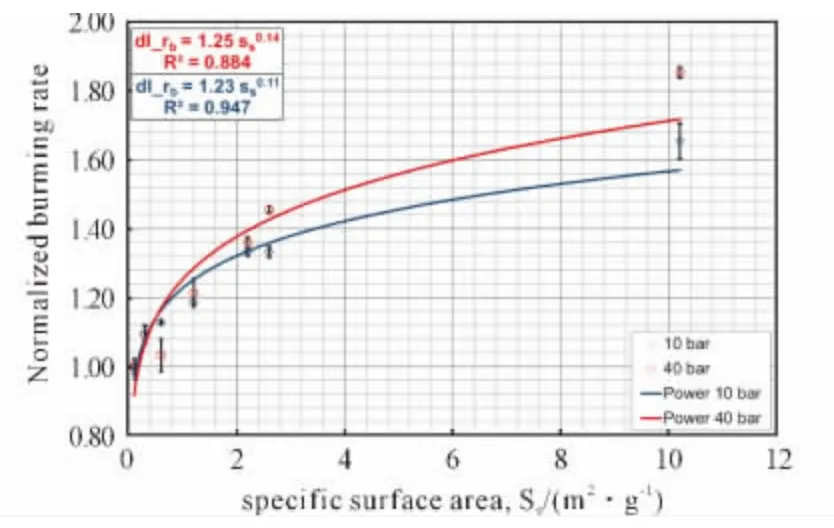
Fig.7 Normalized regression rate versus specific surface at different pressures.Normalization is performed per pressure and with respect to the baseline composition[32]
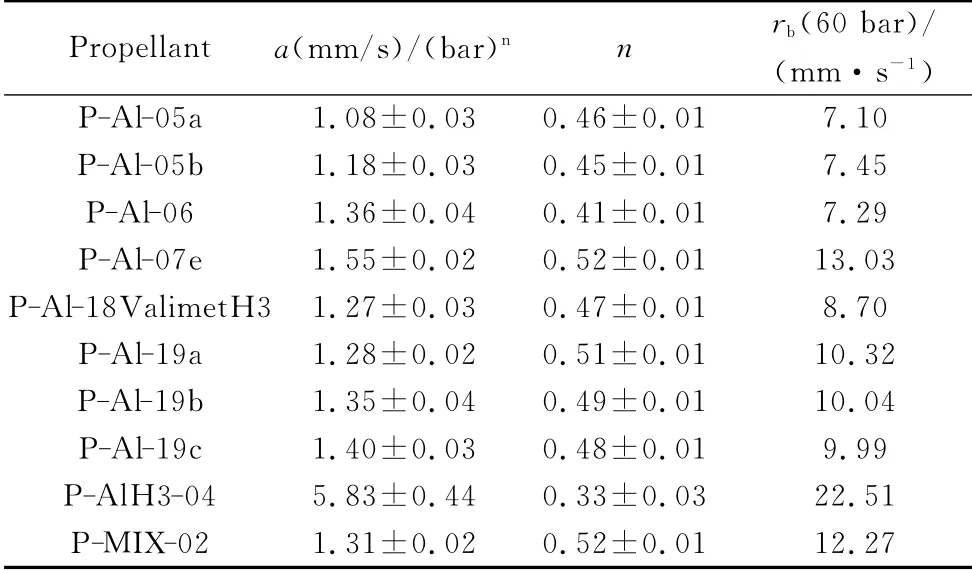
Table 14 Vieille ballistic parameters and burning rates at 60 bar at ambient initial temperature for AP/HTPB/Al and AP/HTPB/AlH3 propellants[3,32,33,37]
7.2 Steady State Burning of Pure ADN
A good survey of pure ADN burning rate data collected by a host of international investigators over a wide range of pressure was offered by Gross et al.at BYU[22];see Figure 8.ADN monopropellant is much faster,say,at least 3times more than AP,and has a much lower PDL(say,2instead of 20bar for AP)than other solid oxidizers.Burning rate is around 30mm/s at 60bar with pressure sensitivity n≈0.6,while over the pressure interval 2-40bar sensitivity is slightly reduced,say[24]in the rangen=0.46-0.6.

Fig.8 Steady burning rate vs.pressure for ADN from various sources[22]and overlapping data by Sinditskii et al.[23-24]
When used in HTPB-based propellants as partial substitution of AP,ADN proved to be very effective for burning rate increments even in small quantities and especially for coarse rather than fine particles;however,an excessive pressure sensitivity was also revealed[20-21].A total replacement of AP by ADN leads to a good increase of burning rates,but with a pressure exponent approaching the critical value of unity.These literature results are confirmed in the experiments discussed hereinafter.
The flame structure of pure ADN was extensively investigated by Sinditskii and coworkers[23-24].Their results are well illustrated in Figure 9:three extended and essentially gaseous zones(like for DB)follow the melt layer floating over the burning surface(like for AN).ADN flame is not tightly anchored to the burning surface as for AP.This implies a complex multistep heat release mechanism whose role and interplay mainly depend on pressure;representative thermal wave values are reported in Table 15.The surface temperatureTsis first uniform for some distance and then increases moderately in a stepwise manner;above 20bar it becomes difficult to detect.The aerosol zone-roughly corresponding to the DB fizz zone-has a temperatureTaonly about 120-145 K higher thanTsand its thickness quickly shrinks for increasing pressure.At low pressures,ADN burns with copious fumes but no visible flame.The first gas flame zone(the DB dark zone)appears at 5bar with the temperature rising to 1 325-1 475Kover the range 5-40bar.Like for DB,full heat release and maximum combustion temperatures need larger pressures.The aerosol zone exists concurrently with the first flame zone at pressures up to 40bar.As shown in Table 15 ,like for DB,high-temperature zones stand far off the surface for most of the pressure range.When pressure is large enough,say above 100bar,the flame structure collapses to only one zone and the final temperature Tf2has to match the adiabatic flame temperatureTf(for example,2 036Kat 68bar).Overall,condensed-phase(liquid)heat release controls low-pressure burning(below 10bar),while gas-phase heat release controls highpressure burning(above 100bar);unstable burning is observed in between.Surface temperature grows up to,say 630[19]K or 750[24]K at 20bar;final temperature is limited to,say 1 500Kup to 40bar(chemical kinetics limitations)and 2 060up to 100bar(thermochemical limitations).
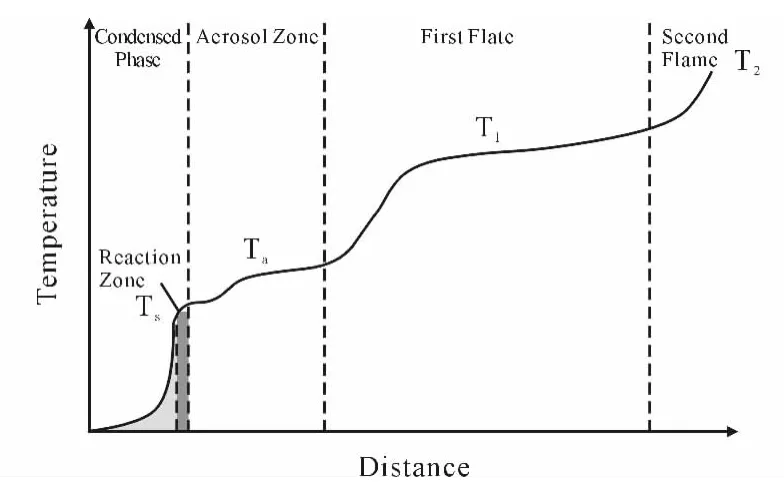
Fig.9 Sketch of ADN multistep flame structure[24].Burning appears flameless below1 bar,condensed-phase controlled up to 10 bar,unstable up to 100 bar,and collapses to one,gas-phase controlled zone above 100 bar
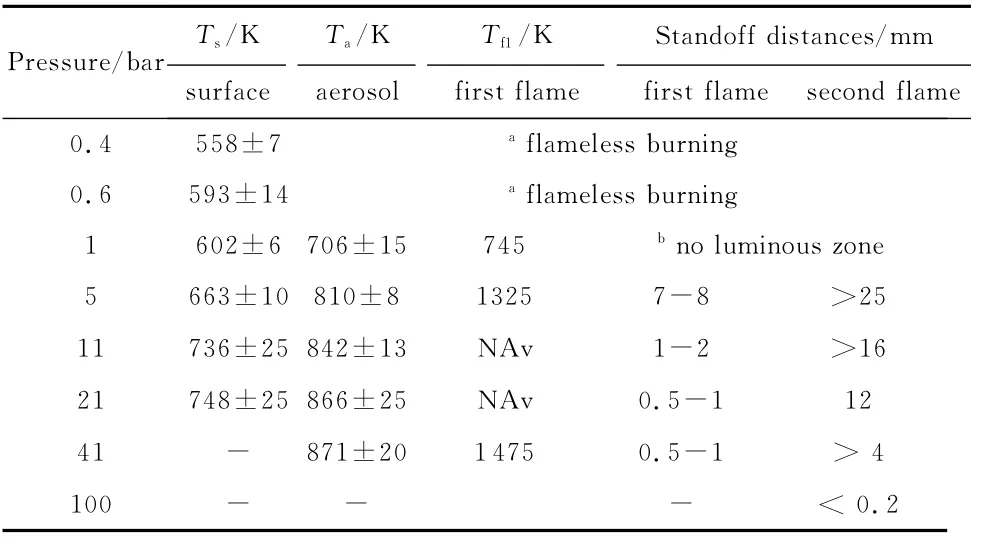
Table 15 Surface temperature Ts,aerosol zone temperature Ta,first flame temperatures Tf1,and standoff distances of ADN flame zones[24]
7.3 Steady State Burning Rate of ADN/HTPB/Al
Steady ballistics of the intermediate formulation ADN/HTPB/Al was investigated at FOI and TNO.For this class of propellant experiments confirmed moderate burning rates with however a large pressure sensitivity.The exact compositions and the obtained Vieille parameters of three propellants tested at FOI are reported[39]in Table 16.Two Al powders were tested:H10(d50=13.2μm)and H30(d50=30.0μm).The two formulations with 75%solid fraction had the same pressure exponent ofn=0.87and a similar burning rate at 60bar(12.1and 11.5mm/s).The formulation with 80%solid fraction had an higher pressure exponent ofn=0.91and a burning rate of 12.8mm/s at 60bar.Burning rate vs.pressure plots are shown in Figure 10.
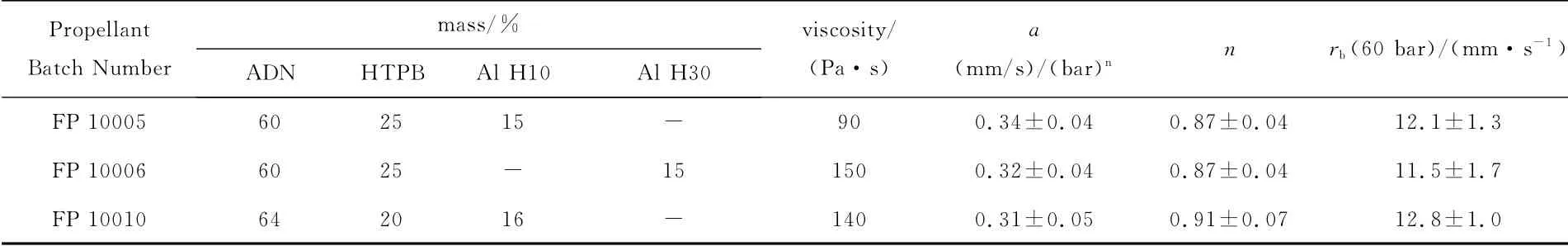
Table 16 Composition,viscosity,and Vieille parameters ADN/HTPB/Al[39]
Recent investigations conducted at JAXA[40]for bimodal prilled ADN/HTPB=70/30in mass%confirmed burning rates around 12 mm/s with a prohibitiven=1.1.Replacing HTPB with a thermoplastic elastomer caused a dramatic increase of the pressure sensitivity ton=2.7.Both formulations behave much better with AP instead of ADN,bringing the slope down to n=0.3.Smoldering and unstable combustion with a thick molten layer were observed at 20bar,while stable combustion with a thin molten layer occurred at ≥50bar.Still at 50bar,the final flame temperature of AP/HTPB does not reach 1500K.
Although AP/HTPB/Al propellants tested at PoliMi and ADN/HTPB/Al propellants tested at FOI are not exactly comparable,at least limited to micrometric Al a broad estimate can be drawn:with ADN replacing AP not only burning rates get quite larger(close to twice)but also pressure sensitivity becomes dangerously high.The well-known multiple flames structure of AP/HTPB/Al formulations is probably kept,with the intense ADN self-deflagration replacing the much weaker AP self-deflagration.The presence of an inert binder adds to the complex(structured)ADN premixed monopropellant flame a second and possibly important diffusive flame with its associated heat release zone in the gas-phase.This causes the pressure exponent to increase from the otherwise modest value related to the ADN self-deflagration.The presumably low surface temperatures of this class of ADN-based composite propellants make difficult the ignition of micrometric size Al particles.In turn,this implies thick flames and little effects on the burning rates.This tentative picture may drastically change if active binders and/or nanometric size Al are used.
7.4 Steady State Burning Rate of ADN/GAP
ADN propellants with a GAP-based binder,containing 70%bimodal prilled ADN,were manufactured and tested at FOI[29].A low solid fraction loading was chosen to ensure low viscosity and good quality of the casted propellant.Identical ballistic results were obtained for four different batches,as shown in Table 17.The resulting burning rate vs.pressure plot is again shown in Figure 10.

Table 17 Composition,properties,and Vieille parameters ADN/GAP propellants[29]

Fig.10 Steady burning rate vs.pressure for ADN/HTPB/Al and ADN/GAP propellants tested at FOI at ambient initial temperature[29,39].Few exploratory tests(·)with ADN/HTPB propellants conducted at TNO are also reported
Under a different program several ADN propellants with a GAPbased binder,cured either with stabilized N100(NCO/OH=1.0)isocyanate or BPS,were manufactured and tested at ICT[28].Samples were made with and without TMETN as a nitrate ester plasticizer.To augment propellant performance(including solid fraction and density),60-120μm prilled ADN was used together with 3.9μm HMX(both being median particle size).The exact compositions and the obtained burning rate parameters of three propellants tested at ICT are reported in Table 18.With respect to BPS curing,N100isocyanate yields lower burning rates but also lower glass transition temperatures(-43.9℃for ADN-59against-28.5℃for ADN-99and-38.2℃for ADN 109).

Table 18 Composition,properties,and Vieille parameters ADN/HMX/GAP[28]
The two families of ADN/GAP formulations,in spite of many dif-ferent details,share similar ballistic properties:burning rates are large but with an acceptable pressure sensitivity in the rangen=0.49-0.52.The complex(structured)ADN premixed monopropellant flame gets even more complicated by the active decomposition of GAP augmenting the surface temperature and making the overall flame probably shorter.However,the active GAP binder,being controlled by condensed-phase decomposition N3→N-N2,appears less disruptive to ADN self-deflagration in the gas-phase and does not generate the pressure dependence introduced by the inert HTPB binder.The possible presence of plasticizer and stabilizers does not seem to sensibly affect the ballistic behavior.Impact and friction sensitivities of ADN/GAP propellants equal those of AP/GAP,while sensitivity to detonation might be significantly higher[28].
7.5 Steady State Burning Rate of ADN/GAP/Al and ADN/GAP/AlH3
All propellant were manufactured and tested at ICT.All samples could be ignited and burnt starting from 1bar up to all the considered pressures,implying that PDL(Pressure Deflagration Limit)falls in the subatmospheric range.At 1bar all samples burnt with a good linear burning rate.Under higher pressure levels,samples ICT-D and ICT-G containingμAl,as well as ICT-AlH3,behaved properly and manifested regular linear burning rates.On the contrary,due to appreciable manufacture porosity,samples ICT-E and ICT-F containing nAl burnt in a regime of porous combustion even expelling fragments.Burning rates could only be measured with uncertainties and may not respect the standard Vieille law[41].
For 1bar tests,movies clearly indicate a regular propellant combustion and no porous behavior.At this pressure a clear ranking is given for the tested samples differing only in the type of aluminum particles,with an increase of about a factor 2from sample to sample.Sample ICT-G with 18μm Al particles burns slowest followed by ICT-D(5μm Al)and ICT-F(uncoated nAl).Sample ICT-E,with nAl particles coated with stearic acid,features the highest burning rate.Note that at 1bar sample ICT-E burns with a rate close to that of ICT-D and ICT-G at 10to 20bar.
In Figure 11the measured burning rates of all samples are presented as a function of pressure.Burning rate values of ICT-D and ICT-G are of the same order of magnitude.Applying Vieille′s law to the pressure range investigated,a linear fit does not describe the data well,due to the low values at 1bar.Excluding results at 1bar leads to a significantly better agreement.Pressure exponents fall in the upper band withn=0.52for ICT-D(5μm Al)andn=0.64for ICT-G(18μm Al);again the stabilized alane formulation features the lowest pressure sensitivity withn=0.37.Thus,over the pressure range 10to 100bar,the addition of Alespecially for ICT-G-leads to an increase of both burning rates and pressure sensitivity in Figure 11with respect to ADN/GAP withn=0.49in Figure 10.Alane again appreciably mitigates the pressure dependence,without sensibly affecting however burning rates.All of the obtained ballistic data are summarized in Table 19.

Fig.11 Comparing steady burning rates of ADN/GAP/Al and ADN/GAP/AlH3 solid propellants,showing much faster combustion than AP/HTPB/Al compositions.For ICT-F only data at 1 bar are reported
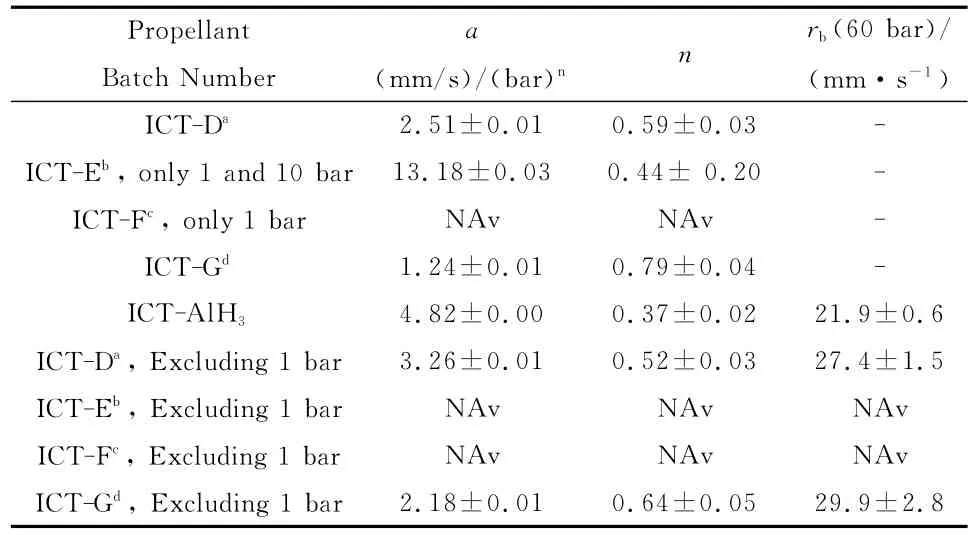
Table 19 Comparing steady burning rates of ADN/GAP/Al and ADN/GAP/AlH3solid propellants,showing much faster combustion than AP/HTPB/Al compositions.For ICT-F only data at 1 bar are reported
Although AP/HTPB/Al propellants tested at PoliMi and ADN/HTPB/Al propellants tested at FOI are not exactly comparable,the following broad portray can be drawn:with GAP replacing HTPB burning rates increase and pressure sensitivity is moderated.The active decomposition of GAP augments the surface temperature and makes the overall flame probably shorter.Under these circumstances,ignition of micrometric size Al particles becomes less impervious,while nAl or AlH3particles might start reacting already at or below the burning surface.At any rate,burning ofμAl still occurs through thick flames,while burning of nAl or AlH3is essentially complete very close to the burning surface.
7.6 Comparative Remarks
Overall,under the explored operating conditions,no formulation looks readily suitable for practical applications:either burning rates are too high because of fast ADN self-deflagration or the pressure sensitivity is too large due to some not yet well understood modifications in the gas-phase flame structure.In order to moderate burning rates,if control of the ADN grain size is not enough,apossible way out is either a double oxidizer such as ADN/AP and ADN/AN or a doping of ADN by a small mass fraction(say,1%)of hydrocarbon fuels,rubber,plasticizer greatly reducing its burning rate,especially at low pressures[42].On the other hand,to mitigate the burning rate pressure sensitivity,the couple GAP/AlH3is much preferable to HTPB/Al,being both ingredients capable of surface or near surface reactions.In fact,due to the low ADN decomposition temperatures,slowly reactive inert binders will preferably affect the flame regions beyond the aerosol zone and thus promote an augmented pressure sensitivity.
8 Conclusions and Future Work
While ideal thermochemistry analyses overall favor ADN/GAP/Al formulation with respect to AP/HTPB/Al,at this time a full understanding of its flame structure and associated agglomeration phenomena at the surface layer is still missing and is sorely needed.ADN-based double oxidizer and/or ADN loaded with a small amount of organic fuels are recommended to make ballistic properties suitable to rocket propulsion applications.Mechanical properties,chemical stability,and hazard features have to be better assessed and maybe improved.
Ideal thermochemistry analyses also point out Al as the most suitable metal fuel in terms of gravimetric specific impulse.But considerations about the generated CCP amount and average size often indicate more promising directions for the actually delivered specific impulse.As a matter of fact,optimum dual metal formulations(μAlnAl,μAl-MgxBy,etc.)can in general be identified leading to improved ballistic properties under real operating conditions.ForμAl-MgxBydual metal formulations,in the case of AP/HTPB/Al propellants best results in terms of reduced average agglomerate size are obtained with 25% Mg coating.
Attention was also devoted to AlH3as a very promising high-energy metallic fuel[16,37].In spite of some density loss,ideal gravimetric specific impulse and measured pressure sensitivities are in favor of AlH3rather than Al,but current experimental results regarding agglomeration are not encouraging.Notice that burning rates increase and average agglomerate sizes decrease when Al is replaced by AlH3in the AP/HTPB matrix,on the contrary burning rates are little affected and average agglomerate sizes increase when Al is replaced by AlH3in the ADN/GAP matrix.
Acknowledgements
The authors gratefully acknowledge the crucial contribution of ENI Donegani Institute,Novara,Italy in performing advanced powder analyses.The authors also wish to thank AVIO,Italy for providingμAl samples;FOI,Sweden for providing actAl samples;and STK,Russia for providing nAl samples.The authors finally wish to thank Dr.W.Waesche for helpful discussions.
This work was partially supported by the HISP project(High performance solid propellants for In-Space Propulsion)of the European Community′s Seventh Framework Programme(FP7/2007-2013),under Grant Agreement No.262099,coordinated by FOI.
NOMENCLATURE
Roman Letters
a= multiplier factor solid propellant burning law,(mm/s)/(bar)n
Ab= burning area,cm2
d10= number weighted mean diameter,μm
d43= mass weighted mean diameter,μm
Is= gravimetric specific impulse,s
Iv= volumetric specific impulse,s g/cm3
n= steady burning rate pressure sensitivity
p= pressure,bar
rb= steady burning rate,mm/s
T= temperature,K
Tf= adiabatic flame temperature,K
Tign= ignition temperature,K
Greek Letters
ΔHf= standard enthalpy of formation,kJ/mole
ρ= density,g/cm3
Symbols
M= molecular mass,g/mole
Subscripts
a= aerosol
c= combustion gas
cc= combustion chamber
p= solid propellant
s= surface
Abbreviations
actAl = activated Aluminum
ADN = Ammonium DiNitramide
AlexTM= nanosized Aluminum obtained by electrical explosion of wires
AN = Ammonium Nitrate
AP = Ammonium Perchlorate
BET = Brunauer-Emmett-Teller specific surface area
BPS = Bis Propargyl Succinate
CCP = Condensed Combustion Products
DB = Double-Base propellants
DOA = DiOctyl Adipate
EEW = Electrical Explosion of Wires
FOI = Swedish Defence Research Agency
GAP = Glycidyl Azide Polymer
HMX = cyclotetramethylenetetranitramine
HNF = Hydrazinium Nitroformate
HNIW = 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-Hexaazai
sowurtzitane,also CL-20
HTPB = Hydroxyl-Terminated PolyButadiene
ICT = Institut für Chemische Technologie
IPDI = IsoPhorone Di-Isocyanate
L-AlexTM= nanosized Aluminum coated with stearic acid
nAl = nano-sized Aluminum
OB = Oxygen Balance
PDL = Pressure Deflagration Limit
RDX = cyclotrimethylenetrinitramine
SEM = Scanning Electron Microscopy
SPLab = Space Propulsion and Nanoenergetics Laboratory
SRI = Stanford Research Institute
STK = SibTermoKhim
TEM = Transmission Electron Microscopy
TMETN = Trimethylolethanetrinitrate
TMD = Theoretical Maximum Density
XPS = X-ray Photoelectron Spectroscopy
XRD = X-Ray Diffraction
2P = Two-Phase
μAl = micron-sized Aluminum
[1]Wingborg N.High Performance Solid ADN Propellant for Space Applications.5th EuCASS-Propulsion Physics,Munich,Germany,01-05 Jul 13.
[2]Calabro M.FP7 HISP Program:Selection of Reference Missions and Definition of Propellant Requirements.5th EuCASS-Propulsion Physics,Munich,Germany,01-05Jul 13.
[3]Maggi F,Dossi S,Reina A,et al.Advanced aluminum powders for solid propellants.5th EuCASS-Propulsion Physics,Munich,Germany,01-05Jul 13.
[4]Weiser V,Franzin A,Gettwert V,et al.Combustion of Metallised ADN/GAP Solid Rocket Propellants with Focus on Agglomeration Effects.5th EuCASS-Propulsion Physics,Munich,Germany,01-05Jul 13.
[5]Weiser V,De Luca L T,Franzin A,et al.Combustion Behaviour of Aluminium Particles in ADN/GAP Composite Propellants.HEM Workshop,Tokyo,Japan,07-09Oct 13.
[6]Lovett J R.Hydrazine Nitroform and Method of Preparation:US,3 378 594[P].1968.
[7]Atwood A I,Boggs T L,Curran P O,et al.Burning Rate of Solid Propellant Ingredients,Part 1:Pressure and Initial Temperature Effects[J].Journal of Propulsion and Power,1999,15(6):740-747.
[8]Atwood A I,Boggs T L,Curran P O,et al.Burning Rate of Solid Propellant Ingredients,Part 2:Determination of Burning Rate Temperature Sensitivity[J].Journal of Propulsion and Power,1999,15(6):748-752.
[9]Davenas A.Development of Modern Solid Propellants[J].AIAA JPP,2003,19(6):1108-1128.
[10]Meyer R,Köhler J,Homburg A.Explosives[M].6thedition Wiley-VCH,2007.
[11]Kubota N.Propellants and Explosives:Thermochemical Aspects of Combustion[M].2nd edition.Wiley-VCH,2007.
[12]Kuo K K,Achirya R.Fundamentals of Turbulent and Multiphase Combustion[M].Wiley,2012.
[13]Östmark H,Bemm U,Langlet A,et al.The Properties of Ammonium Dinitramide(ADN):Part 1,Basic Properties and Spectroscopic Data[J].Journal of Energetic Materials,2000,18:123-128.
[14]Kon′kova T S,Yu N Matyushin,Miroshnichenko E A,et al.Thermochemical properties of dinitramidic acid salts[J].Russian Chemical Bulletin,International Edition,2009,58(10):2020-2027.
[15]Talawar M B R,Sivabalan M,Anniyappan G M,et al.Emerging Trends in Advanced High Energy Materials[J].FGV,2007,43(1):62-72.
[16]Pak Z P.Some Ways to Higher Environmental Safety of Solid Rocket Propellant Application,93-1755[R].New York:AIAA,1993.
[17]Fogel′zang A E,Sinditskii V P,Egorshev V Y,et al.Combustion Behavior and Flame Structure of Ammonium Dinitramide[C]//28th International Annual Conference of ICT.Karlsruhe:ICT,1992:99.
[18]Manelis G B.Thermal Decomposition of Dinitramide Ammonium Salt[C]//26th Int Annual Conference of ICT,Karlsruhe:ICT.1995:15.
[19]Zenin A A,Puchkov V M,Finjakov S V.Physics of ADN combustion,99-0595[R].1999.
[20]Price E W,Chakravarthy S R,Freeman J M,et al.Combustion of Propellants with Ammonium Dinitramide,AIAA-1988-3387[R].New York:AIAA,1988.
[21]Chakravarthy S R,Freeman J M,Price E W,et al.Combustion of propellants with Ammonium Dinitramide[J].Propellant,Explosive,Pyrotechnics,2004,29(4):220-230.
[22]Gross M L,Beckstead M W,Puduppakkam K V,et al.Multi-Phase Combustion Modeling of Ammonium Dinitramide Using Detailed Chemical Kinetics,2006-4747[R].New York:AIAA,2006.
[23]Sinditskii V P,Egorshev V Y,Levshenkov A I,et al.Combustion of Ammonium Dinitramide,Part 1:Burning Behavior[J].Journal of Propulsion and Power,2006,22(4):769-776.
[24]Sinditskii V P,Egorshev V Y,Levshenkov A I,et al.Combustion of Ammonium Dinitramide,Part 2:Combustion Mechanism[J].Journal of Propulsion and Power,2006,22(4):777-785.
[25]Langlet A,Östmark H,Wingborg N.Method of Preparing Dinitramideic Acid and Salts Thereof:WO,97/06099[P].1997.
[26]Johansson M,de Flon J,Pettersson Å,et al.Spray Prilling of ADN,and Testing of ADN-Based Solid Propellants[C]//3rd International Conference on Green Propellants for Space Propulsion.ESA,Poitiers,France,2006.
[27]Heintz T,Pontius H,Aniol J,et al.Ammonium Dinitramide(ADN)-Prilling,Coating,and Characterization[J].Propellants,Explosive and Pyrotechnics,2009,34:231-238.
[28]Menke K,Heintz T,Schweikert W,et al.Formulation and Properties of ADN/GAP Propellants[J].PEP,2009,34:218-230.
[29]Wingborg N,Andreasson S,de Flon J,et al.Development of ADN-based Minimum Smoke Propellants,AIAA 2010-6586[R].New York:AIAA,2010.
[30]Trunov M A,et al.Ignition of Aluminum Powders Under Different Experimental Conditions[J].Propellants,Explosives,Pyrotechnics,2005,30(1):36-43.
[31]Beckstead M.W.A Summary of Aluminum Combustion,NATO RTO EN 023,2004.
[32]Dossi S,Reina A,Maggi F,et al.Innovative metal fuels for solid rocket propulsion[J].Int.J.Energetic Materials.Chem.Prop,2012,11(4):299-322.
[33]Maggi F,Dossi S,Paravan C,et al.Activated aluminum powders for space propulsion[J].submitted for publication.
[34]Wingborg N,Wanhatalo M,Petterson A.Solid Propellants Containing Activated Aluminum[C]//3rd Int.Conf.on Green Propellant for Space Propulsion,9th Int.Hydrogen Peroxide Propulsion Conference.Poitiers,France,2006,ESA SP-635,Dec 2006.
[35]Hahma A,Gany A,Palovuori K.Combustion of activated aluminium[J].Combustion and Flame,2006(145):464-480.
[36]Kuo K K,Achirya R.Fundamentals of Turbulent and Multiphase Combustion[M].Wiley,2012.
[37]DeLuca L T,Galfetti L,Severini F,et al.Physical and Ballistic Characterization of AlH3-based Space Propellants[J].Aerospace Science and Technology,2007,11(1):18-25.
[38]DeLuca LT,Rossettini L,Kappenstein C,et al.Ballistic Characterization of AlH3-Based Propellants for Solid and Hybrid Rocket Propulsion,2009-4874[R].New York:AIAA,2009.
[39]de Flon J,Andreasson S,Liljedahl M,et al.Solid Propellants based on ADN and HTPB,AIAA Paper 2011-6136[R].New York:AIAA,2011.
[40]Koji Fujisato,Hiroto Habu,Hidefumi Shibamoto,et al.Combustion characteristics of ADN-based solid propellants[J].Trans JSASS Aerospace Tech,2012,10(28):89-92.
[41]Eisenreich N,Fischer T S,Langer G,et al.Burn Rate Models for Gun Propellants[J].Propellants,Explosives,Pyrotechnics,2002,27(3):142-149.
[42]Strunin V A,D'Yakov A P,Manelis G B.Combustion of Ammonium Dinitramide[J].Combustion And Flame,1999,117:429-434.
[43]DeLuca L T,Galfetti L,Maggi F,et al.Innovative Metallized Formulations for Solid or Hybrid Rocket Propulsion[J].Chinese Jour-nal of Energetic Materials,2012,20(4):465-474.
