Neural bases of reappraisal regulatory effect on negative emotion in high reappraisers*******☆
2012-01-04WencaiZhangJinLuo
Wencai Zhang, Jin Luo,
1 Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing 100101, China
2 Beijing Key Laboratory of Learning and Cognition, Department of Psychology, Capital Normal University, Beijing 100048, China
Neural bases of reappraisal regulatory effect on negative emotion in high reappraisers*******☆
Wencai Zhang1, Jin Luo1,2
1Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing 100101, China
2Beijing Key Laboratory of Learning and Cognition, Department of Psychology, Capital Normal University, Beijing 100048, China
Previous studies have reported that individual differences in reappraisal use are associated with particular patterns of neural activity. We hypothesized that if ‘high reappraisers’ (individuals who use reappraisal well in a behavioral experiment) completed two training sessions, they would exhibit more reliable patterns of neural activity related to cognitive reappraisal. In the present study, 13 high reappraisers were selected from 27 healthy volunteers through an initial behavioral experiment (first training) followed by a functional MRI experiment (second training). Emotional images selected from the International Affective Picture System were used for both the behavioral and functional MRI sessions of the experiment. The behavioral results revealed that reappraisal reduced subjective unpleasantness. The functional MRI results revealed that the cognitive reappraisal used by high reappraisers decreased the activation of emotion-responsive regions, including the amygdala, insula, and cingulate gyrus, and increased the activation of regulation-related regions, including the inferior prefrontal cortex, orbital prefrontal cortex, and dorsal medial prefrontal cortex. These findings suggest the involvement of inferior orbital and dorsal medial prefrontal cortex in constructing reappraisal strategies that modulate activity in emotion-processing systems.
cognitive reappraisal; negative emotion; amygdala; functional MRI; neural regeneration
Research Highlights
(1) High reappraisers (individuals who use reappraisal well in a behavioral experiment) were selected to test whether the reliability of the neural correlates of cognitive reappraisal increased with multiple training sessions using a functional MRI.
(2) Cognitive reappraisal with high reappraisers was found to decrease the activation of emotion-responsive regions including the amygdala and insula, and activate a smaller area in the dorsal prefrontal cortex.
Abbreviations
PFC, prefrontal cortex; fMRI, functional MRI
INTRODUCTlON
Reappraisal refers to the reframing or recontextualization of a negative stimulus in less emotional terms[1-6]. Previous studies indicate that reappraisal depends upon interactions between prefrontal and cingulate regions associated with cognitive control and systems implicated in emotional responses[7-11], such as the amygdala and insula. However, in previous studies, the time allotted for reappraisal training was typically short and individual differences inreappraisal were not controlled. These factors may complicate the examination of the reappraisal effect and reappraisal-related brain activation[12]. For example, high reappraisers (individuals who use reappraisal well) reported less anger, less negative emotion, more positive emotion, greater cardiac output and less total peripheral resistance, compared to low reappraisers[13]. Furthermore, they observed a positive relationship between reappraisal ability, reappraisal frequency, and wellbeing[14].
Moreover, women exhibiting high reappraisal ability exhibited fewer depressive symptoms than those with low reappraisal ability during high levels of stress[15]. Importantly, it was found that greater reappraisal use in daily life was associated with a smaller activated area in the dorsal lateral prefrontal cortex (PFC) compared to initial use in most reappraisal studies[16]. Moreover, several other studies reported that individual differences in trait rumination and dispositional mindfulness are associated with reappraisal-related neural activity[17-18]. Cognitive reappraisal has received more attention than other modes of emotion regulation, at least partly, because current theorists propose that deficits in cognitive reappraisal characterize psychopathology[19-20]. Thus, a better understanding of cognitive reappraisal and its neural bases has implications for understanding emotion as well as the treatment of psychopathology.
We hypothesized that increasing reappraisal training and reducing individual differences would lead to a more reliable reappraisal effect and neural activity correlated with cognitive reappraisal. As such, to obtain a reliable reappraisal effect in the present study, we conducted two sessions of reappraisal training to guarantee effortless use of reappraisal strategies. Like past studies[21-23], we first conducted a behavioral experiment to find participants exhibiting a reliable reappraisal effect, then examined them using functional MRI (fMRI), minimizing inter-individual variation.
RESULTS
Quantitative analysis of subjects
A total of 27 participants took part in the initial behavioral experiment, and high reappraisers were defined as people who used cognitive reappraisal strategy well in the reduction of unpleasantness rating scores. These high reappraisers (n=14) were included in the fMRI experiment. One participant was excluded from the fMRI study due to an experimental error. A total of 13 participants were included in the final fMRI analyses.
Unpleasantness ratings in the behavioral and fMRl experiments
The behavioral experiment revealed a significant interaction effect [F(1, 25)=20.10,P<0.001] between condition (control and reappraisal) and reappraiser type (high and low). Further pairedt-tests revealed that unpleasantness ratings were significantly lower in the reappraisal condition compared to the control condition in the high-reappraiser group [t(1, 13)=8.20,P<0.001], whereas low reappraisers did not exhibit this pattern. This finding indicates that a reappraisal effect was indeed induced in these participants. In the fMRI experiment, unpleasantness ratings were provided both before and after each block of images. A significant reappraisal effect was also found for unpleasantness associated with actually viewing the images [t(12)=6.33,P<0.001; Figure 1]. These results indicate an anxiety-relieving effect of reappraisal during the scanning experiment.

Figure 1 Unpleasantness ratings in the control condition (passively viewing negative images) and reappraisal condition (reappraising the negative images in a less negative way).
Brain regions exhibiting attenuated activity in the reappraisal condition
A comparison of brain activity in the control and reappraisal conditions during unpleasant image viewing and neutral image viewing [(CU–CN)–(RU–RN)] revealed several clusters of significantly attenuated activity (Figure 2) in the right amygdala, right insula and right cingulate.
Modulation network exhibiting increased activity in the reappraisal condition
A contrast between the reappraisal and control conditions during the viewing of unpleasant images (RU–CU) revealed significant activity in the left inferior frontal gyrus, right medial frontal gyrus and left orbital frontal gyrus (Figure 3).
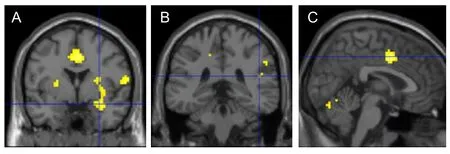
Figure 2 Activated regions during unpleasant image viewing and neutral image viewing were contrasted between the control and reappraisal conditions [(CU–CN)–(RU–RN)](functional MRI). Yellow indicates the activated clusters and the blue line intersection indicates the location of the maximum activation in the specific clusters.

Figure 3 Activated regions when contrasting the reappraisal and control conditions separately for the viewing of unpleasant images (functional MRI).
DISCUSSION
The present results support the findings of previous studies of cognitive reappraisal reporting that a significant reappraisal effect was associated with significantly decreased activity in the amygdala and increased activity in multiple prefrontal regions[9-10,24-27]. We found that cognitive reappraisal reduced activation of the amygdala in response to negative stimuli, and reduced the difference between negative and neutral stimuli in the reappraisal condition relative to the control group. This decreased amygdala activation is consistent with reports of therapeutic treatments, including anxiolytic placebo effects[23,28], antidepressants, and ataractics for major depression and panic disorder, respectively[29-32]. In addition, we found that reappraisal regulation was associated with increased activity in the left inferior frontal gyrus, left orbital frontal gyrus and right dorsal medial frontal gyrus. Previous reports have suggested that reappraisal is associated with activation in dorsal portions of the PFC that are involved in working memory and selective attention, ventral portions of the PFC that are implicated in language processing, dorsal portions of the anterior cingulate cortex that are implicated in monitoring control processes, and dorsal portions of the medial PFC that are implicated in reflection about the affective states of the self or other[9,16,32-36]. The results of the present study were generally consistent with these findings.
Importantly, the present findings have important implications for revealing a stable mechanism of cognitive reappraisal. To control individual differences, we conducted reappraisal training twice, selecting high reappraisers as participants so that brain activation would be more reliable compared to studies in which participants completed only one reappraisal training session or differences in reappraisal use were not accounted for. Previous studies reported that individual differences in dispositional mindfulness may modulate activity in the neural systems involved in the effective cognitive reappraisal of negative emotion[19]. Furthermore, individual differences in rumination are reported to be correlated with greater activity decreases in prefrontal regions implicated in self-focused thought, when participants decreased negative effect[17]. These findings indicate the importance of controlling for the influence of differences in reappraisal use. In the current study, we found a smaller area of activation in the dorsal PFC compared to previous studies that did not control for individual differences and conducted reappraisal training only once. These findings indicate that high reappraisers with more training may require less effort to complete reappraisal. These results are consistent with a previous study reporting that reappraisal predicted decreased amygdala activation and increased activation in the dorsal lateral/medial PFC, orbital PFC, and parietal cortex compared to non-reappraisal, whereas attenuated activation was observed in the dorsal PFC in people exhibiting greater everyday reappraisal use compared to those exhibiting less everyday reappraisal use[16]. Taken together with the current results, these findings indicate that more reliable, reappraisal-related brain structures are likely to be found among high reappraisers. In conclusion, the current fMRI results revealed that cognitive reappraisal with high reappraisers significantly decreased the activation of emotion-responsive regions including the amygdala and insula, and activated a smaller area in the dorsal prefrontal cortex compared to that reported in previous studies. Overall, these findings indicate that high reappraisers with more training may require less effort to complete reappraisal.
SUBJECTS AND METHODS
Design
Comparative observation of behaviors and imaging.
Time and setting
The fMRI experiment was conducted in an fMRI scanning room and the behavioral experiment was conducted in an ordinary room in the Institute of Biophysics, Chinese Academy of Science in September 2011.
Subjects
We recruited volunteers through the University BBS network. Inclusion criteria: (1) normal health; (2) right handed; (3) normal/corrected-to-normal vision; (4) no history of psychosis or neurosis; (5) no history of serious physical or emotional trauma; (6) provided written informed consent. A total of 27 participants with an average age of 20.7±1.1 years were included (23 females and four males).
Methods
High reappraisers were selected using an initial behavioral experiment (first training) to identify participants exhibiting a significant reappraisal regulatory effect. These participants were then included in an fMRI experiment (second training). We compared the reappraisal condition with the nonreappraisal (control) condition using a within-group design to identify reappraisal-related brain areas.
Emotional stimuli
Emotional images for both the behavioral and fMRI experiments were selected from the International Affective Picture System. In the behavioral experiment, only emotionally unpleasant images were used (mean±SEM: valence and arousal were 2.62±0.87 and 5.74±0.42, respectively). In the fMRI experiment, both high-arousal unpleasant (arousal: 5.84±0.10; valence: 2.60±0.10) and low-arousal neutral (arousal: 3.67±0.15; valence: 5.35±0.11) images were used; these types of images differed significantly from each other in terms of both arousal [t(59)=10.588,P<0.001] and valence [t(59)=62.910,P<0.001].
Behavioral experiment
Participants were instructed to use a cognitive reappraisal strategy by generating an interpretation of, or a story about, each image that would explain apparently negative events in a less negative way[11]. Participants completed 10 practice trials to ensure that they were able to use their reappraisal strategies effectively. They were then presented with two blocks of unpleasant images, one passive-viewing block and one reappraising block. The arousal and valence values of the images were equivalent and the presenting order was counterbalanced between the two blocks. Each block contained four high-arousal unpleasant images. Each image was presented for 3 seconds, followed by a fixation cross (“+”) for 3 seconds. Blocks were separated by a one-minute rest period, to avoid the potential confounding influence of emotional fatigue. Immediately after each block, participants reported unpleasantness ratings using an 11-point scale (from 0=no unpleasantness to 10=unbearably unpleasant)[23].
fMRI experiments
Fourteen participants exhibited greater reduction of reported unpleasantness in the reappraisal condition compared to the mean group reduction, and were invited to return for follow-up fMRI scanning. These participants were trained and completed another 10 practice trials. There were three runs of alternating reappraisal and control condition stimulus blocks. The runs began with a reappraisal condition block for half of the participants and with a control condition block for the other half. Each block consisted of 10 unpleasant and 10 neutral images. The arousal and valance values of the images were equivalent across all blocks. Participants were instructed to carefully view each image during its 3-second presentation period. Unpleasant and neutral images were pseudo-randomly intermixed, and images from the same category were presented no more than three times in a row in order to avoid habituation effects. After each block, participants reported unpleasantness ratings on an 11-point scale.
The fMRI imaging was performed with a 3.0 Tesla MR scanner (Siemens, Magnetom Trio, Germany), using the standard radio frequency headcoil. Each participant’s head was fixed with foam pads throughout the experiment to minimize head movement. Thirty-two transversal slices of functional images that covered the whole brain were acquired with a T2*-weighted echo-planar imaging sequence based on blood oxygenation level-dependent contrast (repetition time=2 000 ms; echo time=30 ms; image matrix=64×64; slice thickness=4 mm; gap=0.4 mm; field of view=200×200 mm2; flip angle=90°). For each participant, a high-resolution anatomical scan was acquired at the end of the experiment with a T1-weighted 3D magnetization-prepared rapid gradient-echo pulse sequence (repetition time=2 530 ms, echo time=3.37 ms, flip angle=7°, field of view=256×256 mm2, voxel size=1×1×1.33 mm3, 144 contiguous 1.33-mm thick sagittal slices, slice matrix size=256×256).
The preprocessing and statistical analysis of images was performed using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm). The first four functional echo-planar imaging volumes were discarded to allow for T1 equilibration. Preprocessing of the remaining functional echo-planar imaging images included slice correction, motion correction, and normalization. Functional images were transformed into a standard anatomical space (3×3×3 mm3isotropic vexes) based on the Montreal Neurological Institute template. Functional images were spatially smoothed using a Gaussian filter with 8-mm full width half maximum. The data were statistically analyzed using the general linear model and statistical parametric mapping.
To assess the neural activity corresponding to the processing of the two different types of images under each of the experimental conditions, four separate regressors were created (CU, viewing unpleasant images in the control condition; CN, viewing neutral images in the control condition; RU, viewing unpleasant images in the reappraisal condition; RN, viewing neutral images in the reappraisal condition). These conditions were time-locked to the onset of image presentation and convolved with a canonical hemodynamic function. In addition, motion realignment parameters were modeled to account for variability related to head movements. A high-pass filter with a cut-off frequency of 1/128 Hz was used to correct for low-frequency components, and serial correlations were accounted for with an autoregressive AR (1) model.
The relevant parameter contrasts generated on an individual level were submitted to a group analysis using a random effects model. A 2×2 full factorial analysis of variance was conducted with data from all participants, with experimental condition (reappraisalvs. control) and emotional image type (unpleasant vs. neutral) as factors. Whole brain search results from the random effects analysis with a threshold atP<0.001 (uncorrected) are reported here. The MNI coordinates were converted into Talairach coordinates.
Statistical analysis
Unpleasantness ratings were analyzed with repeated measures analysis of variance and pairedt-tests using SPSS version 10.0 (SPSS Corporation, Chicago, IL, USA).
Funding:This research was supported by the National Natural Science Foundation of China, No. 30970890, 30770708 and 31100746; the Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences, No. Y0CX451S01; the National Basic Research Program of China, No. 2010CB833904; the Knowledge Innovation Program of the Chinese Academy of Sciences, No. KSCX2-EW-J-8; and Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences.
Author contributions:Wencai Zhang and Jin Luo conceived and designed the study and wrote the manuscript. Wencai Zhang conducted the experiments and analyzed the data.
Conflicts of interest:None declared.
Ethical approval:The procedures of the present study were approved by the Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences, China.
Author statements:The manuscript is original, has not been submitted to or is not under consideration by another publication, has not been previously published in any language or any form, including electronic, and contains no disclosure of confidential information or authorship/patent application disputations.
[1] Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998; 74(1):224-237.
[2] McRae K, Ochsner KN, Mauss IB, et al. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Process Intergroup Relat. 2008;11(2):143-162.
[3] Gross JJ, Thompson RA. Emotion regulation: conceptual foundation. In: Gross JJ, ed. Handbook of Emotion Regulation. New York: Guilford Press. 2007.
[4] Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3): 281-291.
[5] Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348-362.
[6] Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci. 2006;6(4):291-297.
[7] Costafreda SG, Brammer MJ, David AS, et al. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58(1):57-70.
[8] Blair KS, Smith BW, Mitchell DG, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430-440.
[9] Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci. 2008;17(2):153-158.
[10] Ochsner KN, Bunge SA, Gross JJ, et al. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215-1229.
[11] Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11(7): 307-316.
[12] Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, ed. Handbook of Emotion Regulation. New York: Guilford Press. 2007.
[13] Mauss IB, Cook CL, Cheng JY, et al. Individual differences in cognitive reappraisal: experiential and physiological responses to an anger provocation. Int J Psychophysiol. 2007;66(2):116-124.
[14] McRaea K, Jacobsb SE, Rayc DR et al. Individual differences in reappraisal ability: links to reappraisal frequency, well-being, and cognitive control. J Res Pers. 2012;46(1):2-7.
[15] Troy AS, Wilhelm FH, Shallcross AJ, et al. Seeing the silver lining: cognitive reappraisal ability moderates the relationship between stress and depressive symptoms. Emotion. 2010;10(6):783-795.
[16] Drabant EM, McRae K, Manuck SB, et al. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol Psychiatry. 2009;65(5): 367-373.
[17] Ray RD, Ochsner KN, Cooper JC, et al. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn Affect Behav Neurosci. 2005;5(2):156-168.
[18] Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc Cogn Affect Neurosci. 2010; 5(4):369-377.
[19] Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, ed. Handbook of Emotion Regulation. New York: Guilford Press. 2007.
[20] Werner KW, Gross JJ. Emotion regulation and psychopathology: a conceptual framework. In: Kring AS, Sloan D, eds. Emotion Regulation and Psychopathology. New York: Guilford Press. 2009.
[21] Wager TD, Matre D, Casey KL. Placebo effects in laserevoked pain potentials. Brain Behav Immun. 2006;20(3): 219-230.
[22] Zhang W, Luo J. The transferable placebo effect from pain to emotion: changes in behavior and EEG activity. Psychophysiology. 2009;46(3):626-634.
[23] Zhang W, Qin S, Guo J, et al. A follow-up fMRI study of a transferable placebo anxiolytic effect. Psychophysiology. 2011;48(8):1119-1128.
[24] Goldin PR, McRae K, Ramel W, et al. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577-586.
[25] McRae K, Hughes B, Chopra S, et al. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22(2): 248-262.
[26] Phan KL, Fitzgerald DA, Nathan PJ, et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210-219.
[27] Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15(9):657-666.
[28] Petrovic P, Dietrich T, Fransson P, et al. Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46(6):957-969.
[29] Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
[30] Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159(5):728-737.
[31] Goldin PR, Manber-Ball T, Werner K, et al. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry. 2009; 66(12):1091-1099.
[32] Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60(4):337-343.
[33] Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11(8):880-881.
[34] Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242-249.
[35] Schaefer SM, Jackson DC, Davidson RJ, et al. Modulation of amygdalar activity by the conscious regulation of negative emotion. J Cogn Neurosci. 2002;14(6):913-921.
[36] Delgado MR, Nearing KI, Ledoux JE, et al. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829-838.
10.3969/j.issn.1673-5374.2012.32.009 [http://www.crter.org/nrr-2012-qkquanwen.html]
Zhang WC, Luo J. Neural bases of reappraisal regulatory effect on negative emotion in high reappraisers. Neural Regen Res. 2012;7(32):2542-2547.
Wencai Zhang☆, M.D., Associate researcher, Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing 100101, China
Jin Luo, M.D., Professor, Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing 100101, China; Beijing Key Laboratory of Learning and Cognition, Department of Psychology, Capital Normal University, Beijing 100048, China luoj@psych.ac.cn; luoj@cnu.edu.cn
2012-04-10
2012-09-08 (N20111223002/YJ)
(Edited by Xiao YY, Wei PX/Su LL/Song LP)
杂志排行
中国神经再生研究(英文版)的其它文章
- Characteristics of vascular lesions in patients with posterior circulation infarction according to age and region of infarct*☆
- Morphological differences in skeletal muscle atrophy of rats with motor nerve and/or sensory nerve injury*★
- A standardized method to create peripheral nerve injury in dogs using an automatic non-serrated forceps***★
- Rapid genetic screening of Charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsies patients**★
- Ultrasound guided neural stem cell transplantation through the lateral ventricle for treatment of cerebral palsy in children☆
- Correlation between spina bifida manifesta in fetal rats and c-Jun N-terminal kinase signaling**★
