Ultrasound guided neural stem cell transplantation through the lateral ventricle for treatment of cerebral palsy in children☆
2012-01-04ShengHeZuoLuanSuqingQuXuanQiuDaqingXinWenkaiJiaYanhuaShenZehuiYuTaoXu
Sheng He, Zuo Luan, Suqing Qu, Xuan Qiu, Daqing Xin, Wenkai Jia, Yanhua Shen, Zehui Yu, Tao Xu
Department of Ultrasonography, Navy General Hospital of Chinese PLA, Beijing 100048, China
Ultrasound guided neural stem cell transplantation through the lateral ventricle for treatment of cerebral palsy in children☆
Sheng He, Zuo Luan, Suqing Qu, Xuan Qiu, Daqing Xin, Wenkai Jia, Yanhua Shen, Zehui Yu, Tao Xu
Department of Ultrasonography, Navy General Hospital of Chinese PLA, Beijing 100048, China
A total of 24 children with cerebral palsy were enrolled in this study and underwent ultrasound guided transplantation of neural stem cells through the lateral ventricle. Neural stem cells (3.8×106–7.3×107) were injected into the lateral ventricles. Mild injury of lateral ventricular blood vessels occurred in only two cases (8.3%). Seven cases (29.2%) experienced a fever. Clinical manifestations were improved to varying degrees in eight cases (28.0%) within 3 months after transplantation. Patient condition did not worsen, and no patient experienced severe adverse reactions.
cerebral palsy in children; puncture of the lateral ventricle; neural stem cells; transplantation; blood vessel injury; ultrasonography; neural regeneration
Research Highlights
(1) Neural stem cell transplantation, for treatment of cerebral palsy in children, was performed through the lateral ventricle and was guided by ultrasonography.
(2) The effective rate of improvement was approximately 50%.
(3) At 3 months following transplantation, patient condition did not worsen, and none experienced severe adverse reactions.
(4) These findings indicated that this method has a good safety profile for clinical application.
INTRODUCTION
Research institutes in various countries have investigated cell transplantation therapy for central nervous system diseases since the 1980s[1]. Cell transplantation therapy has been suggested as a possible safe and efficient therapy for many diseases in both animal and clinical research. These diseases include stroke, traumatic central nervous system injury, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis. Previous studiesindicated the effectiveness of using neural progenitor cells to treat cortical blindness[2-4], severe hypoxic-ischemic encephalopathy in neonates and encephalopathy from severe carbon monoxide poisoning. Although the curative effects are not entirely satisfactory, experiments show that newborn animals have a better internal environment for transplantation of neural stem cells and the effects of transplantation are greater compared with adult animals[5-7]. Therefore, it is worth investigating whether thetransplantation of neural stem cells could become an effective clinical treatment for cerebral palsy, including hypoxic-ischemic encephalopathy[7-10]. This paper mainly discusses the method of ultrasound guided neural stem cell transplantation in the lateral ventricle.
RESULTS
Quantitative analysis of subjects
All 24 cases of cerebral palsy initially included entered to the final analysis.
Baseline data of subjects
Clinical data from the 24 cases of cerebral palsy are shown in Table 1.
Identification results of neural stem cells
Neural stem cells culturedin vitroformed a suspended sphere (Figure 1A), and were positive for nestin (Figure 1B). After induction by fetal calf serum,
neurofilament-positive cells (Figure 1C) or glial fibrillary acidic protein-positive cells (Figure 1D) were present.
Safety of ultrasound guided neural stem cell transplantation in the lateral ventricle
Among the 24 children with cerebral palsy, seven children successively underwent neural stem cell transplantation twice. Altogether the lateral ventricle was punctured 31 times. The width of the anterior horn of the right lateral ventricle was 1.9–10.5 (4.1±3.2) mm, the amount of neural stem cells injected into ventricles was 0.4–1.2 (mean: 0.8±0.4) mL, the cell count was 3.8×106–7.3×107, and all the punctures were a success on the first attempt.
Among the punctures, the cerebrospinal fluid of two cases (6.45% of the total punctures) was light red, indicating that some blood vessels in the ventricle were injured.
After the operation, patient ventricles were examinedviaultrasound, whereupon all ventricles were found to be normal and not widened. Patient hemostasis was corrected and their condition began to stabilize. Seven cases (29.17%) experienced fever after surgery with a temperature range of 37.5–39.2 (mean: 38.7±0.2)°C. Temperature was restored to normal through physical cooling and symptomatic treatment in 2–11 (mean: 6.3±5.5) days. Hospitalization extended from 12–92 (mean: 39.1±12.3) days. One case (4.17%) vomited. This patient displayed stable normal vital signs and cardiopulmonary examination. Vomiting ceased following symptomatic treatment. Sphenotresia was performed in seven cases (29.17%), two (6.45%) undergoing this procedure twice. Neural stem cell transplantation was conducted one week after the drilling in these cases.
Short-term curative effect after transplantation
A short time after transplantation (1–3 months after surgery), the muscular tension in the limbs of eight patients (about 33.3%) decreased. Furthermore, the head-controlling action of eight children (about 33.3%) improved, visual acuity was enhanced in two patients (about 8.3%), and the communicative competence of three children (12.5%) improved. Overall clinical impression improved by 28.0%, and no patients worsened.
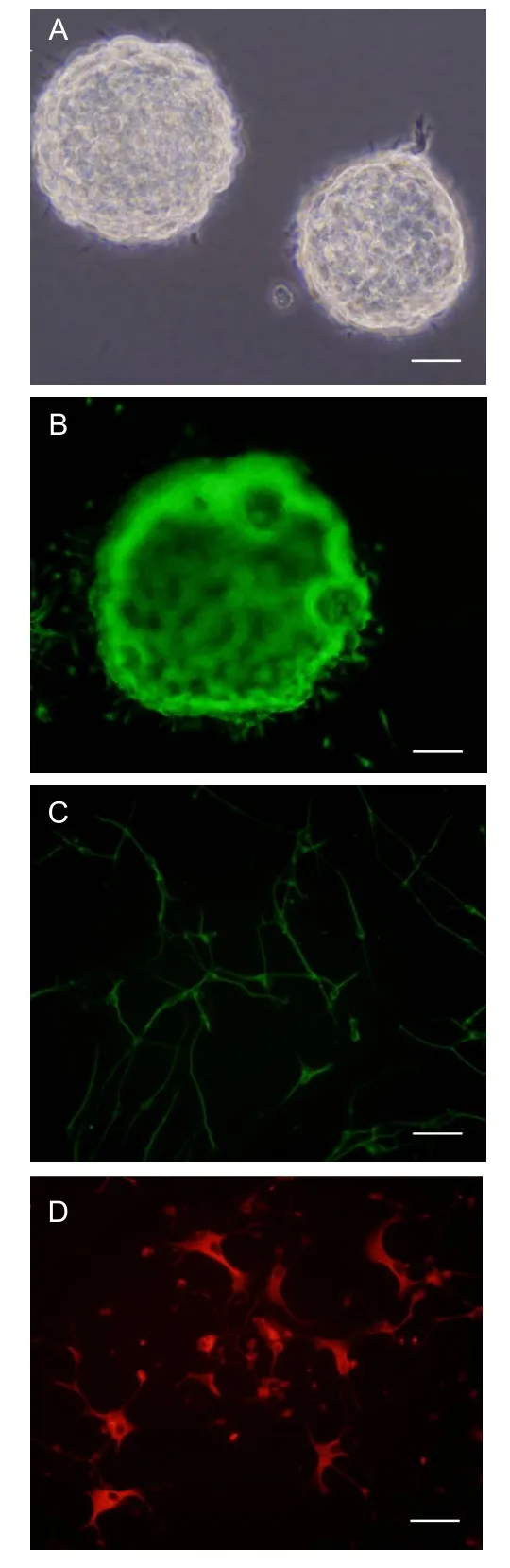
Figure 1 Neural stem cell identification (immunofluorescence).
DISCUSSION
The mechanism by which different grafts may treat hypoxic/ischemic brain damage is not yet clear. Our theory includes the possibility that the transplanted cells and activated endogenous neural stem cells work together to substitute injured or necrotic nerve cells, thereby reconstructing the nerve net and secreting neurotrophic factors necessary for recovering from injuries. The renovation and regeneration of myelin tissue would aid in neuronal recovery. During this process, spontaneous regeneration initiated by endogenous neural stem cells is limited. However, exogenous neural stem cells can promote the spontaneous renovation of endogenous neural stem cells after ischemia. Given that neural stem cells culturedin vitrohave the capability of neural differentiation after being transplanted into the host, it is considered that the process of differentiation presents obvious region specificityin vivo. The transplantation sites chosen in relevant experiments have been different from this study. The sites are mainly frontal lobe, occipital lobe, hippocampus and lateral ventricle, and a certain transplantation effect has been achieved. Although these reports do not have the relevant experimental controlled observations, a suitable transplantation site is very important for acquiring better curative effects[6,11-13].
Some concerns were apparent on choosing the ventricle as the transplantation pathway for this group of patients with cerebral palsy. In brain injuries caused by different pathologies, endogenous neural stem cells repaired injuries through migrating from the subventricular zone to the damaged area along the callosum and external capsule. Exogenous neural stem cells finish the migration in the host body under the guidance of the endogenous neural stem cells. The hypoxic-ischemic encephalopathy seen in neonates, however, is a multi-site, bihemispheric injury, and not confined to a certain region. Clinical multi-site transplantation creates a large amount of injury and is a difficult operation that cannot achieve region specificity; however, this process can make use of the circulation of the cerebrospinal fluid in the ventricular system. Transplantation into the ventricle only requires puncture once to achieve the effects of renewing the injury site through migration. Furthermore, microglia and macrophages are in an activated state in the damaged area, and the regional microenvironment of brain is so complex that neural stem cells implanted in the damaged zone may be eliminated. Puncture of the ventricle can avoid direct implantation in the damaged area and also bypasses the influences of an inflammatory environment on the survival of implanted cells[14-17].
Former experiences of treating cerebral palsy in children using an ultrasound guided puncture through an anterior fontanel and lateral ventricle have been described[18-21]. Changes in this procedure need to occur in order to accommodate the physiological and pathological characteristics of older children where cranial bones are thicker, anterior fontanels have closed and suture lines of the skull are narrow. For these patients, sphenotresia was performed before the puncture to provide a better acoustic window and puncture pathway, so that the accuracy of the injection site was guaranteed and the risk of possible injuries during the puncture was greatly reduced. The 0.5 cm hole drilled in the skull had no negative impact on patient condition. The success rate of one puncture was 100% for the 31 successive punctures in the 24 cases, in which mild injury of the lateral ventricle blood vessels occurred in only two cases (incidence rate of 6.5%). However, non-broadening and mild-broadening of the lateral ventricle occurred in 15 cases (about 62.5%), indicating that the puncture of the lateral ventricle guided by ultrasonography possessed the advantages of safety, high success rate, simple and convenient surgery, light injury and limited complications. This implies that the shortcomings of traditional clinical blind puncture through the anterior fontanel, and the lateral ventricle of children can now be avoided[21-24].
As shown in Table 1, we can see that the patients with cerebral palsy had different pathogenesis, typing, complications and states of illness. The clinical evaluation on the effects of neural stem cell transplantation mainly depended on the recovery index of patient function, which was also the objective of transplantation[6,17,23].
In terms of this study, the physiological function of some patients improved after neural stem cell transplantation. Although no electroencephalogram became more favorable for any patient, the index of patient condition in some children did improve or the original condition was partly controlled. However, whether such curative effects could be consolidated or further improved requires coordination and observation of other relative treatments[20,22]. Some children experienced a fever of varying degrees. Most were one-off low grade fever fluctuating between 38.7±0.2°C. Indications of acute infection were not found through routine blood examination, and temperatures returned to normal following symptomatic treatment. Furthermore, original symptoms were not aggravated. Vomiting occurred in one case, which was inferred to be related to the medication used during surgery. Lateral ventricles did not broaden in the two cases that had mildly injured lateral ventricle blood vessels. The acoustic reflection of the ventricle did not change, and intracranial pressure did not rise during or after surgery.
In addition, convulsions reoccurred in children who originally experienced convulsions because of cell migration in the early days following transplantation. These convulsions gradually disappeared and did not need special treatment[1,17].
SUBJECTS AND METHODS
Design
Retrospective analysis.
Time and setting
Experiments were performed at the Department of Ultrasonography, Navy General Hospital of Chinese PLA between May 2005 and March 2009.
Subjects
According to the definition of cerebral palsy in children[25], the diagnostic conditions included: (1) the cerebral injury that led to cerebral palsy was non-progressive; (2) the lesion leading to dyskinesia was in the brain; (3) the symptoms appeared during infancy; (4) there were some complications, such as mental deficiency, epilepsy, sensory disturbance and other anomaly; (5) central dyskinesia induced by progressive disease and temporary motor retardation were ruled out.
马老用赞许的眼光望着高潮,说,很好,很好嘛。接着,扭头看了一眼田卓,继续说,下一期的《NEW商圈》,看样子要做“十大本土最具发展潜力房地产企业”评选活动的专号了,你们现在就要与拟入选的企业联系,为他们编写形象宣传稿件,一定要突出每家企业的闪光点,让他们感觉入选得值,钱花得也值,让他们感觉,我们的《NEW商圈》,不比《福布斯》中文版做得差。小田、小高啊,你们重任在肩啊。这次活动圆满结束之后,我安排你们两个一次减压之旅,国内也好,国外也好,地点由你俩确定。
Twenty-four cases of children with cerebral palsy presented to the Navy General Hospital of Chinese PLA from May 2005 to March 2009, comprising 20 males and 4 females, aged 1 month to 7 years (mean: 5.5±4.3 months). General data are shown in Table 1.
Inclusion criteria: (a) diagnosed with definite cerebral palsy clinically, including quadriplegic, diplegic, dyskinetic, and mixed cerebral palsy; (b) classified to level IV–V according to Gross Motor Function Classification Scale[1]; (c) making no improvement after routine treatment and rehabilitation training; (d) advancing genetic metabolic disease was excluded.
Exclusion criteria: (a) severe heart disease, cardiac functional insufficiency; (b) severe pulmonary disease, respiratory failure; (c) complicated with acute or chronic infection; (d) frequent epileptic seizures in the last 6 months; (e) blood coagulation deficiency; (f) brain tumor or other malignant tumors.
The protocol for this study was approved by the Scientific Council and Ethics Committee of the Navy General Hospital of Chinese PLA, and was conducted in accordance with the guidelines ofAdministrative Regulations on Medical Institution, issued by State Council of the People’s Republic of China[26]. Informed consent was signed by patient guardians following being fully informed about the sources, separation and cultivation of the cells, the methods of cell therapy, risks of the surgery, the possible side effects, and safety measures.
Methods
Cell source and identification
The forebrain tissues of naturally-aborted 12-week old fetuses were taken to prepare a unicellular solution using a mechanical dispersion method. This corresponded with the provision of Hospital Management Committee of Ethics. The women knew, and agreed with, the circumstances of use of these fetuses.
Forebrain tissue was incubated in N2medium (17502-048; Gibco, New York, USA) in a dish with a diameter of 35 mm (DMEM/F12 + N2+ B27; Gibco). The concentration of inoculated cells was 1×105/mL, and the medium was added with 20 ng/mL of epidermal growth factor (100-15; Peprotech, New Jersey, USA), 10 ng/mL of basic fibroblast growth factor (100-18B; Peprotech) and 1 ng/mL of leukemic inhibitory factor (300-05; Peprotech). The equivalent above-mentioned culture fluid was added on the fifth day of culture. Until the eleventh day, a small eugenic colony of neural stem cells (small cellular balls, each with several cells) was obtained to prepare the cellular solution containing 1×105cells/μL. Cellular spheres were also used for immunocytochemistry, with the polyclonal rabbit antihuman nestin antibody (1:100; sc-20978; Santa Cruz Biotechnology, Santa Cruz, CA, USA) being used to identify the characteristics of neural stem cells. Simultaneously, 10% calf serum (10099-141, Gibco) was added to induce and differentiate the spheres. Finally, monoclonal mouse anti-human neurofilament protein (1:500; MAB5294; Chemicon, MA, USA) and monoclonal mouse anti-human glial fibrillary acidic protein (1:300; MAB360; Chemicon) were used to determine if cells had differentiated into neurons or astrocytes, respectively[5-6,8]. Immunofluorescence staining results were observed using fluorescence microscopy (IX51; Olympus, Tokyo, Japan).
Before transplantation, an examination was conducted to ascertain whether the child’s temperature, pulse, breathing rate, routine bloods and coagulation were normal. The pathway of puncture depended on the level of closure of the anterior fontanel and the acoustic reflection coefficient of the cranium. For those patients whose anterior fontanel and coronal suture were not closed, the anterior fontanel and coronal suture could be directly chosen as the puncture and ultrasound observation point (acoustic window). For those patients who had a closed anterior fontanel but whose cranium had good acoustic reflection, clearly reflecting the intracranial structures, a bone drill was used, under ultrasound guidance, to drill a hole on the parietal bone with a diameter of 0.3–0.5 cm until the cerebral dura mater was reached. This opening was used as the puncture point. For those patients who had a closed anterior fontanel and whose cranium had poor acoustic reflection, a hole with a diameter of 0.5 cm was drilled into the parietal bone 1.5–2.0 cm from the midline for use as the observation window for ultrasound guidance. The puncture position was then measured through the guidance window. For those patients with brain trauma, the position of the skull defect was used as the acoustic window and/or puncture point. For convenience, the observation point was located at the parietal bone on the left of the midline of the cranium, and the puncture point was located at the parietal bone on the right of the cranial midline. The instrument used was an AcusonSequia 512 (Siemens, Acuson, Germany) with a 4V1 probe and frequency of 2.0–4.0 MHz. The patients’ pulse and respiration were monitored during the surgery. Patients were fasted for 2–4 hours prior to surgery to avoid aspiration of gastric contents during anesthesia.
Neural stem cell transplantation in the lateral ventricle under the guidance of ultrasonography
The patient was in the supine position, and the transplantation was performed under ketamine anesthesia. In the sterilized region, a 22G puncture needle (Hakko, Nagano-Ken, Japan) was fixed on the needle holder of the ultrasound probe and the leading line was turned on. The reflected image of the brain structure was observed again, and the width of the anterior horn of the right lateral ventricle was measured. The puncture pathway was determined using Color Doppler Flow Imaging to view the presence of blood flow. If blood flow was detected, the angle and direction of the probe were adjusted in order to preserve encephalic blood vessels. Under the guidance of ultrasound, the puncture needle tip and part of the needle body presented a strong echo and was steadily guided into the ventricle. The stylet was then extracted to allow visualization of cerebrospinal fluid flow, of which 0.5–2.0 mL was reserved for laboratory examination. Then, after gentle shaking, the neural stem cell suspension was slowly infused into the ventricle. Two-dimensional imaging showed the gas integrated in the suspension appearing as a high echo and Color Doppler Flow revealed that the colorful Doppler signals appeared as patchy glittering around the needle tip (Figure 2).
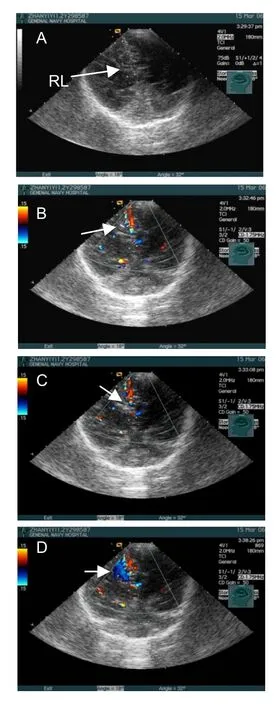
Figure 2 Transplanting neural stem cells into the lateral ventricle was guided by ultrasonography in a child with cerebral palsy.
Afterwards, 0.3–0.5 mL saline solution was used to wash the residual suspension in the needle. Dexamethasone (5%; 0.2 mL) was added to the saline if intraventricular hemorrhage appeared. After the puncture stylet was replaced, the puncture needle was extracted and the operation region was partially disinfected and bandaged. During and after surgery, the patients received continuous oxygen and electrocardioscope monitoring. After surgery, the patients received intravenous injection of cerebrolysin (except those who had ever had a convulsion), vitamin B12, ganglioside and cyclosporine A.
Indexes in evaluating the effectiveness of the transplantation
In the evaluation of curative effects of the transplantation, we depended mainly on the patients’ clinical appearance and then on electroencephalograms (Sd32, Micromed, Ital) and images[1,25]. The clinical observations included whether: the muscular tension of the limbs decreased, the hands were opened, and whether the patient showed or regained grasping action. Control of head movement, straight kneeling, turning over, lumbar muscle strength, posture-controlling, balance during walking, visual acuity and object-tracking, attention and communicative competence, epileptic seizures, sleep, and irritation were also monitored for changes post-surgery.
Author contributions:Sheng He performed majority of the ultrasound guided transplantation. He was the chief author of the article, including the idea, design and data analysis. Zuo Luan and Suqing Qu provided clinical and laboratory assistance. Xuan Qiu, Daqing Xin, Wenkai Jia, Yanhua Shen, Zehui Yu and Tao Xu all assisted Sheng He during operations.
Conflicts of interest:None declared.
Ethical approval:The protocol for this study was approved by the Scientific Council and Ethics Committee of Navy General Hospital of Chinese PLA.
Author statements:The manuscript is original, has not been submitted to or is not under consideration by another publication, has not been previously published in any language or any form, including electronic, and contains no disclosure of confidential information or authorship/patent application disputations.
[1] Luan Z, Liu W, Qu S, et al. Effects of neural progenitor cells transplantation in children with severe cerebral palsy. Cell Transplant. 2012;21 Suppl 1:91-98.
[2] Luan Z, Qu SQ, Liu WP, et al. Treatment of Heteroptics after cerebral palsy with transplantation of human neural stem cells into cerebral ventricle in infants: 7 case report. Zhongguo Kangfu Lilun yu Shijian. 2007;13(12): 1103-1105.
[3] Luan Z, Yin GC, Hu XH, et al. Treatment of an infant with severe neonatal hypoxic-ischemicencephalopathy sequelae with transplantation of human neural stem cells into cerebral ventricle. Zhonghua Er Ke Za Zhi. 2005; 43(8):580-583.
[4] Qu SQ, Luan Z, Yin GC, et al. Case report of a neonate with severe carbon monoxide poisontreated with transplantation of human neural stem cells. Zhongguo Xinsheng Erke Zazhi. 2007;22(6):334-336.
[5] Bentlage C, Nikkahah G, Cunningham MG, et al. Reformation of the nigrostriatal pathway by fetal dopaminergic micrografts into the substanianigra is critically dependent on theage of the host. Exp Neurol. 1999;177(2):177-190.
[6] Ansari SA, Nachanakian A, Biary NM. Current surgical treatment of Parkinson's disease. Saudi Med J. 2002; 23(8):1319-1323.
[7] Arias CO, Yuan TF. Autologous neural stem cell transplantation: a new treatment option for Parkinson's disease? Med Hypotheses. 2009;73(5):757-759.
[8] Tian ZM, Chen T, Zhong N, et al. Clinical study of transplantation of neural stem cells in therapy of inherited cerebellar atrophy. Beijing Da Xue Xue Bao. 2009;41(4): 456-458.
[9] Qu SQ, Luan Z, Yin GC. Experimental study of neonatal hypoxic-ischemic brain injury by intraventricular transplantation of human neural stem cells. Zhonghua Xiaoer Waike Zazhi. 2005;43(8):576-579.
[10] Yang CR, Yu RK. Intracerebral transplantation of neural stem cells combined with trehalose ingestion alleviates pathology in a mouse model of Huntington's disease. J Neurosci Res. 2009;87(1):26-33.
[11] Englund U, Fricker GRA, Lundberg C, et al. Transplantation of human neural progenitor cells into the neonatal rat brain: extensive migration and differentiation with long distance axonal projections. Exp Neurol. 2002; 173(1):1-21.
[12] Zheng XS, Yang XF, Liu WG, et al. Transplantation of neural stem cells into the traumatized brain induces lymphocyte infiltration. Brain Inj. 2007;21(2):275-278.
[13] Garzon MT, Quinones HA. Neural stem cell niches and homing: recruitment and integration into functional tissues. ILAR J. 2009;51(1):3-23.
[14] Carpentino JE, Hartman NW, Grabel LB, et al. Region-specific differentiation of embryonic stem cell-derived neural progenitor transplants into the adult mouse hippocampus following seizures. J Neurosci Res. 2008;86(3):512-524.
[15] Harting MT, Sloan LE, Jimenez F, et al. Subacute neural stem cell therapy for traumatic brain injury. J Surg Res. 2009;153(2):188-194.
[16] Trujillo CA, Schwindt TT, Martins AH, et al. Novel perspectives of neural stem cell differentiation: from neurotransmitters to therapeutics. Cytometry A. 2009; 75(1):38-53.
[17] Kennea NL, Melmet H. Perinatal applications of neural stem cells. Best Pract Res Clin Obstet Gynaecol. 2004; 18(4):997-994.
[18] Roh JK, Jungn KH, Chu K. Adult stem cell transplantation in stroke: its limitations and prospects. Curr Stem Cell Res Ther. 2008;3(3):185-196.
[19] Miljan EA, Sinden JD. Stem cell treatment of ischemic brain injury. Curr Opin Mol Ther. 2009;11(4):394-403.
[20] Luan Z, Yin GC, Hu XH, et al. Treatment of an infant with severe neonatal hypoxic-ischemic encephalopathy sequelae with transplantation of human neural stem cells into cerebral ventricle. Zhonghua Erke Zazhi. 2005;43(8): 580-583.
[21] He S, Duan YY, Cao TS. Ultrasound-guided puncture treatment of premenstrual skull lateral ventricle meningitis: 12 cases of infant. Zhonghua Erke Zazhi. 2001;8(3): 496-497.
[22] Bai J, Luan Z, Zhou CH. Influence of hyperbaric oxygen on the transplantation of exogenous human neural stem cells in vivo differentiation of neurons. Zhongguo Dangdai Erke Zazhi. 2008;10(2):195-198.
[23] Sugaya K. Potential use of stem cells in neuroreplacement therapies for neurodegenerative diseases. Int Rev Cytol. 2003;228(1):1-30.
[24] Perry RNW, Bowman ED, Morton LJ, et al. Ventricular size in newborn infants. J Ultrasound Med. 1985;4(3): 475-477.
[25] Shi W, Yang H, Shi BP, et al. Approaches for definition,clinical types and function classification of cerebral palsy domestic and abroad (review). Chin J Rehabil Theory Pract. 2009;15(9):801-803
[26] State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994-09-01.
10.3969/j.issn.1673-5374.2012.32.007 [http://www.crter.org/nrr-2012-qkquanwen.html]
He S, Luan Z, Qu SQ, Qiu X, Xin DQ, Jia WK, Shen YH, Yu ZH, Xu T. Ultrasound guided neural stem cell transplantation through the lateral ventricle for treatment of cerebral palsy in children. Neural Regen Res. 2012;7(32):2529-2535.
Sheng He☆, M.D., Chief physician, Department of Ultrasonography, Navy General Hospital of Chinese PLA, Beijing 100048, China
Sheng He, Department of Ultrasonography, Navy General Hospital of Chinese PLA, Beijing 100048, China shenghecn2008292@sina. com
2012-06-11
2012-09-10 (NY20110830001/WJ)
(Edited by Chen X, Guo YQ/Qiu Y/Song LP)
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Characteristics of vascular lesions in patients with posterior circulation infarction according to age and region of infarct*☆
- Morphological differences in skeletal muscle atrophy of rats with motor nerve and/or sensory nerve injury*★
- A standardized method to create peripheral nerve injury in dogs using an automatic non-serrated forceps***★
- Rapid genetic screening of Charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsies patients**★
- Correlation between spina bifida manifesta in fetal rats and c-Jun N-terminal kinase signaling**★
- Tacrolimus reduces scar formation and promotes sciatic nerve regeneration*☆
