Tacrolimus reduces scar formation and promotes sciatic nerve regeneration*☆
2012-01-04JunQueQuanCaoTaoSuiShihaoDuAiliangZhangDechaoKongXiaojianCao
Jun Que, Quan Cao, Tao Sui, Shihao Du, Ailiang Zhang, Dechao Kong, Xiaojian Cao
1 Intensive Care Unit, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China
2 Department of Orthopedics, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China
Tacrolimus reduces scar formation and promotes sciatic nerve regeneration*☆
Jun Que1, Quan Cao1, Tao Sui2, Shihao Du2, Ailiang Zhang2, Dechao Kong2, Xiaojian Cao2
1Intensive Care Unit, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China
2Department of Orthopedics, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China
A sciatic nerve transection and repair model was established in Sprague-Dawley rats by transecting the tendon of obturator internus muscle in the greater sciatic foramen and suturing with nylon sutures. The models were treated with tacrolimus gavage (4 mg/kg per day) for 0, 2, 4 and 6 weeks. Specimens were harvested at 6 weeks of intragastric administration. Masson staining revealed that the collagen fiber content and scar area in the nerve anastomosis of the sciatic nerve injury rats were significantly reduced after tacrolimus administration. Hematoxylin-eosin staining showed that tacrolimus significantly increased myelinated nerve fiber density, average axon diameter and myelin sheath thickness. Intragastric administration of tacrolimus also led to a significant increase in the recovery rate of gastrocnemius muscle wet weight and the sciatic functional index after sciatic nerve injury. The above indices were most significantly improved at 6 weeks after of tacrolimus gavage. The myelinated nerve fiber density in the nerve anastomosis and the sciatic nerve functions had a significant negative correlation with the scar area, as detected by Spearman’s rank correlation analysis. These findings indicate that tacrolimus can promote peripheral nerve regeneration and accelerate the recovery of neurological function through the reduction of scar formation.
tacrolimus; scar; myelinated nerve fiber; sciatic nerve; peripheral nerve injury; neural regeneration; neurological function
Research Highlights
(1) Tacrolimus can reduce collagen fiber content and scar area in nerve anastomosis of sciatic nerve injury in rats.
(2) Tacrolimus increases myelinated nerve fiber density, average axon diameter and myelin sheath thickness in nerve anastomosis of sciatic nerve injury in rats.
(3) Tacrolimus promotes neuronal functional recovery in rats with sciatic nerve injury.
(4) The myelinated nerve fiber density and sciatic nerve function index are negatively related to the scar area.
(5) Tacrolimus reduces scar formation, thus promoting peripheral nerve regeneration and accelerating the recovery of neurological function.
INTRODUCTlON
Delayed anastomosis at the nerve stump after trauma-induced peripheral nerve injury may lead to permanent loss of neurological function[1-2]. Even when axons are repaired immediately after injury, they are still influenced by scar formation[3-4]. Sunderland[5]found that the speed of neural regeneration was 1–3 mm/d, but the time taken for regenerating axons to crossthrough the nerve anastomosis was 20–40 days, so the formation of anastomotic scars hinders axonal penetration and slows down the speed of neural regeneration. Therefore, reducing the collagen fiber content and scar formation in nerve anastomosis could promote neural regeneration and functional recovery.
Tacrolimus has powerful effects on the promotion of neural regeneration[6-12], and the underlying mechanism is mainly associated with immune inhibition[13]and neurotrophic activity[14-21]. However, the effect of tacrolimus on scar formation after nerve injury is not well studied.
This study aimed to observe the effect of tacrolimus on scar formation in nerve anastomosis and to investigate its correlation with neural regeneration and functional recovery.
RESULTS
Quantitative analysis of experimental animals
Fifty rats were included in this study, of which 40 were used to establish models of sciatic nerve transection and repair by transecting the sciatic nerve and suturing the nerve ends with nylon sutures at the tendon of obturator internus muscle in the greater sciatic foramen. The remaining 10 rats served as a normal control group. The 40 successful models were randomly and equally divided into a model group, which received saline gavage, and three tacrolimus groups, receiving tacrolimus gavage for 2, 4 and 6 weeks, followed by normal saline at the other time points. Samples were harvested at 6 weeks after model establishment. All 50 rats were involved in the final analysis.
Tacrolimus reduced collagen fiber content and scar area in nerve anastomosis of rats with sciatic nerve injury
At 6 weeks after establishing the nerve injury model, Masson staining of the longitudinal sections of injured sciatic nerve showed that the collagen fiber content in the model group was extremely high, as seen by the dark blue staining and the very low level of red-stained nuclei (Figure 1). With increased tacrolimus gavage, the number of blue collagen fibers gradually reduced and the number of red nuclei gradually increased. There was no significant difference at 4 and 6 weeks of tacrolimus gavage, while the blue collagen fibers were scarcely visible in the normal control group and the vast majority of neurons showed red nuclear staining (Figure 1).

Figure 1 Collagen fiber proliferation in longitudinal sections of injured sciatic nerve at the nerve anastomosis site (Masson staining, light microscope,×40).
Measurements of the scar area showed that the area of the anastomosis site was significantly increased in the model group compared with the normal control group (P<0.01), and significantly decreased in the tacrolimus gavage groups compared with the model group (P<0.05 orP<0.01). The scar area decreased more noticeably as the time post-gavage increased; however, there was no significant difference between 4 and 6 weeks of tacrolimus gavage (P>0.05; Table 1).
Tacrolimus increased the myelinated nerve fiber density, average axonal diameter and myelin sheath thickness in rats with sciatic nerve injury
Using light microscopy, hematoxylin-eosin staining of the transverse sciatic nerve after injury showed that the number of myelinated nerve nuclei (blue) was minimal and there were a large number of red collagen fibers in the model group (Figure 2). As the period of tacrolimus gavage increased, the number of myelinated nerve nuclei gradually increased, but there was no significant difference between 4 and 6 weeks of tacrolimus gavage. In normal control group, a large number of blue myelinated nerve nuclei were visible (Figure 2).

Table 1 Effect of tacrolimus on cross-sectional scar area (mm2), recovery rate of gastrocnemius muscle wet weight (%) and sciatic functional index (%) at the anastomotic site of the sciatic nerve in rats

Figure 2 Myelinated nerve fiber formation in the transverse sciatic nerve at the site of anastomosis (hematoxylin-eosin staining, light microscope,×40).
Statistical results showed that the myelinated nerve fiber density, average axonal diameter and myelin thickness in the model group were significantly lower than those in the normal control group (P<0.01), and these indices gradually increased in the tacrolimus gavage groups compared with model group (P<0.05 orP<0.01). The difference increased significantly with increased tacrolimus administration. However, there were no significant differences in myelinated nerve fiber density, average axonal diameter and myelin thickness between groups receiving 4 or 6 weeks of tacrolimus gavage (P>0.05; Table 2).
Tacrolimus improved the recovery rate of gastrocnemius muscle wet weight in rats with sciatic nerve injury
The recovery rate of gastrocnemius muscle wet weight can reflect muscle atrophy and recovery during nerve injury and regeneration, and thus indirectly reflects the degree of motor functional recovery[22]. Results of this study showed that the recovery rate of gastrocnemius muscle wet weight in the model group was significantly lower than that in the normal control group (P<0.01). As the period of tacrolimus gavage was increased, the recovery rate of gastrocnemius muscle wet weight increased significantly compared with the model group (P<0.05 orP<0.01). Furthermore, the recovery rate for 4 and 6 weeks of tacrolimus gavage was similar to the normal level in the normal control group (Table 1).
Tacrolimus increased sciatic functional index in rats with sciatic nerve injury
Sciatic functional index was –9.16±2.35% in the normal control group and –126.55±7.88% in the model group at 6 weeks after sciatic nerve injury, indicating complete loss of nerve function in the model group (P<0.01). With increased period of tacrolimus gavage, the sciatic functional index significantly improved compared with the model group (P<0.05 orP<0.01; Table 1).
Correlation of myelinated nerve fiber density and sciatic functional index with scar area
Spearman’s rank correlation analysis results showed that the myelinated nerve fiber density decreased with increased scar area (rs=–1.0,P<0.01).
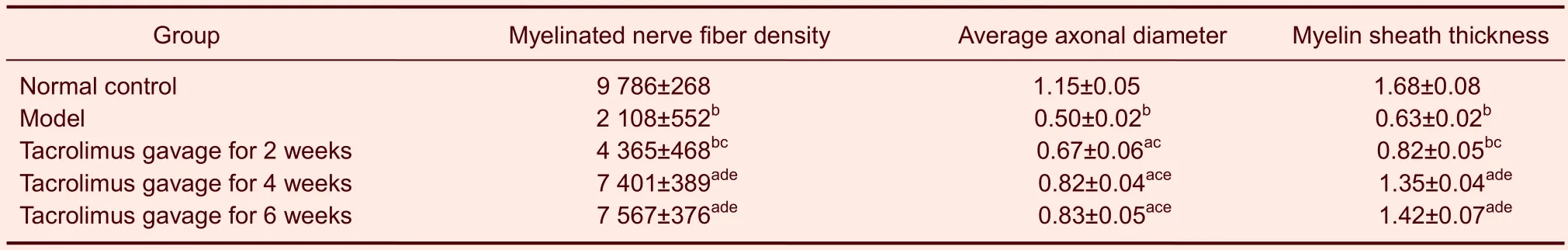
Table 2 Effect of tacrolimus on myelinated nerve fiber density (n/mm2), average axonal diameter (μm) and myelin sheath thickness (μm) in transverse sciatic nerve injury at the anastomosis site
Thus, the myelinated nerve fiber density negatively correlated with scar area, suggesting that tacrolimus promotes peripheral nerve regeneration by reducing scar formation (Figure 3A).
Spearman’s rank correlation analysis results showed that the absolute value of the sciatic functional index increased with an increase in scar area (rs=1.0,P<0.01), and the higher absolute value of the sciatic functional index indicated the poorer the recovery of neurological function (Figure 3B).
Therefore, sciatic nerve function negatively correlated with scar area, indicating that tacrolimus accelerates the recovery of neurological function by reducing scar formation (Figure 3B).
DISCUSSION
With nerve injuries that are caused by a slight crush injury, where a nerve intimal rupture is not visible, axonal regeneration can be rapid[5]. After partial or complete nerve transection injury, axonal regeneration is difficult because of a rupture in the endoneurium and scar formation[3,5,23].
Wound healing processes consist of inflammation, granulation formation, transformation and remodeling, and scar formation[5]. Scar formation is crucial for wound healing, but it may impede wound growth, lead to deformities and impair normal functions in many clinical cases[24-25]. Intraneural scar formation prevents axonal regeneration and the crossing of regenerating axons through the nerve anastomosis[26-27]. Grahamet al[4]found that the local application of triamcinolone acetonide can reduce scar formation in the squirrel or monkey, thus promoting neural regeneration. Bora[28]found a negative correlation between the degree of neurological functional recovery and the scar area formed. In addition, Atkinset al[25]showed that the scar may prevent neural regeneration and that by reducing scar formation you can promote neurological functional recovery. Thus, reducing scar formation within the nerve is beneficial for axons crossing the nerve anastomotic site and contributes to establishing synaptic connections with the target organ, thus promoting the recovery of neurological function.
This study observed the effect of tacrolimus on scar formation after nerve injury. Results found that tacrolimus apparently reduced collagen fiber content and scar area at the nerve anastomotic site and its effect was proportional to the period of tacrolimus administration.
With an increase in the number of tacrolimus gavage treatments over time, the transverse nerve myelinated fiber density, average axonal diameter and myelin sheath thickness gradually increased and the recovery rate of gastrocnemius muscle wet weight and sciatic functional index were significantly improved. Correlation analysis indicated that the myelinated nerve fiber density and sciatic functional index were negatively correlated with the scar area, which further confirmed that tacrolimus can promote peripheral nerve regeneration and accelerate the recovery of neurological function by reducing scar formation.
The collagen fiber content and scar area at the site of nerve anastomosis showed no significant difference between 4 and 6 weeks of tacrolimus gavage. This may be because of an inhibition of scar formation due to the early delivery of tacrolimus, which was maintained at a steady state for 4 weeks. Indicators for neural regeneration and functional recovery also reached a steady level at 4 weeks after administration, following which they did not improve with longer periods of administration. There are two possible reasons why recovery reached a steady level at 4 weeks: (1) scar formation within the nerve was stable and its impact on neural regeneration maintained at a stable level, thus preventing any further improvement; and (2) the vast majority of regenerated axons could successfully cross the nerve anastomosis after 4 weeks of tacrolimus, thus discarding the theory of Sunderland[5], who suggested that the anastomotic scar is the main factor responsible for preventing axonal regeneration. These results provide an experimental basis for the clinical short-term use of tacrolimus for promoting neural regeneration, thereby avoiding the adverse consequences induced by the long-term use of immunosuppressive agents and reducing treatment costs.
In summary, this study was the first demonstration and verification that tacrolimus can promote peripheral nerve regeneration by reducing scar formation, which may provide useful information for the production of a new clinical drug.
MATERlALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
This study was performed at Nanjing Medical University, China from January 2011 to August 2011.
Materials
Fifty clean healthy Sprague-Dawley male rats, aged 2–3 months, weighing 250–300 g, were provided from the Experimental Animal Center of Nanjing Medical University, China under the license No. SYXK (Su) 2008-0007. All procedures were performed in accordance with theGuidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[29].
Methods
Establishment of rat sciatic nerve transection anastomosis models
Sprague-Dawley rats were anesthetized by 30 mg/kg sodium pentobarbitalviaintraperitoneal injection and fixed in an abduction position at room temperature. After the right thigh and buttock were shaved, a longitudinal incision was made along the median femur to expose approximately 2 cm of the sciatic nerve. The greater sciatic foramen was cut at the tendon level of the obturator internus muscle. The incision was sutured with three sutures under 10×magnification using a surgical microscope (Latric Optical Instrument Factory, Shanghai, China) and 10/0 nylon monofilament (Latric Suture Needle Company Limited, Ningbo, Zhejiang Province, China). Rats were postoperatively injected with penicillin 1 600 000 U/kg at the non-operated side to prevent infection.
Drug intervention
Tacrolimus was purchased from Sigma (St. Louis, MO, USA), containing 1 mg per capsule. After the capsule dressing was removed, the powder was dissolved in saline and prepared into a homogeneous suspension with a final concentration of 0.5 mg/mL by microwaving for 5 minutes. Rats were intragastrically injected with the suspension at a dose of 4 mg/kg per day after model establishment. Model group: saline for 6 weeks; tacrolimus gavage for 2 weeks group: tacrolimus gavage for 2 weeks + saline for 4 weeks; tacrolimus gavage for 4 weeks group: tacrolimus gavage for 4 weeks + saline for 2 weeks; tacrolimus gavage for 6 weeks group: tacrolimus gavage for 6 weeks; normal control group: saline for 6 weeks without sciatic injury.
Harvesting the injured sciatic nerve
At 6 weeks after model establishment, five rats of each group were sacrificed. The bilateral sciatic nerve was completely dissected, a 5-mm-long nerve trunk was cut from 2.5 mm lateral to the distal and proximal ends of the anastomosis, followed by labeling, conventional fixation, dehydration, transparency, permeation, embedding, sectioning, coating, paraffin mounting and slicing into 4-μm thick sections.
Masson staining for anastomotic scar formation
Slices were dewaxed and mordanted (10% potassium dichromate + 10% trichloroacetic acid) for 30 minutes, nuclei were stained with hematoxylin for 20 minutes, differentiated with hydrochloric acid and ethanol for 15 seconds, returned to blue with weak ammonia for 15 seconds, stained with Masson solution (Cell Signaling Technology, Irvine, CA, USA) for 1 minute, rinsed with 1% acetic acid, dehydrated with an increasing ethanol series, cleared with xylene I and II for 10 minutes to make sections transparent and finally sealed in resin. Under light microscopy (Olympus, Tokyo, Japan), cell nuclei were stained red and collagen fibers were blue. Collagen fiber proliferation in longitudinal sections was observed under light microscopy. Three transverse images were collected from each rat and the transverse scar area (mm2) was measured using Image-pro plus 5.0 image analysis software (Media Cybernetics Shanghai Office, Shanghai, China).
Hematoxylin-eosin staining
Slices were dewaxed, nuclei were stained with hematoxylin for 20 minutes, differentiated with hydrochloric acid and ethanol for 15 seconds, returned to blue with weak ammonia for 15 seconds, dyed with eosin for 20 minutes, then dehydrated with an increasing ethanol series, treated with xylene I and II for 10 minutes to make sections transparent and finally sealed in resin. Under a light microscope, the nuclei were stained a purplish blue and collagen fibers were pink. The myelinated nerve fiber density and connective tissue were observed under a light microscope. Three transverse images were collected from each rat, and the myelinated nerve fiber density, average axonal diameter and myelin sheath thickness were measured using Image-pro plus 5.0 image analysis software.
Determination of the recovery rate of gastrocnemius muscle wet weight
After nerve specimen sampling was completed, the gastrocnemius muscle was excised from both sides and stripped of implicative fat and fascia on the muscle surface. The wet weight of each sample was weighed on an electronic balance (0.1 mg; Mettler Toledo, Shanghai, China). The recovery rate (%) of gastrocnemius muscle wet weight was calculated by comparing the weight at the operated side and the autologous normal muscle weight at the contralateral side.
Determination of sciatic functional index with the de Medinaceli method
At 6 weeks after modeling, five rats in each group were selected, and the sciatic functional index was detected using the de Medinaceli method[30]. The podogram of the left and right feet (E: experimental side foot; N: normal lateral foot) were selected to measure four parameters as follows: TOF (distance to the contralateral foot), PL (podgram length), TS (toe width) and IT (middle toe distance). Sciatic functional index=[(ETOF–NTOF)/NTOF + (NPL–EPL)/EPL + (ETS–NTS)/NTS + (EIT– NIT)/NIT]×220/4. Sciatic functional index of 0±11%: normal neurological function; below –100%: complete loss of neurological function.
Statistical analysis
Statistical processing was performed using SPSS 13.0 statistical software (SPSS, Chicago, IL, USA) and measurement data were expressed as mean±SD. Grouping data were compared with one-way analysis of variance and theqtest was applied for the intergroup comparisons if analysis of variance showed statistically significant differences. APvalue <0.05 was considered statistically significant. The correlation of myelinated nerve fiber density and sciatic functional index with scar area was analyzed using Spearman’s rank correlation analysis, withP<0.05 indicating correlation.
Funding:This study was supported by the National Natural Science Foundation of China, No. 81171694.
Author contributions:All authors participated in the study design, conduction, and evaluation. Jun Que and Quan Cao drafted the manuscript. All authors contributed to preparation of the manuscript.
Conflicts of interest:None declared.
Ethical approval:Experiments on animal disposal were approved by the Animal Ethics Committee of Nanjing Medical University in China.
Author statements:The manuscript is original, has not been submitted to or is not under consideration by another publication, has not been previously published in any language or any form, including electronic, and contains no disclosure of confidential information or authorship/ patent application disputations.
[1] Rustemeyer J, Dicke U. Allografting combined with systemic FK506 produces greater functional recovery than conduit implantation in a rat model of sciatic nerve injury. J Reconstr Microsurg. 2010;26(2):123-129.
[2] Sosa I, Reyes O, Kuffler DP. Immunosuppressants: neuroprotection and promoting neurological recovery following peripheral nerve and spinal cord lesions. Exp Neurol. 2005;195(1):7-15.
[3] Lane JM, Bora FW, Pleasure D. Neuroma scar formation in rats following peripheral nerve transection. J Bone Joint Surg Am. 1978;60(2):197-203.
[4] Graham WP 3rd, Pataky PE, Calabretta AM, et al. Enhancement of peripheral nerve regeneration with triamcinolone after neurorrhaphy. Surg Forum. 1973;24: 457-459.
[5] Sunderland S. Nerve and Nerve Injury. New York: Churchill Livingston. 1987.
[6] Yan Y, Sun HH, Hunter DA, et al. Efficacy of short-term FK506 administration on accelerating nerve regeneration. Neurorehabil Neural Repair. 2012;26(6):570-580.
[7] Jifeng H, Dezhong L, Qiongjiao Y, et al. Evaluation of PRGD/FK506/NGF conduits for peripheral nerve regeneration in rats. Neurology India. 2010;58(3): 384-391.
[8] Li X, Wang W, Wei G, et al. Immunophilin FK506 loaded in chitosan guide promotes peripheral nerve regeneration. Biotechnol Lett. 2010;32(9):1333-1337.
[9] Costantini LC, Isacson O. Immunophilin ligands and GDNF enhance neurite branching or elongation from developing dopamine neurons in culture. Exp Neurol. 2000;164(1):60-70.
[10] Navarro X, Udina E, Ceballos D, et al. Effects of FK506 on nerve regeneration and reinnervation after graft or tube repair of long nerve gaps. Muscle Nerve. 2001;24(7): 905-915.
[11] Jost SC, Doolabh VB, Mackinnon SE, et al. Acceleration of peripheral nerve regeneration following FK506 administration. Restor Neurol Neurosci. 2000;17(1):39-44.
[12] Chunasuwankul R, Ayrout C, Dereli Z, et al. Low dose discontinued FK506 treatment enhances peripheral nerve regeneration. Int Surg. 2002;87(4):274-278.
[13] Becker DB, Jensen JN, Myckatyn TM, et al. Effects of FKBP-12 ligands following tibial nerve injury in rats. J Reconstr Microsurg. 2000;16(8):613-620.
[14] Lagoda G, Xie Y, Sezen SF, et al. FK506 neuroprotection after cavernous nerve injury is mediated by thioredoxin and glutathione redox systems. J Sex Med. 2011;8(12): 3325-3334.
[15] Li Q, Shen TG, Wu YM, et al. Experimental study of electrophysiologic effects of regenerative nerve fibres affected by control releasing FK506. Zhongguo Gu Shang. 2010;23(11):841-844.
[16] Fansa H, Keilhoff G, Horn T, et al. Stimulation of Schwann cell proliferation and axonal regeneration by FK506. Restor Neurol Neurosci. 2000;16(2):77-86.
[17] Gold BG, Densmore V, Shou W, et al. Immunophilin FK506-binding protein 52 (not FK506-binding protein12) mediates the neurotrophic action of FK506. J Pharmacol Exp Therap. 1999;289(3):1202-1210.
[18] Sulaiman OA, Voda J, Gold BG, et al. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic schwann cell denervation. Exp Neurol. 2002;175(1):127-137.
[19] Chabas JF, Alluin O, Rao G, et al. FK506 induces changes in muscle properties and promotes metabosensitive nerve fiber regeneration. J Neurotrauma. 2009;26(1):97-108.
[20] Chen B, Song Y, Liu Z. Promotion of nerve regeneration in peripheral nerve by short-course FK506 after end-to-side neurorrhaphy. J Surg Res. 2009;152(2):303-310.
[21] Haisheng H, Songjie Z, Xin L. Assessment of nerve regeneration across nerve allografts treated with tacrolimus. Artif Cells Blood Substit Immobil Biotechnol. 2008;36(5):465-474.
[22] Nakazato K, Song H, Waga T. Dietary apple polyphenols enhance gastrocnemius function in Wistar rats. Med Sci Sports Exerc. 2007;39(6):934-940.
[23] Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000;25(3):391-414.
[24] O'Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol. 1997;29(1): 63-78.
[25] Atkins S, Smith KG, Loescher AR, et al. Scarring impedes regeneration at sites of peripheral nerve repair. Neuroreport. 2006;17(12):1245-1249.
[26] Albayrak BS, Ismailoglu O, Ilbay K, et al. Doxorubicin for prevention of epineurial fibrosis in a rat sciatic nerve model: outcome based on gross postsurgical, histopathological, and ultrastructural findings. J Neurosurg Spine. 2010;12(3):327-333.
[27] Ngeow WC. Scar less: a review of methods of scar reduction at sites of peripheral nerve repair. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(3): 357-366.
[28] Bora FW. Peripheral nerve repair in cats. The fascicular stitch. J Bone Joint Surg Am. 1967;49(4):659-666.
[29] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[30] de Medinaceli L, Church AC, Wang YN. Posttraumatic autoimmune reaction in peripheral nerve: effect of a single injury. Exp Neurol. 1985;88(2):372-384.
10.3969/j.issn.1673-5374.2012.32.003 [http://www.crter.org/nrr-2012-qkquanwen.html]
Que J, Cao Q, Sui T, Du SH, Zhang AL, Kong DC, Cao XJ. Tacrolimus reduces scar formation and promotes sciatic nerve regeneration. Neural Regen Res. 2012;7(32):2500-2506.
Jun Que☆, M.D., Lecturer, Associate chief physician, Intensive Care Unit, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China
Jun Que and Quan Cao contributed equally to this work.
Xiaojian Cao, M.D., Professor, Chief physician, Department of Orthopedics, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, Jiangsu Province, China xiaojiancao@gmail.com
2012-07-10
2012-09-20 (N20120410003/WLM)
(Edited by Liang LM, Feng YQ/Yang Y/Song LP)
杂志排行
中国神经再生研究(英文版)的其它文章
- Characteristics of vascular lesions in patients with posterior circulation infarction according to age and region of infarct*☆
- Morphological differences in skeletal muscle atrophy of rats with motor nerve and/or sensory nerve injury*★
- A standardized method to create peripheral nerve injury in dogs using an automatic non-serrated forceps***★
- Rapid genetic screening of Charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsies patients**★
- Ultrasound guided neural stem cell transplantation through the lateral ventricle for treatment of cerebral palsy in children☆
- Correlation between spina bifida manifesta in fetal rats and c-Jun N-terminal kinase signaling**★
