Expression of gamma-aminobutyric acid type A receptor α2 subunit in the dorsal root ganglion of rats with sciatic nerve injury*★
2012-01-04YuLianYangWangKetaoMaLeiZhaoZhongshuangZhangYuanyuanShangJunqiangSiLiLi
Yu Lian, Yang Wang,, Ketao Ma, Lei Zhao, Zhongshuang Zhang, Yuanyuan Shang, Junqiang Si,, Li Li
1 Department of Physiology, Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China
2 Key Laboratory of Xinjiang Endemic and Ethnic Disease, Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China
3 Fundamental Medical School of Wuhan University, Wuhan 430071, Hubei Province, China
Expression of gamma-aminobutyric acid type A receptor α2subunit in the dorsal root ganglion of rats with sciatic nerve injury*★
Yu Lian1,2, Yang Wang1,2,3, Ketao Ma1,2, Lei Zhao1,2, Zhongshuang Zhang1,2, Yuanyuan Shang1,2, Junqiang Si1,2,3, Li Li1,2
1Department of Physiology, Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China
2Key Laboratory of Xinjiang Endemic and Ethnic Disease, Shihezi University School of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China
3Fundamental Medical School of Wuhan University, Wuhan 430071, Hubei Province, China
The γ-aminobutyric acid neurotransmitter in the spinal cord dorsal horn plays an important role in pain modulation through primary afferent-mediated presynaptic inhibition. The weakening of γ-aminobutyric acid-mediated presynaptic inhibition may be an important cause of neuropathic pain. γ-aminobutyric acid-mediated presynaptic inhibition is related to the current strength of γ-aminobutyric acid A receptor activation. In view of this, the whole-cell patch-clamp technique was used here to record the change in muscimol activated current of dorsal root ganglion neurons in a chronic constriction injury model. Results found that damage in rat dorsal root ganglion neurons following application of muscimol caused concentration-dependent activation of current, and compared with the sham group, its current strength and γ-aminobutyric acid A receptor protein expression decreased. Immunofluorescence revealed that γ-aminobutyric acid type A receptor α2subunit protein expression decreased and was most obvious at 12 and 15 days after modeling. Our experimental findings confirmed that the γ-aminobutyric acid type A receptor α2subunit in the chronic constriction injury model rat dorsal root ganglion was downregulated, which may be one of the reasons for the reduction of injury in dorsal root ganglion neurons following muscimol-activated currents.
γ-aminobutyric acid; γ-aminobutyric acid type A receptor α2subunit; neuropathic pain; dorsal root ganglion; whole-cell patch clamp; immunofluorescence; primary afferent depolarization; paw withdrawal latency; muscimol
Research Highlights
(1) Gamma-aminobutyric acid type A receptor α2subunit participates in the pathogenesis of neurological pain.
(2) Gamma-aminobutyric acid type A receptor α2subunit expression was downregulated in the dorsal root ganglion in rats with chronic constriction injury, which may be one of the reasons for the decreased current of dorsal root ganglion activation at the ipsilateral side after chronic constriction injury in the sciatic nerve.
(3) The activation current of the gamma-aminobutyric acid type A receptor α2subunit was decreased on the ipsilateral side, but increased on the contralateral side. This change resulted from the compensation mechanism of nerves.
Abbreviations
GABA, γ-aminobutyric acid; GABAAR, γ-aminobutyric acid type A receptor; CCI, chronic constriction injury
lNTRODUCTlON
Neuropathic pain is defined as ‘pain initiated or caused by a primary lesion or dysfunction in the nervous system’. Neuropathic pain is often reported as having a lancinating or continuous burning character and is often associated with the appearance of abnormal sensory signs, such as spontaneous pain-related behavior, allodynia (pain as a result of a stimulus that does not normally provoke pain) or hyperalgesia (an increased response to a stimulus which is normally painful)[1-4]. In normal primary afferent neurons, it is rare for a firing threshold to be reached without the input of a stimulus. However, following nerve injury, it has been demonstrated that there is a large increase in the level of spontaneous firing in afferent neurons linked to the injury site. This has been termed ectopic discharge and has also been demonstrated in humans suffering from neuropathic pain.
γ-aminobutyric acid (GABA) is a classical inhibitory neurotransmitter. GABA receptors can be divided into GABAAR, GABABR and GABACR. GABAAR are ligand-gated ion channel receptors. Spinal dorsal horn GABAAreceptors are found both postsynaptically on central neurons and presynaptically on axons and/or terminals of primary sensory neurons, where they mediate primary afferent depolarization and presynaptic inhibition. GABA can act at GABAAR localized on primary afferent neurons to inhibit presynaptic neurotransmitter release and produce analgesiaviaa process of primary afferent depolarization. GABA mediates fast synaptic inhibition by activating ionotropic GABAAR, which are assembled from a large family of constituent subunits[5-6]. GABAAR is made up from 19 known subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π and ρ1-3)[7-8]with an integral channel that is permeable to Cl-ions. The majority of GABAAR consist of α2β3γ2[9]that can interact with a range of compoundsviaspecific binding sites that include agonists (e.g. GABA and muscimol). After injury, GABAAR positive allosteric modulators inhibit robust analgesia in animalsviarestoration of post-synaptic GABAAR α2and GABAAR α3subunit function within the spinal cord[10].
Subunit α2is expressed at pre- and post-synaptic elements within the spinal dorsal horn and is predominantly found within superficial layers that receive nociceptive input from primary afferents[11]. Recent molecular, genetic, and pharmacological data point to α1-containing GABAAR as the "sedative" and α2- and/or α3-containing receptors as the "anxiolytic" subtypes[12].
The contribution of primary afferent depolarization to the processing of nociceptive signals and to the antihyperalgesic effect of GABAAR modulators is unknown, mainly because of the lack of suitable tools for the specific targeting of presynaptic GABAAR. In this study, through the establishment of a chronic constriction injury CCI model of sciatic nerve, we monitored GABAAR α2subunit function and expression changes in L4-6dorsal root ganglion neurons using whole-cell patch-clamp and immunofluorescence methods to further understand the contribution of the GABAAR α2subunit in pathological pain.
RESULTS
Quantitative analysis of experimental animals
Ninety experimental Sprague-Dawley rats were randomly divided into three groups: sham-surgery group (n= 30), CCI ipsilateral side model group (n= 30) and CCI contralateral side model group (n= 30). CCI models were established on the ipsilateral side and contralateral side of the latter groups, and the number of modeling success was both 26. The sham-surgery group was treated the same as that in the CCI modeling method, with the exception of mp ligation with chromium suture, and 26 rats were randomly selected from the sham group. A total of 78 rats in three groups were involved in the final experiment, 20 in each group for electrophysiology experiments and six in each group for immunofluorescence experiments.
Thermal hyperalgesia of the rat CCl model
We explored the behavioral performance of hyperalgesia after CCI. Thermal hyperalgesia was measured before operation (baseline) and on 1, 3, 7, 12 and 15 days after operation. Thermal hyperalgesia was assessed using the hot plate test. Heat-evoked thermal withdrawal latency of CCI rats began to significantly decrease from 1 to 15 days post-surgery. The sham-operated and CCI contralateral side showed no changes in baseline and after surgery (P> 0.05); the thermal withdrawal latency of the CCI ipsilateral side were significantly different before and after surgery (P< 0.01), and compared with sham-operated rats, thermal withdrawal latency of CCI rats significantly decreased (P< 0.05; Figure 1).
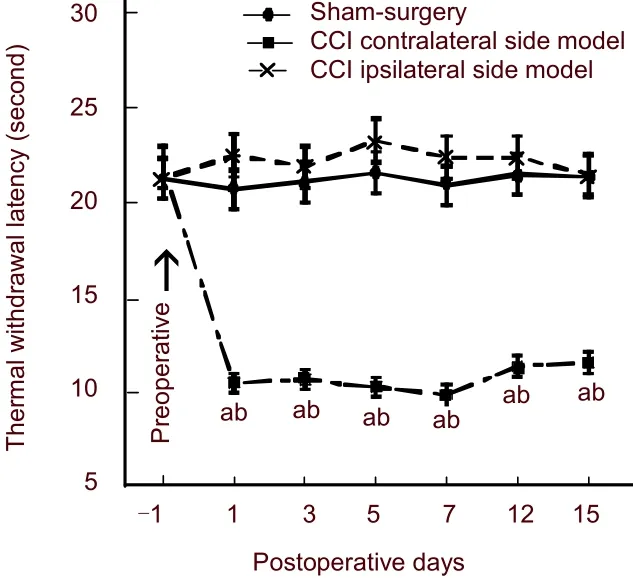
Figure 1 Paw withdrawal latency in rats following chronic constriction injury (CCI) in the sciatic nerve.
Changes in GABAAR electrophysiological features on dorsal root ganglion neurons in CCl rats
Freshly isolated neurons from the L4-6dorsal root ganglion in the diameter range of 15-45 μm were used in the present study. In the majority of neurons examined (82.4%, 89/108), muscimol (10-8-10-3M) induced a concentrationdependent inward current. We recorded three typical types of muscimol-activated currents: fast desensitization type (3/108), mixed (93/108) and slow desensitization type (12/108) (Figure 2A). This muscimol- activated current could be reversibly blocked by bicuculline (10-4M), a selective antagonist of GABAAR, indicating this current was mediated by GABAAR (Figure 2B).
Muscimol induced a concentration-dependent (10-8-10-3M) inward current on L4-6dorsal root ganglion neurons from the sham-operated, CCI ipsilateral side model, and CCI contralateral side model. Figure 3A shows the sham-operated, CCI ipsilateral side, and CCI contralateral side curve traces for muscimol. The muscimol-activated current amplitude (Imuscimol) was significantly reduced in the CCI ipsilateral side model, whileImuscimolsignificantly increased on the CCI contralateral side.Imuscimol(10-4M) in the sham-operated, CCI ipsilateral side model, and CCI contralateral side model rats was 1 487 ± 92.9 pA (n= 6), 541 ± 63.3 pA (n= 6) and 1 945 ± 28.4 pA (n= 6), respectively, among the three groups, which were significantly different (P<0.01). From concentration-response curves of muscimolactivated neurons (Figure 3B), it can be seen that, (a) the dose-response curve for the CCI ipsilateral side is shifted downwards compared with sham-operated rats; the CCI contralateral side is shifted upwards; (b) the EC50value in the sham-operated, CCI ipsilateral side model, and CCI contralateral side model groups are very close (14.7 ± 1.8 μM; 14.3 ± 0.9 μM; 14.4 ± 1.6 μM;n= 6), and are not significantly different between groups (P> 0.05).

Figure 2 Muscimol-activated currents in rat dorsal root ganglion neurons.
Distribution of GABAAR α2 subunit expressing neurons in the dorsal root ganglion of CCl rats
Immunofluorescence staining displayed that GABAAR α2subunit expression was widely distributed in dorsal root ganglion neurons of all three groups, and were mainly located in the cell membranes of various diameters at 12 days after modeling (Figure 4). Quantitative analysis demonstrated that GABAAR α2subunit expression was decreased in the CCI ipsilateral side model group when compared with the sham-surgery group at 12 days after modeling (P< 0.01), and expression levels were highest in the CCI contralateral side model group.
Based on the above experiments, GABAAR α2subunit levels in sensory neurons may underlie the development of pain. We examined alterations to GABAAR α2subunits in the ipsilateral L4-6dorsal root ganglion on days 3, 7, 12 and 15 after operation using immunofluorescence. GABAAR α2subunit-positive signals in the membrane of dorsal root ganglion neurons decreased (Figure 5).

Figure 3 Comparison of muscimol-activated currents of dorsal root ganglion neurons.
Changes in absorbance values on the CCI contralateral side were not seen on day 3 (34.9 ± 5.21;n= 6) or day 7 (31.1 ± 3.50;n= 6) after surgery. However, the mean absorbance began to significantly attenuate by day 12 (20.2 ± 2.24;n= 6) after operation when compared with the sham-surgery group (37.2 ± 5.31;n= 6;P< 0.05). This significant decrease continued from 12 to 15 days (20.2±2.24;n= 6).

Figure 4 Confocal images of γ-aminobutyric acid type A receptor α2 (GABAAR α2) subunit expression in the chronic constriction injury (CCI)-induced L4-6 dorsal root ganglion (immunofluorescence staining, fluorescence microscope, × 20) at day 12 after injury.
DlSCUSSlON
In the present study, we found that the CCI ipsilateral side muscimol-activated current amplitudes were significantly lower than that in the sham-surgery group, while the CCI contralateral side model group was greater. Although there was no nerve damage to the contralateral side, it still produced a series of changes in the electrophysiological properties of GABAAR and the expression of the GABAAR α2subunit. Such compensatory changes may be due to injury information from the unilateral nerve into the bilateral spinal cord. Several possible explanations could account for this compensatory enhancement. Synaptic plasticity of neurons is believed to be fundamental to pathological pain and the importance of spinal sensitization is well recognized. This synaptic arrangement can be the substrate of local disinhibition, which may play a key role in sensory processing[13-14]. In the spinal cord, the GABAAR α2subunit is densely expressed in the superficial layers of the dorsal horn, the main termination area of primary nociceptors[15].

Figure 5 Changes in γ-aminobutyric acid type A receptor α2 (GABAAR α2) subunit-positive cells in the ipsilateral L4-6 dorsal root ganglion from day 3 before injury to day 15 after injury.
At this site, the GABAAR α2subunit is found not only postsynaptically on central neurons, where they cause classical hyperpolarization, but most likely presynaptically on the terminals of primary sensory neurons[15-16]. These terminals are depolarized by GABAAreceptors[17], because their intracellular chloride concentration is kept above the electrochemical equilibrium by the chloride importer NKCC1. This primary afferent depolarization causes presynaptic inhibition,i.e., a reduction in synaptic glutamate release, possibly through inactivation of presynaptic sodium channels and/or through activation of a shunting conductance, both of which can result in inhibition of action potential propagation into presynaptic terminals[18]. Both processes will result in the reduction of nociceptive input to the spinal cord.
Indeed, GABAergic interneurons intercalated within the circuitry linking primary afferents and spinal projection neurons may play a dual role in controlling network excitability[13,19]. This study found that the GABAAR α2subunit was involved in neuropathic pain. The GABAAR containing α2subunit and α3subunit are current targets in the battle to develop new pain medications, as they are expressed in the spinal cord where increasing inhibitory drive should result in analgesia[20]. We have found by immunofluorescence staining that the GABAAR α2subunit is widely distributed in dorsal root ganglion neuron cell membranes of various diameters. Being consistent with electrophysiological experiments, at 12 days after CCI, the positive signal of the CCI ipsilateral side is reduced, however the contralateral side shows a compensatory increase. Fukuokaet al[21]examined the effects of unilateral L5spinal nerve ligation using in situ hybridization, and found that GABAAR α2subunit mRNA decreased in ipsilateral L5dorsal root ganglion neurons but did not reach statistical significance. This result may explain why different damage models cause varying degrees of neuropathic pain. The CCI model is reported to be more sensitive to mechanical stimuli than the spinal nerve ligation model[22]. According to time-dependent changes of GABAAR α2subunit expression, a significant decrease was observed at day 12 and continued until day 15. However, in the hot plate test, values for heat-evoked thermal withdrawal latency of CCI rats began to significantly decrease from 1 to 15 days post-surgery. This result may be due to the fact that there was no damage to dorsal root ganglion GABAAR involved in neuropathic pain occurrence and development of hyperalgesia, and that thermal hyperalgesia could not afford a leading role.
GABA, the most abundant and important inhibitive neurotransmitter in the central nervous system, plays a regulatory role in the regeneration of various nerve cells[23-24]. GABA receptor activation has been shown to lead to the depolarization of primary afferent terminals in the spinal dorsal horn, and significantly reduce the transmission of excitatory impulses in primary afferent fibers[25]. If GABA receptor-mediated presynaptic inhibition reduced, this may cause damage to transfer to the message center and the spinal cord, causing sensitization of neurons, leading to neuropathic pain. We have found a loss of presynaptic GABAAR-mediated inhibition in the dorsal root ganglion of CCI rats. This can be regarded as a key feature that contributes to the signs and symptoms of pain associated with neuropathic injury. After CCI, there is no specific loss to GABAergic neurons in the rat spinal dorsal horn after CCI in the sciatic
nerve[26]. Other studies have shown this in the spared nerve injury model (e.g. partial sciatic nerve injury) suggesting that GABA synthesis is down-regulated[27]. However, in wild-type (α2fl/fl) mice and mice lacking α2-GABAAR, specifically in primary nociceptors (sns-α2-/-), GABAAR currents and dorsal root potentials are of normal amplitudein vitro, and mice show normal response thresholds to thermal and mechanical stimulationin vivo, and develop normal inflammatory and neuropathic pain sensitization. However, the effect of benzodiazepines such as diazepam and midazolam was significantly reduced[28].
Studies have demonstrated that the GABAAR α2subunit participates in pain perception conduction in CCI rats. In patch clamp experiments, we found that there is a change in GABAAR function in CCI rats. At the same time, molecular biology studies confirmed the change in GABAAR α2subunit expression. In addition, weakening of presynaptic inhibition of primary afferents can be due to the reduction in presynaptic GABAAR expression. On the other hand, weakening of presynaptic inhibition of primary afferents can also be interpreted as presynaptic GABAAR phosphorylation or dephosphorylation to reduce the activity of GABAAR[29]. In the progression of neuropathic pain, a large number of intracellular signaling pathways are involved in GABAAR subunit phosphorylation, such as cAMP, PKA, cGMP/PKC, NO, CaMKII and tyrosine kinase[30-31]. GABAAR phosphorylation or dephosphorylation will be our future research direction, and we hope to shed further light on neuropathic pain development.
MATERlALS AND METHODS
Design
A randomized, c ontrolled, animal experiment.
Time and setting
Animal experiments were performed at the Department of Physiology, Shihezi University Medical College, China from August 2010 to November 2011.
Materials
Female, healthy, Sprague-Dawley rats, aged 8-10 weeks, weighing 250-280 g, were provided by the Experimental Animal Center of Xinjiang Medical University, China (license No. SCXK 2003-0001). Rats were housed in separate cages with a specific pathogen-free level barrier environment at 24 ± 3°C, relative humidity of 40-70%, 100-120 lx/12-hour light illumination, and allowed free access to food and water. The protocol was conducted in accordance with theGuidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[32].
Methods
Establishment of the rat CCI model
Surgical procedures were performed as previously described by Bennettet al[33]. Rats were anesthetized with an intraperitoneal injection of 1% (w/v) sodium pentobarbital, and the left sciatic nerve trunk was exposed under sterile conditions. Proximal to the sciatic trifurcation, approximately 7 mm of nerve was freed, and 4 tight ligatures of chrome catgut were placed around the sciatic nerve with about 1 mm spacing. The same surgical procedure was followed until the sciatic nerve was exposed, but no ligatures were applied. All surgical procedures were performed by the same person under aseptic conditions. There were significant differences between the CCI contralateral side model and sham-surgery groups, and paw withdrawal latency values in the CCI ipsilateral side model group were declined by at least 30%, which indicated modeling success. Six rats were randomly selected from each group.
Behavioral testing of thermal sensitivity of the rat CCI model
Thermal hyperalgesia was measured using a 58°C hot plate[34-35], and expressed as paw withdrawal latency of the left hind paw. The constant temperature water bath apparatus was produced in Jintan, China (type SHZ-82). Three measurements of thermal nociceptive threshold were taken for each rat, at 4-minute intervals, and the mean of three measurements was regarded as the paw withdrawal latency. Maximum latency was defined as 30 seconds, after which time the animals were removed from the hot plate to prevent tissue damage. Behavioral testing at the following different time points was performed before operation on 1, 3, 5, 7, 12 and 15 days after operation. After behavioral testing at each time point, all six rats from each group were killed, and L4-6dorsal root ganglions were dissected out for experimentation.
Immunofluorescence staining for GABAAR α2 expression in the dorsal root ganglion
Rats were anaesthetized with 1% (w/v) sodium pentobarbital, and then perfused through the aorta with 0.9% (w/v) normal saline, followed by fresh 4% (w/v) paraformaldehyde in PBS for 10 minutes for tissue fixation. The lumbar dorsal root ganglion at the level of L4-6to nerve injury was removed rapidly and placed in 4% (w/v) paraformaldehyde in PBS for 24 hours. The L4-6dorsal root ganglia were cut into 5 μm slices with a freezing microtome. The sections were treated with 0.3% (v/v) H2O2for 10 minutes and blocked with 3% (w/v) bovine serum albumin in 0.3% (v/v) H2O2. Immunofluorescence staining was performed with goat anti-GABAAR α2polyclonal antibody (1:200; Santa Cruz Biotechnology, CA, USA), overnight at 4°C. The sections were incubated for 1 hour in solution containing FITC-labeled rabbit anti-goat secondary antibody (1:100; Zhongshan Golden Bridge, Beijing, China) at room temperature. Slides were then examined by confocal microscopy. Quantitative analysis of GABAAR α2expression in the dorsal root ganglion was performed by measuring the mean absorbance following laser confocal microscopy (LSM510; Carl Zeiss, Jena, Germany) and using analysis software (hp9001; Carl Zeiss, Jena, Germany).
Electrophysiological recordings of dorsal root ganglion neurons
Whole-cell patch clamp recordings were carried out at room temperature (22-24°C) using a whole-cell patch clamp amplifier[36]. Currents were recorded from single dorsal root ganglion neuronsin vitrousing an Axon 700B amplifier (Axon, San Jose, USA) and the pCLAMP 10.2 hardware and software (Axon, Silicon Valley, USA). The micropipettes were filled with internal solution containing (mM): KCl 140, MgCl22.5, HEPES 10, EGTA 11 and ATP 5.
The pH was adjusted to 7.2 with KOH, and osmolarity was adjusted to 310 mOsm/L with glucose. Cells were bathed in an external solution containing (mM): NaCl 150, KCl 5, CaCl22.5, MgCl22, HEPES 10, D-glucose 10. Osmolarity was adjusted to 340 mOsm/L with glucose, and pH was adjusted to 7.4 with NaOH. The resistance of the recording pipette was in the range of 2-5 MΩ. A small patch of membrane underneath the tip of the pipette was aspirated to form a gigaseal, and then a negative pressure was applied to rupture it, thus establishing a whole-cell configuration. The adjustment of capacitance compensation and series resistance compensation was carried out before the membrane currents were recorded. The holding potential was set at -60 mV, except when indicated otherwise. Membrane currents were filtered at 10 kHz.
Statistical analysis
Data were analyzed with SPSS 13.0 software (SPSS, Chicago, IL, USA) and presented as mean ± SEM. A homogeneity test for variance was performed followed by one-way analysis of variance, and two-group comparison was conducted using the least significant differencet-test. AP< 0.05 was considered statistically significant.
Finding: This study was supported by the Youth Science and Technology Innovation Special Foundation of Xinjiang Production and Construction Corps, China, No. 2010JC33.
Author contributions: Li Li and Junqiang Si designed the research study. Yu Lian, Yang Wang, Yuanyuan Shang, Ketao Ma and Li Li performed the research. Lei Zhao, Zhongshuang Zhang and Li Li contributed to new analytical reagents and tools. Yu Lian, Yang Wang and Li Li analyzed the data. Yu Lian, Li Li and Junqiang Si wrote the paper.
Conflicts of interest: None declared.
Ethical approval: The experiment is approved by the Policy for Rodent Survival Surgery provided by the Animal Care Committee of Shihezi University School of Medicine, China.
Author statements: The manuscript is original, has not been submitted to or is not under consideration by another publication, has not been previously published in any language or any form, including electronic, and contains no disclosure of confidential information or authorship/ patent application disputations.
[1] Bridges D, Thompson SW, Rice AS. Mechanisms of neurpathic pain. Br J Anaesth. 2001;87(1):12-26.
[2] Wilson M. Overcoming the challenges of neuropathic pain. Nurs Stand. 2002;16(33):47-53.
[3] Randic M, Hecimovic H, Ryu PD. Substance P modulates glutamate-induced currents in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1990;117(1-2):74-80.
[4] Yang HW, Hu XD, Zhang HM, et al. Roles of CaMKII, PKA, and PKC in the induction and maintenance of LTP of C-fiber-evoked field potentials in rat spinal dorsal horn. J Neurophysiol. 2004;91(3):1122-1133.
[5] Barnard EA, Skolnick P, Olsen RW, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and function. Pharmacol Rev. 1998;50(2):291-313.
[6] Duveau V, Laustela S, Barth L, et al. Spatiotemporal specificity of GABAAreceptor-mediated regulation of adult hippocampal neurogenesis. Eur J Neurosci. 2011;34(3):362-373.
[7] Olsen RW, Sieghart W. GABAAreceptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56(1):141-148.
[8] Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243-260.
[9] Marutha Ravindran CR, Ticku MK. Tyrosine kinase phosphorylation of GABAAreceptor subunits following chronic ethanol exposure of cultured cortical neurons of mice. Brain Res. 2006;1086(1):35-41.
[10] Munro G, Erichsen HK, Rae MG, et al. A question of balance-positive versus negative allosteric modulation of GABA(A) receptor subtypes as a driver of analgesic efficacy in rat models of inflammatory and neuropathic pain. Neuropharmacology. 2011;61(1-2):121-132.
[11] Knabl J, Witschi R, Hösl K, et al. Reversal of pathological pain through specific spinal GABAAreceptor subtypes. Nature. 2008;451(17):330-334.
[12] Atack JR. GABAAreceptor subtype-selective modulators. I. α2/α3-selective agonists as non-sedatinganxiolytics. Curr Top Med Chem. 2011;11(9):1176-1202.
[13] Labrakakis C, Lorenzo LE, Bories C, et al. Inhibitory coupling between inhibitory interneurons in the spinal cord dorsal horn. Mol Pain. 2009;12(5):24-31.
[14] Zeilhofer HU, Wildner H, Yévenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92(1):193-235.
[15] Bohlhalter S, Weinmann O, Mohler H, et al. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16(1):283-297.
[16] Persohn E, Malherbe P, Richards JG. In situ hybridization histochemistry reveals a diversity of GABAAreceptor subunit mRNAs in neurons of the rat spinal cord and dorsal root ganglia. Neuroscience. 1991;42(2):497-507.
[17] Labrakakis C, Tong CK, Weissman T, et al. Localization and function of ATP and GABAAreceptors expressed by nociceptors and other postnatal sensory neurons in rat. J Physiol. 2003;549(Pt 1):131-142.
[18] Kullmann DM, Ruiz A, Rusakov DM, et al. Presynaptic, extrasynaptic and axonal GABAAreceptors in the CNS:where and why? Prog Biophys Mol Biol. 2005;87(1):33-46.
[19] Personius KE, Chang Q, Mentis GZ. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc Natl Acad Sci U S A. 2007;104(28):11808-11813.
[20] Nickolls S, Mace H, Fish R, et al. A comparison of the α2/3/5 selective positive allosteric modulators L-838, 417 and TPA023 in preclinical models of inflammatory and neuropathic pain. Adv Pharmacol Sci. 2011;2011:608912.
[21] Fukuoka T, Tokunaga A, Kondo E, et al. Change in mRNAs or neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78(1):13-26.
[22] Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113(2):200-206.
[23] Luyt K, Slade TP, Dorward JJ, et al. Developing oligodendrocytes express functional GABA(B) receptors that stimulate cell proliferation and migration. J Neurochem. 2007;100(3):822-840.
[24] Chiba C, Matsushima O, Muneoka Y, et al. Time course of appearance of GABA and GABA receptors during retinal regeneration in the adult newt. Brain Res Dev Brain Res. 1997;98(2):204-210.
[25] Willis WD. John Eccles’ studies of spinal cord presynaptic inhibition. Prog Neurobiol. 2006;78(3-5):189-214.
[26] Schoffnegger D, Heinke B, Sommer C, et al. Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. J Physiol. 2006;577(3):869-878.
[27] Polgár E, Hughes DI, Arham AZ, et al. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25(28):6658-6666.
[28] Witschi R, Punnakkal P, Paul J, et al. Presynaptic α2-GABAAreceptors in primary afferent depolarization and spinal pain control. J Neurosci. 2011;31(22):8134-8142.
[29] Brandon NJ, Delmas P, Kittler JT, et al. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275(49):38856-38862.
[30] Marutha Ravindran CR, Ticku MK. CaMKII phosphorylation of the GABA(A) receptor: receptor subtype-and synapsespecific modulation. J Physiol. 2009; 587(10):2115-2125.
[31] Marutha Ravindran CR, Mehta AK, Ticku MK. Effect of chronic administration of ethanol on the regulation of tyrosine kinase phosphorylation of the GABAA receptor subunits in the rat brain. Neurochem Res. 2007;32(7):1179-1187.
[32] The Ministry of Science and TechnoIogy of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[33] Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87-107.
[34] Bianchi M, Sacerdote P, Ricciardi-Castagnoli P, et al. Central effects of tumor necrosis factor alpha and interleukin-1 alpha on nociceptive thresholds and spontaneous locomotor activity. Neurosci Lett. 1992; 148(1-2):76-80.
[35] Su L, Wang C, Yu YH, et al. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12(20):1186-1471.
[36] Si JQ, Zhang ZQ, Li CX, et al. Modulatory effect of substance P on GABA-activated currents from rat dorsal root ganglion. Acta Pharmacol Sin. 2004;25(5):623-629.
10.3969/j.issn.1673-5374.2012.32.002 [http://www.crter.org/nrr-2012-qkquanwen.html]
Lian Y, Wang Y, Ma KT, Zhao L, Zhang ZS, Shang YY, Si JQ, Li L. Expression of gamma-aminobutyric acid type A receptor α2subunit in the dorsal root ganglion of rats with sciatic nerve injury. Neural Regen Res. 2012;7(32):2492-2499.
Yu Lian★, Master, Department of Physiology, Shihezi UniversitySchool of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethn ic Disease, Shihezi UniversitySchool of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China
Yu Lian and Yang Wang contributed equally to this work.
Li Li, M.D., Associate professor, Department of Phy siology, Shihezi UniversitySchool of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethn ic Disease, Shihezi UniversitySchool of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Junqiang Si, M.D., Professor, Department of Physiolo gy, Shihezi UniversitySchool of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Key Laboratory of Xinjiang Endemic and Ethn ic Disease, Shihezi UniversitySchool of Medicine, Shihezi 832002, Xinjiang Uygur Autonomous Region, China; Fundamental Medical School of Wuhan University, Wuhan 430071,
Hubei Province, China
Lily7588@163.com; sijunqiang@shzu.edu.cn
2012-08-08
2012-10-23
(N20120625001/WJ)
We wish to thank Dr. Hongzhen Hu from the Department of Integrative Biology and Pharmacology, University of Texas Health Science Center for reading the manuscript and for valuable suggestions.
(Edited by Zou WY, Jia DL/Yang Y/Song LP)
杂志排行
中国神经再生研究(英文版)的其它文章
- Characteristics of vascular lesions in patients with posterior circulation infarction according to age and region of infarct*☆
- Morphological differences in skeletal muscle atrophy of rats with motor nerve and/or sensory nerve injury*★
- A standardized method to create peripheral nerve injury in dogs using an automatic non-serrated forceps***★
- Rapid genetic screening of Charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsies patients**★
- Ultrasound guided neural stem cell transplantation through the lateral ventricle for treatment of cerebral palsy in children☆
- Correlation between spina bifida manifesta in fetal rats and c-Jun N-terminal kinase signaling**★
