New details indicated by different stainings during conjugation of ciliated protozoa Paramecium
2011-12-25GAOXinYANGXianYuZHUJiaJunYUANJinQiangWANGYiWenSONGMinGuo
GAO Xin, YANG Xian-Yu, ZHU Jia-Jun, YUAN Jin-Qiang, WANG Yi-Wen, SONG Min-Guo
(The Nurturing Station for the State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University, Lin’an 311300, China)
New details indicated by different stainings during conjugation of ciliated protozoaParamecium
GAO Xin, YANG Xian-Yu*, ZHU Jia-Jun, YUAN Jin-Qiang, WANG Yi-Wen, SONG Min-Guo
(The Nurturing Station for the State Key Laboratory of Subtropical Silviculture, Zhejiang A & F University, Lin’an 311300, China)
During conjugation ofParameciumcaudatum, nuclear events occur in a scheduled program.Morphological studies on nuclear behavior during conjugation ofP.caudatumhave been performed since the end of the 19th century. Here we report on new details concerning the conjugation ofP.caudatumthrough the staining of conjugating cells with protargol, carbol fuchsin solution, Hoechst 33342 and immunofluorescence labeling with monoclonal antibody of anti-α tubulin. 1) The crescent nucleus is a characteristic of the meiotic prophase ofP.caudatum,has an unstained area. We stained this area with protargol, which was separated from the chromatin area and was not detected by the other stainings. 2) In regards to the four meiotic products, it has long been considered that only one product enters the paroral cone region (PC) and survives after meiosis. However, our protargol and immunofluorescence labeling results indicated that PC entrance of the meiotic product happened before the completion of meiosis instead of after. 3) In our previous study, protargol staining indicated the presence of a swollen structure around the central part of the “U” and “V” shaped spindles connecting the two types of prospective pronuclei. However, immunofluorescence labeling with anti-α tubulin antibodies gave a different image from protargol. All these observations form the basis for further studies of their molecular mechanisms.
Paramecium; Conjugation; Crescent; PC entrance; Connecting spindles
Morphological studies on nuclear behavior during conjugation ofParameciumcaudatumhave been studied since the end of the 19th century (Maupas, 1889; Calkins& Cull, 1907). In addition, many similar studies on otherParameciumand ciliates have been performed before and during the 1980s (Nanney, 1980; Wichterman, 1986).Over the last 30 years, much ciliate research has focused on mechanisms to interpret morphological phenomenaby molecular biological techniques, and some remarkable results have been obtained such as the discoveries of telomere, telomerase and ribozyme(Blackburn & Gall, 1978; Cech & Bass, 1986; Greider &Blackburn, 1985). However, fewer morphological studies have been done. Comparison of images obtained from the same cells by different stainings led to new findings in our previous study (Gao et al, 2010).Therefore, several staining methods were used to restudy the conjugation process ofP.caudatum, including the protargol technique (Shi, 1987), carbol fuchsin solution staining (Carr & Walker, 1961), Hoechst 33342(Santos et al, 2000) and immunofluorescence labeling with monoclonal antibody of anti-α tubulin (Yang &Takahashi, 2002). We discovered new details concerning conjugation ofP.caudatum, which are reported here.
1 Materials and Methods
1.1 Chemicals and preparation of staining solutions
Silver protein from Merck KGaA (Germany),monoclonal antibody of mouse anti-α tubulin, FITC-conjugated goat anti-mouse IgG, propidium iodide (PI)and Hoechst 33342 (HO) purchased from Beyotime Institute of Biotechnology (China) were used. All other chemicals were from Hangzhou Dafang Chemical Reagent Inc (China). Preparations of stock solutions for Hoechst 33342 and carbol fuchsin solution followed previous descriptions (Carr & Walker, 1961; Yang &Takahashi, 1999; Yang et al, 2007).
1.2 Cell culture, conjugation induction and stainings
Two complementary mating types ofP.caudatumcollected from East Lake Campus of Zhejiang A & F University (China) were used. Cell culture, conjugation induction, and collection of synchronized conjugating pairs followed previous studies (Dryl, 1959; Hiwatashi,1968; Sun et al, 2010; Wei et al, 2008; Yang &Takahashi, 1999). Modified protargol (Shi, 1987) briefly reported before (Shi & Frankel, 1990; Yang & Shi, 2007)was used. Immunofluorescence labeling with anti-α tubulin antibody followed Yang & Takahashi (2002).Temporary preparations were made by means of“volume-fixing” (Yang et al, 2008; Lin et al, 2009). All experiments were performed at room temperature (~25 °C).
2 Results and Discussion
2.1 A structure in crescent nuclei indicated by protargol technique
The crescent nucleus is a characteristic of the meiotic prophase ofP.caudatum, in which there is an unstained area (Fujishima & Hiwatashi, 1981; Harumoto& Miyake, 1996). To determine if protargol provides any new indications about this structure, conjugating pairs during the meiotic prophase were stained (Fig. 1C, C′, F,F′). In addition, cells at the same developmental stage were also stained by Hoechst 33342 (Fig. 1A, A′, D, D′)and carbol fuchsin solution (Fig. 1B, B′, E, E′). As in former reports, an unstained structure was observed in the crescent nuclei when cells were stained either by Hoechst 33342 or by carbol fuchsin solution (Fig. 1D, D′,E, E′). In contrast to this, a protargol stained structure separated from chromatin was observed (Fig. 1F, F′),which was exactly the area not detected by the other two stainings (compare Fig. 1D, E, F; D′, E′, F′). Before the crescent stage, micronuclei form a truncated spindle shape when cells were stained by Schiff reagent(Fujishima, 1983). Both Hoechst 33342 and carbol fuchsin solution staining in the current study also showed the micronuclei as truncated spindle shapes (Fig. 1A, B,A′, B′). However, protargol staining indicated that the micronucleus was a fully spindle shape consisting of two parts, thread-like chromatin and evenly stained nonchromatin (Fig. 1C, C′). The non-chromatin area was located at one end of the nucleus corresponding to the truncated area indicated by Hoechst 33342 and carbol fuchsin solution. The nature of this structure might be related with microtubule organizing center (MTOC). To date, immunofluorescence labeling with anti-gammatubulin antibodies indicated that during conjugation ofParamecium, only a transient site of gamma-tubulin and microtubule assembly was observed at the site of nuclear exchange (Klotz et al, 2003). To clarify the relationships among microtubules, gamma-tubulin and MTOCs,further research needs to be done with the help of molecular biological techniques.
2.2 Entrance of meiotic product into paroral cone region
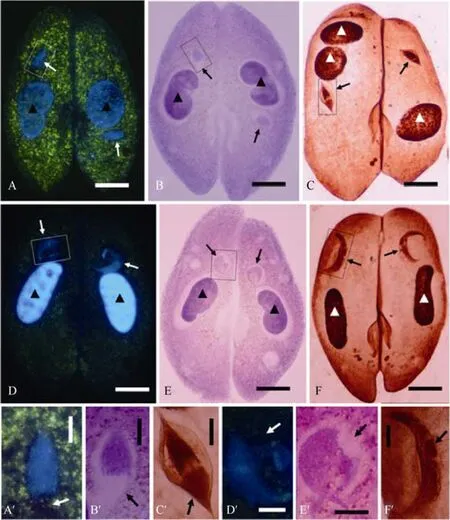
Fig. 1 Micronuclei on meiotic prophase of Parameciumcaudatum
After meiosis, four meiotic products are produced,among which only one enters the paroral cone region(PC) (the area surrounding the disintegrated oral apparatus) and survives to complete the third prezygotic division producing a migratory and stationary pronucleus(Wichterman, 1986; Yanagi, 1987; Yanagi & Hiwatashi,1985), while the remaining three undergo apoptotic degeneration (Yang et al, 2007; Gao et al, 2010).Recently, immunofluorescence labeling with monoclonal antibodies of anti-α tubulin indicated that at least one of the meiotic products was located in the PC area at the telophase of the second meiotic division (Gao et al,2011b). To know the precise timing of PC entrance,many conjugating pairs were stained either by protargol technique or immunofluorescence labeling with anti-α tubulin antibodies. Both stainings indicated two cases of PC entrance (Fig. 2). One was during telophase of the second meiotic division (Fig. 2B, B′, G-I), when the meiotic nuclei showed a teardrop form. The other was soon after meiosis (Fig. 2D, D′, J), when all meiotic products showed spindle shapes without degenerating symptoms. Protargol staining indicated that PC entrance occurred in 70% of conjugants during the telophase of the second meiotic division, and in 96.3% soon after meiosis. In the case of immunofluorescence labeling, PC entrance was observed in 94.6% of conjugants during telophase of the second meiotic division, and 100% of conjugants after meiosis. However, PC entrance was observed neither at the telophase nor after the completion of the first meiotic division (Fig. 2E, F). These observations indicated that PC entrance of meiotic products was a specific phenomenon, which mainly happened during telophase of the second meiotic division instead of after meiosis. Based on electron microscopic analysis of transverse sections of jointedP.caudatumat the junction zone (André & Vivier, 1962; Vivier &André, 1961; Vivier, 1965), it has been suggested that a paroral cone is not present during the conjugation(Wichterman, 1986). In the current experiment, the paroral cone region was clearly observed (Fig. 2A-D),and might be involved in the protection of meiotic products from degeneration. We are not sure whether this observed difference was from different techniques or different syngens ofP.caudatum.
2.3 A protargol staining-indicated structure in the spindles connecting prospective migratory and stationary pronuclei
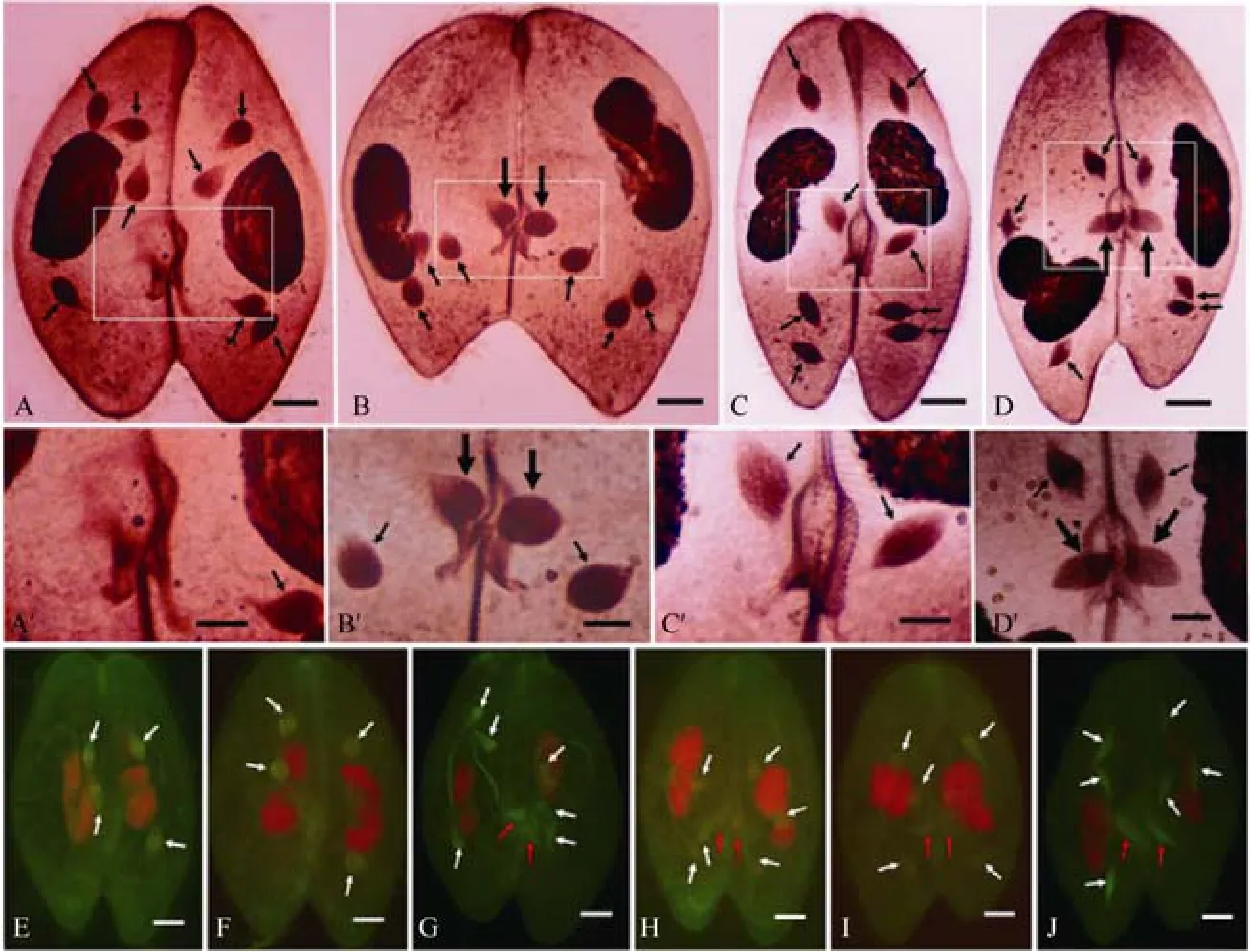
Fig. 2 PC entrance of meiotic products in Parameciumcaudatum
After meiosis, the haploid nucleus located in the PC region divides mitotically (the third prezygotic division)yielding two types of pronuclei (Wichterman, 1986).Recently, it has been indicated by both protargol staining and immunofluorescence labeling with anti-α tubulin antibodies that “U” and “V” shaped spindles connect prospective migratory and stationary pronuclei at the telophase of the third prezygotic division (Gao et al,2011a, b). For easier comparison, the same experiments were repeated in the current study. The “V”-shaped spindles and side-by-side localization of the two prospective pronuclei were observed (Fig. 3). Protargol staining indicated the presence of a swollen structure at the crossing points of the “V”-shaped spindles (circles in Fig. 3A-C, A′-C′), while immunofluorescence labeling with anti-α tubulin antibodies indicated a slender line structure at the same area. In other words, this protargolstained structure consisted of microtubules and some other unknown components. It is not clear if this structure corresponded to the structure in the crescent nuclei indicated in the current study, or functioned as MTOC. Besides these, there were two new points that have never been reported. The first was that the stationary pronuclei were much closer to the migratory pronuclei in Fig. 3C than in Fig. 3A and B. To clarify this, many conjugating pairs covering the third prezygotic division have to be stained and measured and statistically analyzed. The second is that many anti-α tubulin recognized dots were observed surrounding both types of prospective pronuclei. It has been previously reported that both intranuclear and cytoplasmic microtubules play important roles on pronuclear behavior (Nakajima et al, 2001). This current study indicated that both cytoplasmic and intranuclear microtubules appeared much earlier than the stage of pronuclear transfer.
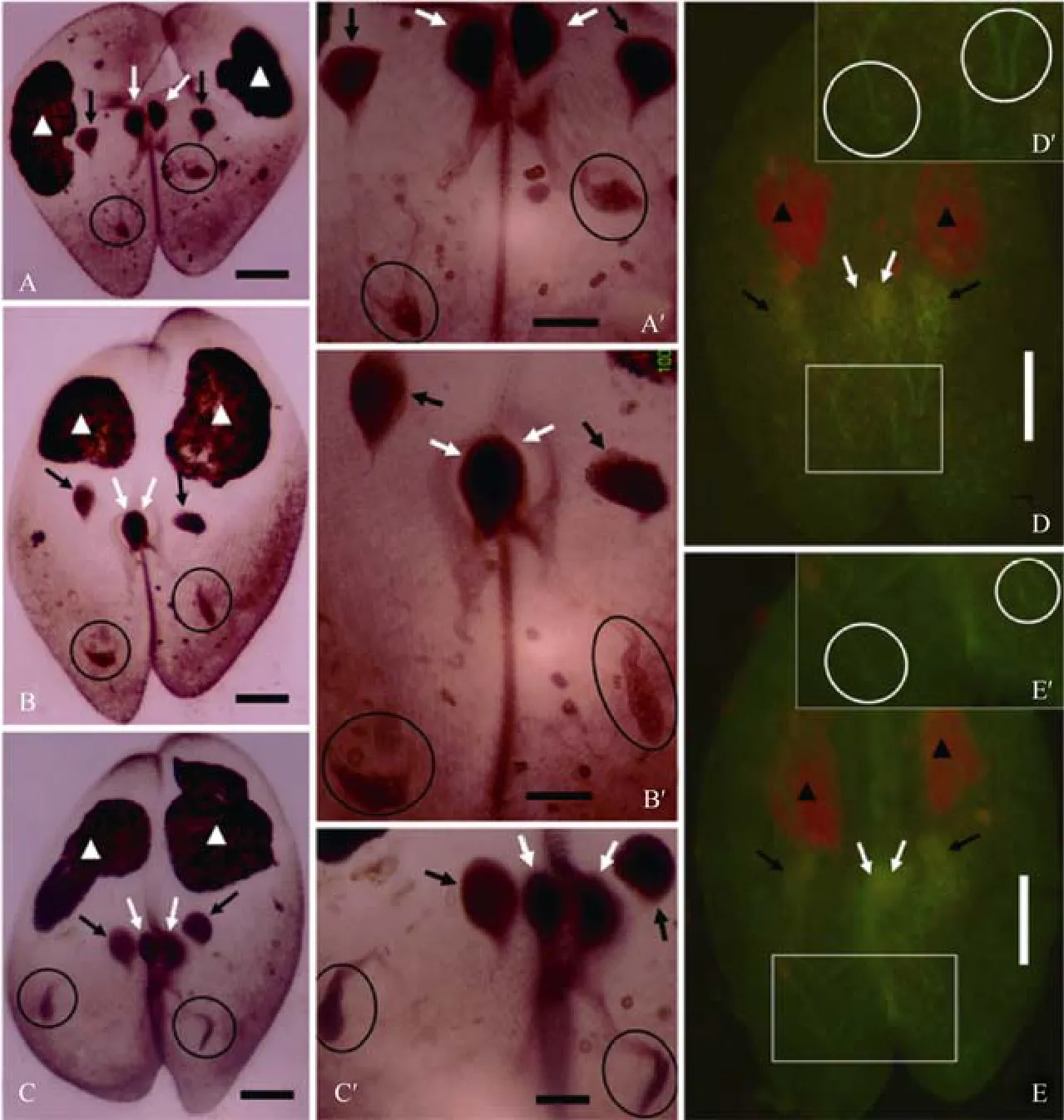
Fig. 3 Telophase of the third prezygotic division of Parameciumcaudatum
André J, Vivier E. 1962. Queleques aspects ultrastructuraux de l’échange micronucleaire lors de la conjugaison chezParamecium caudatum[J].J Ultrastruct Res, 6: 390-406.
Blackburn EH, Gall JG. 1978. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes inTetrahymena[J].J Mol Biol, 120: 33-55.
Calkins GN, Cull SW. 1907. The conjugation ofParameciumaurelia(caudatum) [J].Arch Protistenkd, 10: 375-415.
Carr DH, Walker JE. 1961. Carbol fuchsin as a stain for human chromosome [J].Biotech Histochem, 36(4): 233-236.
Cech TR, Bass BL. 1986. Biological catalysis by RNA [J].Annu Rev Biochem, 55: 599-629.
Dryl S. 1959. Antigen transformation inParamecium aureliaafter homologous antiserum treatment during autogamy and conjugation [J].J Protozool, 6: 25S
Fujishima M. 1983. Microspectrometric and autoradiographic study of the timing and duration of pre-meiotic DNA synthesis inParamecium caudatum[J].J Cell Res, 60: 51-65.
Fujishima M, Hiwatashi K.1981. Transplantation of germ nuclei inParamecium caudatum. II. Induction of meiosis in transplanted interphase nucleus [J].Exp Cell Res, 131: 63-71.
Gao X, Zhang XJ, Yang XY. 2010. Morphological apoptotic characteristics of the post-meiotic micronuclei inParamecium caudatum[J].EurJ Protistol, 46: 243-250
Gao X, Shi XB, Yang XY. 2011a. Dynamic behaviour of stationary pronuclei during their positioning inParameciumcaudatum[J].Eur J Protistol, 47: 235-237.
Gao X, Zhu JJ, Yang XY, Yuan JQ, Wang YW, Song MG. 2011b.Localization of stationary pronuclei during congjugation ofParameciumindicated by immunofluorescence staining [J].Zool Res, 31: 461-464.
Greider CW, Blackburn EH. 1985. Identification of a specific telomere terminal transferase activity inTetrahymenaextracts [J].Cell, 43:405-413.
Harumoto T, Miyake A. 1996. Chemical induction of conjugation by DAPI (4’,-6-diamino-2-phenylin-dole) and other DNA-bingding compounds inParamecium caudatum[J].Eur J Protistol,32(Suppl 1): 37-45.
Hiwatashi K. 1968. Determination and inheritance of mating types inParameciumcaudatum[J].Genetics, 58: 373-386.
Klotz C, Ruiz F, Garreau de Loubresse N, Wright M, Dupuis-Williams P, Beisson J. 2003. Gamma-tubulin and MTOCs inParamecium[J].Protist,154:193-209.
Lin Y, Gao X, Yang XY. 2009. A method for observation of livingParamecium[J].Shichuan J Zool, 28: 276. (in Chinese)
Maupas E. 1889. Le rajeunissement karyogamique chez les ciliés [J].Arch Zool Exp Gen, 7: 149-517.
Nakajima Y, Mikami K, Takahashi M. 2001. Role of the cytoplasmic and the intranuclear microtubules on the behavior of pronuclei during the conjugation inParameciumcaudatum[J].Proc Jpn Acad:Ser B, 77: 172-177.
Nanney DL. 1980. Experimental Ciliatology [M]. New York: John Wiley and Sons.
Santos ML, Lu E, Wolfe J. 2000. Nuclear death in livingTetrahymena:the case of the haploid nuclei [J].J Eukaryot Microbiol, 47: 493-498.
Shi XB. 1987. Improvements of protargol technique in studies of ciliated protozoa [C]// Proc 4th Symp Chinese Protozool Soc.[S.L.]: [s.n.], p19. (in Chinese)
Shi XB, Frankel J. 1990. Morphology and development of mirrorimage doublets ofStylonychiamytilus[J].J Protozool, 37: 1-13.
Sun XT, Wu JW, Gao X, Yang XY. 2010. To enhance the production of magnetic iron-detran particles by ultrasonic treatment and its applications in ciliate studies [J].Chn J Zool, 45(1): 90-93. (in Chinese)
Vivier E. 1965. Sexuallité et conjugaison chez la Paramécie [J].Ann Fac Sci Clermont-Ferrand, 26: 101-114.
Vivier E, André J. 1961. Donnneés structrales et ultrastructurales nouvelles sur la conjugaison deParamecium caudatum[J].JProtozool, 8: 416-426.
Wei JY, Gao X, Yang XY. 2008. Introducing a method for separating ciliate conjugating pairs by magnetic iron-dextran particles [J].Chn J Zool, 43(2): 77-80. (in Chinese)
Wichterman R. 1986. The Biology ofParamecium[M]. 2nd Ed. New York: Plenum Press.
Yanagi A. 1987. Positional control of the fates of nuclei produced after meiosis inParameciumcaudatum: Analysis by nuclear transplantation [J].Dev Biol, 122: 535-539.
Yanagi A, Hiwatashi K. 1985. Intracellular positional control of survival and degeneration of nuclei during the conjugation inP.caudatum.J Cell Sci, 79: 237-246.
Yang XY, Shi XB. 2007. Gametic nuclear exchange during the conjugation ofParameciumpolycaryum[J].Jpn J Protozool, 40:113-121.
Yang XY, Takahashi M. 1999. Disturbance of determination of germinal and somatic nuclei by heat shock inParamecium caudatum[J].J Eukaryot Microbiol, 46: 49-55.
Yang XY, Takahashi M. 2002. Nuclei may anchor at specific locations during nuclear determination inParameciumcaudatum[J].Eur J Protistol, 38: 147-153.
Yang XY, Gao X, Shi XL. 2007. Detection of haploid nuclear death in livingParamecium caudatum[J].Jnp J Protozool, 40: 123-130.
Yang XY, Huang X, Zhang X, Zhou Q. 2008. Methods for observation of livingParameciumby slowing down their movements [J].Biol Teach, 33(9): 46-47. (in Chinese)
不同染色方法揭示纤毛原生动物草履虫接合生殖过程的新细节
高 欣, 杨仙玉*, 朱嘉骏, 袁进强, 王逸雯, 宋敏国
(浙江农林大学 亚热带森林培育国家重点实验室培育基地,浙江 临安 311300)
通过石炭酸品红、Hoechst 33342、蛋白银及免疫荧光标记等染色方法对草履虫接合生殖过程进行了重新观察,结果发现:1)新月核是第一次减数分裂前期小核的主要形态学特征,在核内有一未被石炭酸品红、Hoechst 33342着色区域,蛋白银染色则清楚显示该结构;2)4个单倍体减数分裂产物中的 1个核进入口旁锥完成配前第三次核分裂,其余3核退化。蛋白银染色和抗α微管蛋白单克隆抗体进行免疫荧光标记显示,核进入口旁锥的时期在第二次减数分裂末期而非减数分裂结束后;3)配前第三次分裂末期,核间连丝的中间段有一被蛋白银识别的结构,但免疫荧光标记却无显示, 只表现为纤维状结构与两侧核间连丝相连。观察结果为草履虫接合生殖过程中相关分子生物学机制研究奠定了必要的形态学基础。
草履虫;接合生殖;新月核;进入口旁锥;核间连丝
Q959.117.04;Q954.44
A
0254-5853-(2011)06-0651-06
2011-08-04;接受日期:2011-09-18
国家自然基金项目(30670397; 31071181); 教育部留学回国人员科研启动基金项目([2007]1108); 浙江省教育厅一般项目(Y200804256); 浙江农林大学科研发展基金项目(2292000030); 浙江省大学生科技创新活动计划(新苗人才计划)项目(2010R412003);浙江农林大学大学生科技创新活动项目(100203,100224)
高欣,男,讲师;研究方向:动物学;E-mail:wildlife909@yahoo.cn
10.3724/SP.J.1141.2011.06651
date: 2011-08-04; Accepted date: 2011-09-18
*Corresponding author (通讯作者), E-mail: xianyu_yang@hotmail.com
猜你喜欢
杂志排行
Zoological Research的其它文章
- Histological and immunocytochemical study of deferens ducts in the Chinese rat snake (Zaocys dhumnades)
- PTEN在小鼠卵细胞和早期胚胎发育中的功能研究
- A phylogeny of the Tylototriton asperrimus group (Caudata: Salamandridae) based on a mitochondrial study: suggestions for a taxonomic revision
- 云南新平哀牢山西黑冠长臂猿分布与群体数量
- 杭州湾及钱塘江河口水鸟群落组成、季节动态及种间相关性分析
- 上海市南汇东滩围垦后海岸带湿地冬春季水鸟生境选择
