Decreased contrast sensitivity of visual cortical cells to visual stimuli accompanies a reduction of intracortical inhibition in old cats
2011-12-25ZHOUJunSHIXiaMingPENGQingSongHUAGuoPengHUATianMiao
ZHOU Jun, SHI Xia-Ming, PENG Qing-Song, HUA Guo-Peng, HUA Tian-Miao
(School of Life Science, Anhui Normal University, Wuhu 241000, China)
Decreased contrast sensitivity of visual cortical cells to visual stimuli accompanies a reduction of intracortical inhibition in old cats
ZHOU Jun, SHI Xia-Ming, PENG Qing-Song, HUA Guo-Peng, HUA Tian-Miao*
(School of Life Science, Anhui Normal University, Wuhu 241000, China)
Psychophysical experiments on human and animal subjects have proven that aged individuals show significantly reduced visual contrast sensitivity compared with young adults. To uncover the possible neural mechanisms, we used extracellular single-unit recording techniques to examine the response of V1(primary visual cortex) neurons as a function of visual stimulus contrast in both old and young adult cats (Felis catus). The mean contrast sensitivity of V1neurons to visual stimuli in old cats decreased significantly relative to young adult cats, consistent with findings reported in old primates. These results indicate that aging can affect contrast sensitivity of visual cortical cells in both primate and non-primate mammalian animals, and might contribute to the reduction of perceptual visual contrast sensitivity in aged individuals. Further, V1cells of old cats exhibited increased responsiveness, decreased signal-to-noise ratio, and enlarged receptive field (RF) size compared with that of young adult cats, which indicated that decreased contrast sensitivity of V1neurons accompanied a reduction of intracortical inhibition during senescence.
Contrast sensitivity; V1neurons; Old cats; Young adult cats
Contrast sensitivity refers to the ability to detect subtle luminance contrast in visual signals and is widely accepted as an important index for visual quality assessment (Riusala et al, 2003; Ginsburg, 2006; Piermarocchi et al, 2006; Hua et al, 2010a). Psychophysical studies on human subjects have demonstrated that visual contrast sensitivity decreases significantly with age (Higgins et al, 1988; Santos et al, 2006), and this aged change has been observed at all stimulus spatial frequencies (Nomura et al, 2003). Age-related decline in visual contrast sensitivity cannot be suspended in subjects with corrected visual acuity of 1.0 or better (Nomura et al, 2003). Further, this senescent change cannot be completely attributed to the deterioration in optical factors as young healthy observers with simulated age-related pupil size reduction and ocular absorption and light scatter increase still show higher contrast sensitivity than older counterparts (Whitaker & Elliott, 1992). Also, this kind of decrease cannot be eliminated through increasing stimulus luminance (Higgins et al, 1988). Therefore, contrast sensitivity reduction in aged individuals cannot be accounted for by changes in optical factors, but seems more relevant to alterations occurring in the central nervous system.
Previous investigations on single-cell responses in the visual system of aged primates has shown that receptive field properties, including contrast sensitivity of neurons at the subcortical level, are relatively unaffected by aging (Spear, 1993; Spear et al, 1994; Schmolesky et al, 2000). Some authors suggest that agerelated contrast sensitivity decline may reflect a visual cortex mechanism (Crassini et al, 1988; Pardhan et al, 1996; Bennett et al, 1999), where neurons display prominent contrast gain control (Ohzawa et al, 1982, 1985; Bonds, 1991; Heeger, 1992; Hua et al, 2010b). A recent study has shown that visual cortical cells of old macaque monkeys exhibit worse contrast sensitivity to visual stimuli than that of younger individuals. However, whether this aged change can be generalized to nonprimate mammalian species is unknown. The aim of our research was to determine if visual cortical cells of cats display a similar age-related reduction in contrast sensitivity. Using extracellular single-unit recording techniques, we systematically recorded the response of V1neurons to optimal visual stimuli with varied luminance contrast (0−1) and compared the mean contrast sensitivity of V1neurons in old and young adult cats to provide new evidence for the cortical mechanism underlying the reduction of visual contrast sensitivity during senescence.
1 Materials and Methods
1.1 Subjects
Study subjects consisted of four young adult cats (2−3 a) and four old cats (12−14 a). Subjects were examined ophthalmoscopically before the experiment to confirm that no optical or retinal problems impaired their visual function. All experimental procedures were strictly in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
1.2 Single-unit recording
All cats were prepared for extracellular single-unit recording as described in previous researches (Hua et al, 2006; Hua et al, 2009; Hua et al, 2010b). Briefly, cats were anesthetized with ketamine HCl (20 mg/kg) and xylazine (2 mg/kg). After intubation of intravenous and tracheal cannulae, cats were placed in a stereotaxic apparatus with ear bars, eye bars and a bite bar. Pupils were maximally dilated with atropine (1%) and appropriate contact lenses were used to protect the corneas. Neosynephrine (5%) was administered to retract the nictitating membranes. Glucose (5%)-saline (0.9%) solution containing a mixture of urethane (20 mg/hr/kg body weight) and gallamine triethiodide (10 mg/hr/kg body weight) was infused intravenously to keep the animal anesthetized and paralyzed. Expired pCO2 was maintained at approximately 3.8%. Heart rate (180−220 pulses/min) and ECG were monitored throughout the experiment to assess anesthesia level. The skull and dura over V1(area 17) were removed with a fine operation under light microscope. Single-unit recording was performed using a glass microelectrode (with an impedance of 3−6 MΩ) which was driven by a hydraulic micromanipulator (NARISHIGE, Japan). The small hole over V1was filled with a 4% agar solution in saline and sealed with wax. After the preparation was complete, the optic discs of the two eyes were reflected onto a movable transparent tangent screen positioned 57 cm from the eyes and overlapped with the CRT monitor used for stimulus presentation. The area centralis of each eye was located prior to physiological recording based on the position of the optic discs reflected onto the tangent screen.
1.3 Visual stimuli
Drifting sinusoidal gratings shown on a CRT monitor (resolution 1 024×768, refresh rate 85 Hz) were used as visual stimuli. The program to generate the stimuli was written in MATLAB, using extensions provided by the high-level Psychophysics Toolbox (Brainard, 1997) and low-level VideoToolbox (Pelli, 1997). Once a single unit was isolated, the cell’s receptive field center was carefully located by consecutively presenting a series of computer-generated light spots on the CRT. By comparing the neuron’s response to a series of stimulus, we determined the preferred orientation and motion direction, preferred spatial and temporal frequency, and optimal stimulus size for each cell. The cell’s response to optimal stimulus with varied luminance contrast levels (0−1, starting from 0 with an increment of 0.1) were then systematically recorded. Each stimulus was presented monocularly to the dominant eye and repeated 4−6 times with a 3 min interval between trials for cellular functional recovery. The duration for each stimulus (5 cycles of grating) was less than 5 sec, which varied depending on the cell’s optimal stimulus temporal frequency. Before each stimulus was presented, spontaneous activity (baseline response) was acquired during a 1sec period while a mean luminance was shown on the screen. The mean luminance of each stimulus was 19 cd/m2, and the environmental ambient luminance on the cornea was 0.1 lux.
1.4 Data acquisition and analysis
After the signal was amplified with a microelectrode amplifier (NIHON KOHDEN, Japan) and differential amplifier (Dagan 2400A, USA), action potentials were fed into a window discriminator with an audio monitor. The original voltage traces (Fig. 1A, C) were digitized using an acquisition board (National Instruments, USA) controlled by IGOR software (WaveMetrics, USA), and saved for later analysis. The evoked response of a cell to a drifting sinusoidal grating was defined as the mean firing rate (after subtracting spontaneous activity) corresponding to the time of stimulus modulation, which was used to draw the orientation, spatial frequency, temporal frequency, stimulus size and contrast-response tuning curve (Fig. 1B, D). To assess contrast sensitivity of each cell to visual stimuli, we fitted the contrastresponse function of each cell with the Naka-Rushton equation (Albrecht, 1995) (Fig. 1B, D):
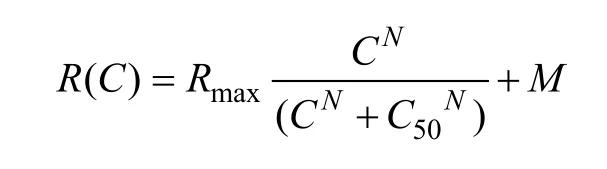
WhereR(C) represents the neuron’s response to a visual stimulus with contrast value of C,Rmaxis the neuron’s maximal visually-evoked response to visual stimuli,Mis the neuron’s spontaneous activity or baseline response, C50corresponds to the stimulus contrast that evokes half of the neuron’s maximal response, andNrepresents the slope of the neuron’s response-contrast tuning curve. Threshold contrast (Tc) of each neuron is defined as the contrast that evokes a response 1.414 (the hypotenuse of a right triangle whose other sides are 1 and 1) times its baseline response (M). Contrast sensitivity of each neuron was assessed by the inverse of C50, called C50-contrast sensitivity (C50CS), and the inverse of Tc, called Tc-contrast sensitivity (TcCS). Cells with less than 95% goodness of fit were not included in our data analysis.
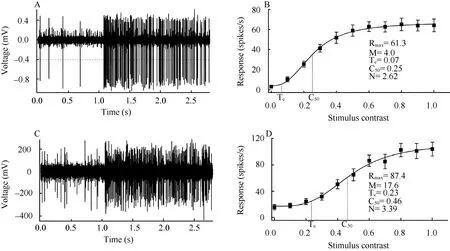
Fig. 1 Response of sample cells to its optimal visual stimulus for young adult (A, B) and old cats (C, D)
All values were expressed as mean±SD. Difference between different individuals and age groups were assessed using one-way or two-way analysis of variance (ANOVA) and Chi-square test.
2 Results
A total of 138 neurons from the four young adult cats and 128 neurons from the four old cats were studied (Tab.1). Neurons recorded from each group of cats were at the same range of depth from the pial surface of the brain, representing a random sample of neurons in all cortical layers. All neurons had receptive field within 8° visual degree from the central area of the dominant eye. No significant difference was found in the eccentricity distribution of neurons between the young adult and old cats (χ2(7)=9.624,P=0.211).
2.1 Contrast sensitivity of V1 neurons
Our results showed that most old cat neurons (84.37%) had a C50-contrast sensitivity value less than 3, whereas the majority of young adult cat neurons (68.12%) had a C50-contrast sensitivity larger than 3 (Fig. 2A). Two-way ANOVA indicated that the mean C50-contrast sensitivity of neurons in each old cat was significantly smaller than in any individual young adult cat (F(1,258)=75.181,P<0.0001) (Tab. 1). This age effect was independent of cat (F(3,258)=1.247,P>0.2). The average C50-contrast sensitivity of neurons in the old cat group was also significantly decreased compared with that in the young cat group (F(1,264)=82.377,P<0.0001).
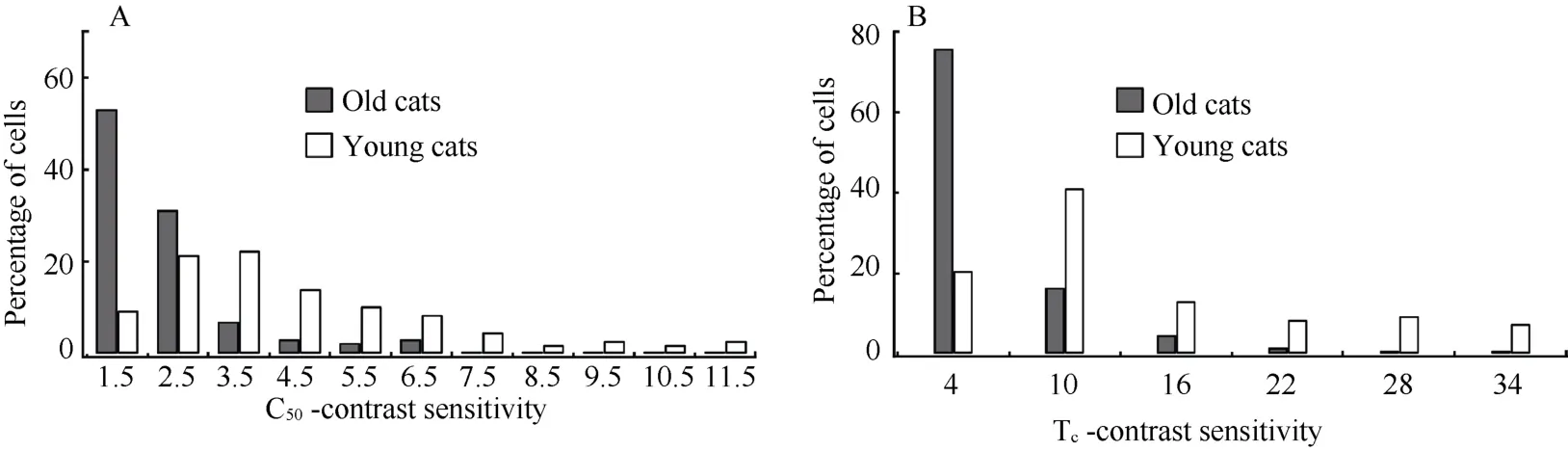
Fig. 2 Percentage of cells with different C50-contrast sensitivity (A) and Tc-contrast sensitivity (B) in old and young adult cats
We also compared Tc-contrast sensitivity of neurons between old and young adult cats. Most old cat neurons (75.78%) had a Tc-contrast sensitivity value lower than 7, whereas most young cat neurons (74.64%) had a Tc-contrast sensitivity value higher than 7 (Fig. 2B). Statistical analysis showed that mean Tc-contrast sensitivity of neurons in each old cat was significantly declined compared with that in any individual young adult cat (F(1,258)=58.391,P<0.0001) (Tab.1). The age effect was independent of subjects (F(3,258)=1.474,P>0.2). Similarly, the average Tc-contrast sensitivity of neurons in the old cat group was significantly lower than in the young cat group (F(1,264)=64.906,P<0.0001).
We concluded, therefore, that the contrast sensitivity of V1neurons in old cats was significantly decreased compared with that in young adult cats.
2.2 Response property changes of V1 neurons
Previous studies have shown that aging may lead to decreased intracortical inhibition, which could account for the changes in response properties of visual cortical cells (Schmolesky et al, 2000; Leventhal et al, 2003; Wang et al, 2005; Hua et al, 2006; Wang et al, 2006; Hua et al, 2008; Hua et al, 2009). To confirm if decreased contrast sensitivity of V1cells to visual stimuli was accompanied with a reduction in intracortical inhibition, we examined the responsiveness and the receptive field size of V1neurons in both age groups.
Statistical analysis showed that maximal visuallyevoked response (Rmax) and spontaneous activity (M) of V1neurons in old cats increased significantly compared with that in young adult cats (Rmax:F(1,264)= 26.185,P<0.0001; M:F(1,264)= 165.608,P<0.0001) (Fig. 3A), but the mean amplitude of M increase (226.7%) was notably higher than that of Rmax(38.4%). This led to a significantly decreased signal-to-noise ratio (SNR) of V1neurons in old cats relative to young adult cats (Age effect:F(1,258)= 81.297,P<0.0001; Interaction of age and subjects:F(3,258)= 2.071,P>0.1) (Fig. 3B).
2.3 Age-related changes of receptive field size
The decreased contrast sensitivity of V1neurons in old cats may be a consequence of presenting improper stimulus size for neurons in old and young adult cats. To clarify this possibility, we evaluated the receptive field size of each neuron by measuring the optimal grating stimulus radius (in degree) from the size-tuning curve (Fig. 4A, B) and the optimal spatial frequency (OSF) from spatial frequency tuning curve.
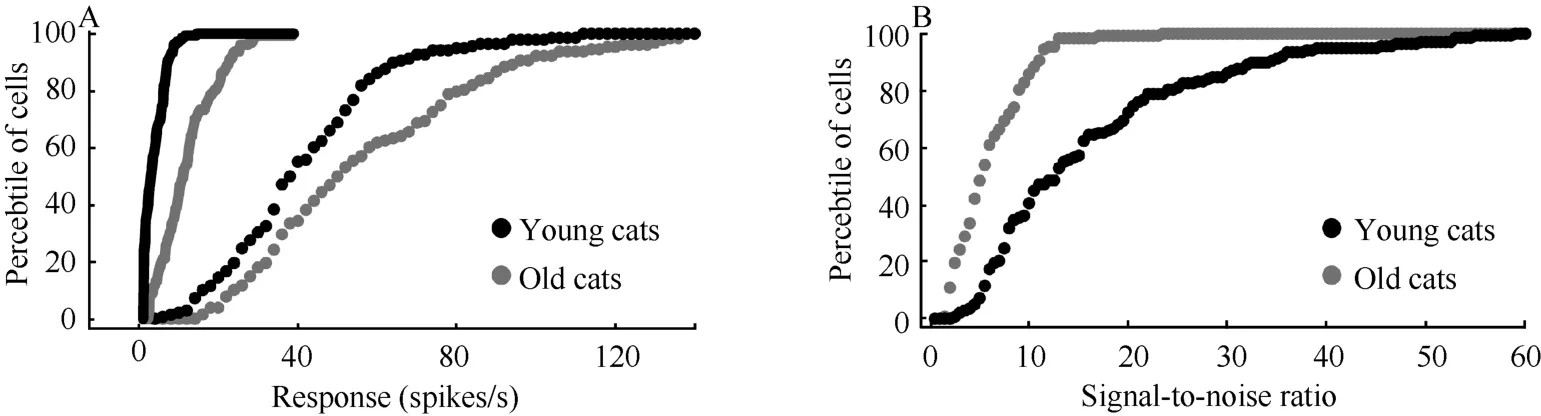
Fig. 3 Percentile of cells with different spontaneous activity (lines) and maximal response (markers) (A) and signal-to-noise ratio (B) of V1 neurons in old and young adult cats

Fig. 4 Response-stimulus size tuning curve of sample cells from old and young adult cats
Most young adult cat cells (63.7%) had an optimal stimulus radius less than 3, whereas the majority of old cat cells (71.1) had an optimal stimulus radius larger than 3 (Fig. 4C, D). The ANOVA results showed that the mean optimal stimulus radius of cells in old cats (3.7±1.3) was significantly higher than in young adult ones (2.8±1.3) (F(1,264)= 27.99,P<0.0001).
Similarly, more than half young adult cat cells (55%) had an optimal spatial frequency higher than 0.4 c/deg. However, most old cat cells (71.1) had an optimal spatial frequency lower than 0.4 c/deg (Fig. 5). Statistical analysis indicated that the mean optimal spatial frequency of cells in old cats (0.26±0.18 c/deg) was significantly decreased compared with that in young adult cats (0.42±0.27 c/deg) (F(1,264)= 33.188,P<0.0001).
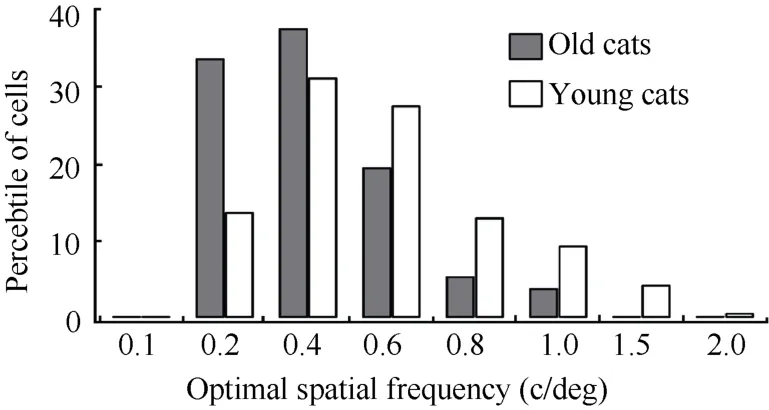
Fig. 5 Percentage of cells with different range of optimal spatial frequencies in old and young adult cats
Therefore, cells in old cats showed a significantly larger receptive field center than cells in young adult cats. This result supports the notion that aging leads to reduced intracortical inhibition.
3 Discussion
Psychophysical studies indicate that visual contrast sensitivity significantly declines during senescence (Higgins et al, 1988; Santos et al, 2006). This aged change is independent of optical factors, and likely relates to alterations in the central nervous system (Higgins et al, 1988; Whitaker & Elliott, 1992; Nomura et al, 2003). We suggested that a decrease of neuronal contrast sensitivity in the visual cortex contributed, at least in part, to visual contrast sensitivity decline in aged individuals because of the following experimental evidences: 1) Investigations have shown that neuronal response properties in the subcortical nucleus (LGN), including contrast sensitivity, are relatively unaffected by aging (Spear et al, 1994; Schmolesky et al, 2000); 2) Neurons in the visual cortex exhibit a distinct contrast gain control in response to varied stimulus contrast (Ohzawa et al, 1982, 1985; Bonds, 1991; Heeger, 1992; Hua et al, 2010b); 3). Our results indicated that the contrast sensitivity of V1neurons in old cats was significantly lower than in young adult cats, which concurred with findings reported in the V1and MT area of macaque monkeys (Yang et al, 2008).
Functional degradation of visual cortical cells in old animals, including increased responsiveness, reduced signal-to-noise ratio, decreased response selectivity for visual stimuli, lagged response latency and increased adaptation to visual stimulation, have been reported in several mammalian species (Schmolesky et al, 2000; Mendelson & Wells, 2002; Wang et al, 2005; Hua et al, 2006; Wang et al, 2006; Zhang et al, 2008; Hua et al, 2009). It is widely suggested that compromised intracortical inhibition during aging could result in functional declines of visual cortical neurons in senescent individuals because: 1) The increased responsiveness and decreased stimulus selectivity of visual cortical neurons in old individuals can be modified by improving intracortical inhibition effects (Leventhal et al, 2003); and 2) Cortex in old animals shows a significantly decreased proportion of GABAergic neurons and GAD immunoreactive neurons compared with young adults (Ling et al, 2005; Hua et al, 2008).
Mechanisms that mediate contrast sensitivity decrease in visual cortical cells to visual stimuli remains unclear. In this study, we observed that the decreased contrast sensitivity of V1neurons in old cats was accompanied with increased spontaneous activity, increased visually-evoked response, and a significantly enlarged receptive field size relative to young adult cats, which suggested compromised intracortical inhibition during aging. Whether a reduction in intracortical inhibition with age also underlies the decreased contrast sensitivity of V1neurons in old individuals needs further clarification.
In summary, our results provide new evidence that aging significantly affected contrast sensitivity of visual cortical cells. This functional degradation was accompanied with a reduced intracortical inhibition during senescence.
Albrecht DG. 1995. Visual cortex neurons in monkey and cat: effect of contrast on the spatial and temporal phase transfer functions [J].Vis Neurosci,12(6): 1191-1210.
Bennett PJ, Sekuler AB, Ozin L. 1999. Effects of aging on calculation efficiency and equivalent noise [J].J Opt Soc Am A Opt Image Sci Vis,16(3): 654-668.
Bonds AB. 1991. Temporal dynamics of contrast gain in single cells of the cat striate cortex [J].Vis Neurosci,6(3): 239-255.
Brainard DH. 1997. The Psychophysics Toolbox [J].Spat Vis,10(4): 433-436.
Crassini B, Brown B, Bowman K. 1988. Age-related changes in contrast sensitivity in central and peripheral retina [J].Perception,17(3): 315-332.
Ginsburg AP. 2006. Contrast sensitivity: determining the visual quality and function of cataract, intraocular lenses and refractive surgery [J].Curr Opin Ophthalmol,17(1): 19-26.
Heeger DJ. 1992. Normalization of cell responses in cat striate cortex [J].Vis Neurosci,9(2): 181-197.
Higgins KE, Jaffe MJ, Caruso RC, deMonasterio FM. 1988. Spatial contrast sensitivity: effects of age, test-retest, and psychophysical method [J].J Opt Soc Am A,5(12): 2173-2180.
Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. 2006. Functional degradation of visual cortical cells in old cats [J].Neurobiol Aging,27(1): 155-162.
Hua T, Kao C, Sun Q, Li X, Zhou Y. 2008. Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex [J].Brain Res Bull,75(1): 119-125.
Hua T, Li G, Tang C, Wang Z, Chang S. 2009. Enhanced adaptation of visual cortical cells to visual stimulation in aged cats [J].Neurosci Lett,451(1): 25-28.
Hua T, Wang Z, Xu J, Diao J. 2010a. Contrast detection learning improves visual contrast sensitivity of cat [J].Zool Res,31(2): 155-162.
Hua T, Bao P, Huang CB, Wang Z, Xu J, Zhou Y, Lu ZL. 2010b. Perceptual learning improves contrast sensitivity of V1 neurons in cats [J].Curr Biol,20(10): 887-894.
Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. 2003. GABA and its agonists improved visual cortical function in senescent monkeys [J].Science,300(5620): 812-815.
Ling LL, Hughes LF, Caspary DM. 2005. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex [J].Neuroscience,132(4): 1103-1113.
Mendelson JR & Wells EF. 2002. Age-related changes in the visual cortex [J].Vision Res,42(6): 695-703.
Nomura H, Ando F, Niino N, Shimokata H, Miyake Y. 2003. Agerelated change in contrast sensitivity among Japanese adults [J].Jpn J Ophthalm,47(3): 299-303.
Ohzawa I, Sclar G, Freeman RD. 1982. Contrast gain control in the cat visual cortex [J].Nature,298(5871): 266-268.
Ohzawa I, Sclar G, Freeman RD. 1985. Contrast gain control in the cat's visual system [J].J Neurophys,54(3): 651-667.
Pardhan S, Gilchrist J, Elliott DB, Beh GK. 1996. A comparison of sampling efficiency and internal noise level in young and old subjects [J].Vision Res,36(11): 1641-1648.
Pelli DG. 1997. The VideoToolbox software for visual psychophysics: transforming numbers into movies [J].Spat Vis,10(4): 437-442.
Piermarocchi S, Sartore M, Bandello F, Lanzetta P, Brancato R, Garattini L, Lumbroso B, Rispoli M, Pece A, Isola V, Pulazzini A, Menchini U, Virgili G, Tedeschi M, Varano M.2006. Quality of vision: A consensus building initiative for a new ophthalmologic concept [J].Eur J Ophthalm,16(6): 851-860.
Riusala A, Sarna S, Immonen I. 2003. Visual function index .VF-14. in exudative age-related macular degeneration of long duration [J].Am J Ophthalmol,135(2): 206-212.
Santos NA, Oliveira AB, Nogueira RM, Simas ML. 2006. Mesopic radial frequency contrast sensitivity function for young and older adults [J].Braz J Med Biol Res,39(6): 791-794.
Schmolesky MT, Wang Y, Pu M, Leventhal AG. 2000. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys [J].Nat Neurosci,3(4): 384-390.
Spear PD .1993. Neural bases of visual deficits during aging [J].Vision Res,33(18): 2589-2609.
Spear PD, Moore RJ, Kim CB, Xue JT, Tumosa N. 1994. Effects of aging on the primate visual system: spatial and temporal processing by lateral geniculate neurons in young adult and old rhesus monkeys [J].J Neurophys,72(1): 402-420.
Wang H, Xie X, Li X, Chen B, Zhou Y. 2006. Functional degradation of visual cortical cells in aged rats [J].Brain Res,1122(1): 93-98.
Wang Y, Zhou Y, Ma Y, Leventhal AG. 2005. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys [J].Cereb Cortex,15(4): 403-408.
Whitaker D, Elliott DB. 1992. Simulating age-related optical changes in the human eye [J].Doc Ophthalm,82(4): 307-316.
Yang Y, Liang Z, Li G, Wang Y, Zhou Y, Leventhal AG. 2008. Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys [J].Neuroscience,156(3): 748-757.
Zhang J,Wang X, Wang Y, Fu Y, Liang Z, Ma Y, Leventhal AG. 2008. Spatial and temporal sensitivity degradation of primary visual cortical cells in senescent rhesus monkeys [J].Eur J Neurosci,28(1): 201-207.
老年猫视皮层细胞对刺激反应的对比敏感度下降伴随皮层内抑制作用减弱
周 俊, 施夏明, 彭青松, 化国鹏, 华田苗*
(安徽师范大学 生命科学学院,安徽 芜湖241000)
对人类和动物的心理学研究证实, 老年个体的视觉对比敏感度相对青年个体显著下降。为揭示其可能的神经机制, 采用在体细胞外单细胞记录技术研究青、老年猫(Felis catus)初级视皮层 (primary visual cortex,V1)细胞对不同视觉刺激对比度的调谐反应。结果显示, 老年猫V1细胞对视觉刺激反应的平均对比敏感度比青年猫显著下降, 这与灵长类报道的研究结果相一致, 表明衰老影响视皮层细胞对视觉刺激反应的对比敏感度是灵长类和非灵长类哺乳动物中普遍存在的现象, 并可能是介导老年性视觉对比敏感度下降的神经基础。另外, 与青年猫相比,老年猫初级视皮层细胞对视觉刺激的反应性显著增强, 信噪比下降, 感受野显著增大, 表明衰老导致的初级视皮层细胞对视觉刺激反应的对比敏感度下降伴随着皮层内抑制性作用减弱。
对比敏感度; 初级视皮层细胞; 老年猫; 青年猫
Q42; R338.8; Q954.671
A
0254-5853-(2011)05-0533-07
2011-02-23;接受日期:2011-07-13
10.3724/SP.J.1141.2011.05533
date: 2011-02-23; Accepted date: 2011-07-13
s: Supported by National Natural Science Foundation of China (31171082), Natural Science Foundation of Anhui Province (070413138) and the Key Research Foundation of Anhui Province Education Department (KJ2009A167)
*Corresponding author (通信作者), E-mail: tmhua@mail.ahnu.edu.cn, tianmiaohua@gmail.com
猜你喜欢
杂志排行
Zoological Research的其它文章
- 白眉山鹧鸪冬季觅食地选择
- Association of RELN promoter SNPs with schizophrenia in the Chinese population
- Complete mitochondrial genome of the laced fritillary Argyreus hyperbius (Lepidoptera: Nymphalidae)
- Possible genetic reproductive isolation between two tilapiine genera and species: Oreochromis niloticus and Sarotherodonmelanotheron
- A new blind loach species, Triplophysa huanjiangensis (Teleostei: Balitoridae), from Guangxi, China
- Seasonal dynamics of wintering waterbirds in two shallow lakes along Yangtze River in Anhui Province
