半夹芯16电子化合物CpCo(S2C2B10H10)中B(3,6)位的选择性分步取代反应
2011-11-09叶红德蒋其柏解铭时丁冠宇李一志
叶红德 蒋其柏 解铭时 丁冠宇 李一志 燕 红*,
(1南京大学化学化工学院,南京 210093)
(2上饶师范学院化学化工学院,上饶 334001)
半夹芯16电子化合物CpCo(S2C2B10H10)中B(3,6)位的选择性分步取代反应
叶红德1,2蒋其柏1解铭时1丁冠宇1李一志1燕 红*,1
(1南京大学化学化工学院,南京 210093)
(2上饶师范学院化学化工学院,上饶 334001)
16e半夹芯化合物CpCo(S2C2B10H10)(Cp:cyclopentadienyl)(1)与炔烃HC≡CC(O)Fc(Fc:ferrocenyl)在物质的量之比为1∶1时反应生成化合物CpCo(S2C2B10H9)(CH=CHC(O)Fc)(2)。在化合物2中,一分子HC≡CC(O)Fc偶合到原料化合物1的碳硼烷笼子的B(3)位点,导致B(3)位的氢原子迁移到炔烃的内部碳原子上形成烯烃取代基。2能继续与另外一分子HC≡CC(O)Fc反应,生成B-双取代产物CpCo(S2C2B10H8)(CH=CHC(O)Fc)2(3)。3仍然是1个16e化合物,并且在B(3,6)位点有2个反式烯烃取代基CH= CHC(O)Fc。在过量炔烃存在情况下,该反应生成化合物3及炔烃环三聚产物1,3,5-{HC=CC(O)Fc}3(4)。化合物2、3、4用红外,核磁,元素分析,质谱和单晶X-射线衍射分析等方法进行了表征。
过渡金属;碳硼烷;B-H键活化;晶体结构
0 Introduction
Over the past decade,a sort of mononuclear 16e half-sandwich complexes of Ru,Os,Co,Rh and Ir bearing a chelating 1,2-dicarba-closo-dodecaborane-1,2-dichalcogenolate ligand have been prepared and studied extensively[1-30].These sterically congested, coordinatively unsaturated compounds have shown rich reaction chemistries towards metal fragments[5],Lewis bases[6-13],and alkynes[14-29].Previous studies have shown that the types and structures of the products are dependant on the influencing factors such as metal center,chalcogen element,substrate,ancillary ligand on metal of the above mentioned 16e complexes, reaction temperature,solvent and the ratio of the reactants.For example,the 16e complexes Cp*M (E2C2B10H10) (Cp*=pentamethylcyclopentadienyl;M= Rh,Ir;and E=S,Se)react with selected alkynes to generate activated B-H activated products in the B(3,6) positions ofcarborane[15-20].However,the reactions of Cp*Co(S2C2B10H10)with alkynes lead almost to insertion products at one Co-S bond[28-29].The treatment of CpCo(S2C2B10H10)(1)with HC≡CC(O)Ph in a ratio of 2∶1 produces a novel 17e product containing a functional group of bicyclo[2.2.1]heptene unit at the B(3,6)site of carborane[26].In contrast,if less CpCo (S2C2B10H10)is used,the reaction gives rise to 16e halfsandwich complexescontainingoneortwoB-H functionalized vinyl groups in the B(3,6)positions of carborane[27].As a continuation of this interesting chemistry,herein we report the stoichio-metric reaction between CpCo(S2C2B10H10)(1)with HC≡CC(O)Fc.
1 Experimental
1.1 Reagents and instruments
All experiments were performed under an argon atmosphere using standard Schlenk techniques.Solvents were dried by refluxing over sodium (petroleum ether,ether,and THF)or calcium hydride (CH2Cl2)under nitrogen and then distilled prior to use.CpCo(S2C2B10H10)(1)[1-2]and HC≡CC(O)Fc[31]were prepared according to the literature methods.Ferrocenecarbaldehyde (Alfa Aesar), and nbutyllithium (2.0 mol·L-1in cyclohexane,Sigma-Aldrich)were used as commercial products without further purification.Elemental analyses were performed in an elementar vario ELⅢelemental analyzer.NMR data were recorded on a Bruker DRX-500 spectrometer.1H NMR and13C NMR spectra were reported in ppm with respect to CHCl3/CDCl3(δ(1H)=7.24,δ(13C)=77.0) and11B NMR spectra were reported in ppm with respect to external Et2O·BF3(δ(11B)=0).The IR spectra were recorded on a Bruker Tensor 27 spectrophotometer with KBr pellets in the 4 000~400 cm-1region.The mass spectra were recorded on Micromass GC-TOF for EIMS(70 eV)or Finnigan MAT TSQ7000 for ESI-MS.
1.2 Synthesis of 2
HC≡CC(O)Fc(71.4 mg,0.3 mmol)was added to the solution of 1 (99.0 mg,0.3 mmol)in CH2Cl2(15 mL),and the mixture was stirred for 12 h at ambient temperature.After removal of solvent,the residue was chromatographed on TLC.Elution with CH2Cl2/ether (10∶1,V/V)gave 2.(brown-red solid).yield 37%(63.1 mg),mp 193℃dec.1H NMR (CDCl3,ppm):δ 7.02(d, 1H,J=18 Hz,B-CH=CH),6.37 (d,1H,J=18 Hz,BCH=CH),5.37(s,5H,Cp),4.87(m,2H,Fc-CH),4.61 (m,2H,Fc-CH),4.23(s,5H,Fc-Cp).11B NMR(CDCl3, ppm):δ-1.3(1B),-3.6(2B),-4.4(2B),-6.7(1B),-7.6 (4B).13C NMR(CDCl3,ppm):δ 192.69(CO),139.56(BCH=CH),135.58 (br,B-CH=CH),96.81(carborane), 81.59(Cp),79.71(Fc-C),73.22(Fc-CH),69.94(Fc-CH),70.74(Fc-Cp).IR(KBr):ν(cm-1):1 642(C=O),2 585(B-H).EI-MS(70 eV):m/z 568.0(M+,100%).Anal.calcd.for C20H25B10OS2FeCo(%):C,42.26;H,4.43.Found(%):C,42.05;H,4.54.
1.3 Synthesis of 3
HC≡CC(O)Fc(23.8 mg,0.1 mmol)was added to the solution of 2 (56.8 mg,0.1 mmol)in CH2Cl2(10 mL),and the mixture was stirred for 12 h at ambient temperature.After removal of solvent,the residue was chromatographed on TLC.Elution with CH2Cl2/ether (10∶1 of volume)gave 3.Brown-red solid,yield 86% (69.3 mg),mp 217℃dec.1H NMR (CDCl3,ppm):δ 7.05(d,2H,J=18 Hz,B-CH=CH),6.26(d,2H,J=18 Hz, B-CH=CH),5.43(s,5H,Cp),4.87(m,4H,Fc-CH),4.61 (m,4H,Fc-CH),4.24(s,10H,Fc-Cp).11B NMR(CDCl3, ppm):δ-2.0 (1B),-4.8 (4B),-8.1 (5B).13C NMR (CDCl3,ppm):δ 192.63 (CO),139.72 (B-CH=HH), 135.32(br,B-CH=CH),98.22(carborane),81.44(Cp), 79.77(Fc-C),73.19(Fc-CH),70.40(Fc-Cp),69.97(Fc-CH).IR(KBr):ν(cm-1):1648(C=O),2579(B-H).ESIMS(negative ion mode,m/z):805.7([M-H]-,63%).Anal.calcd.for C33H35B10O2S2Fe2Co(%):C,49.15;H,4.37.Found(%):C,48.98;H,4.29.
1.4 Synthesis of 4
HC≡CC(O)Fc(119 mg,0.5 mmol)was added to the solution of 1(33 mg,0.1 mmol)in CH2Cl2(15 mL), and the mixture was stirred for 12 h at ambient temperature.After removal of solvent,the residue was chromatographed on TLC.Elution with CH2Cl2/ether (10∶1,V/V)gave 3 and 4.4:brown-red solid,yield 5% (5.9 mg),mp 295℃.1H NMR (CDCl3,ppm):δ 8.84(s, 1H,Ph-CH),4.94(m,2H,Fc-CH),4.66(m,2H,Fc-CH),4.30 (s,5H,Fc-Cp).13C NMR (CDCl3,ppm):δ 197.91(CO),139.65(Ph-CH),131.20(Ph-C),77.75 (Fc-C),73.07(Fc-CH),71.60(Fc-CH),70.48(Fc-Cp).EI-MS(70eV):m/z 714.1(M+,100%).IR(KBr):ν(cm-1): 1 635(CO).Anal.calcd.for C39H30O3Fe3·H2O(%):C, 63.97;H,4.41.Found(%):C,63.79;H,4.35.
1.5 X-ray crystal structure determination
X-ray crystallographic data[32]were collected on a Bruker SMART ApexⅡ CCD diffractometer using graphite-monochromated Mo Kα (λ=0.071 073 nm) radiation.The intensities were corrected for Lorentz polarization effects and empirical absorption with the SADABS program.The structures were solved by direct methods using the SHELXL-97 program.Crystal data, data collection parameters,and the results of the analyses of 2,3 and 4 are listed in Table 1.Selected bond distances and bond angles are listed in Table 2.
CCDC:813827,2;813828,3;802195,4.
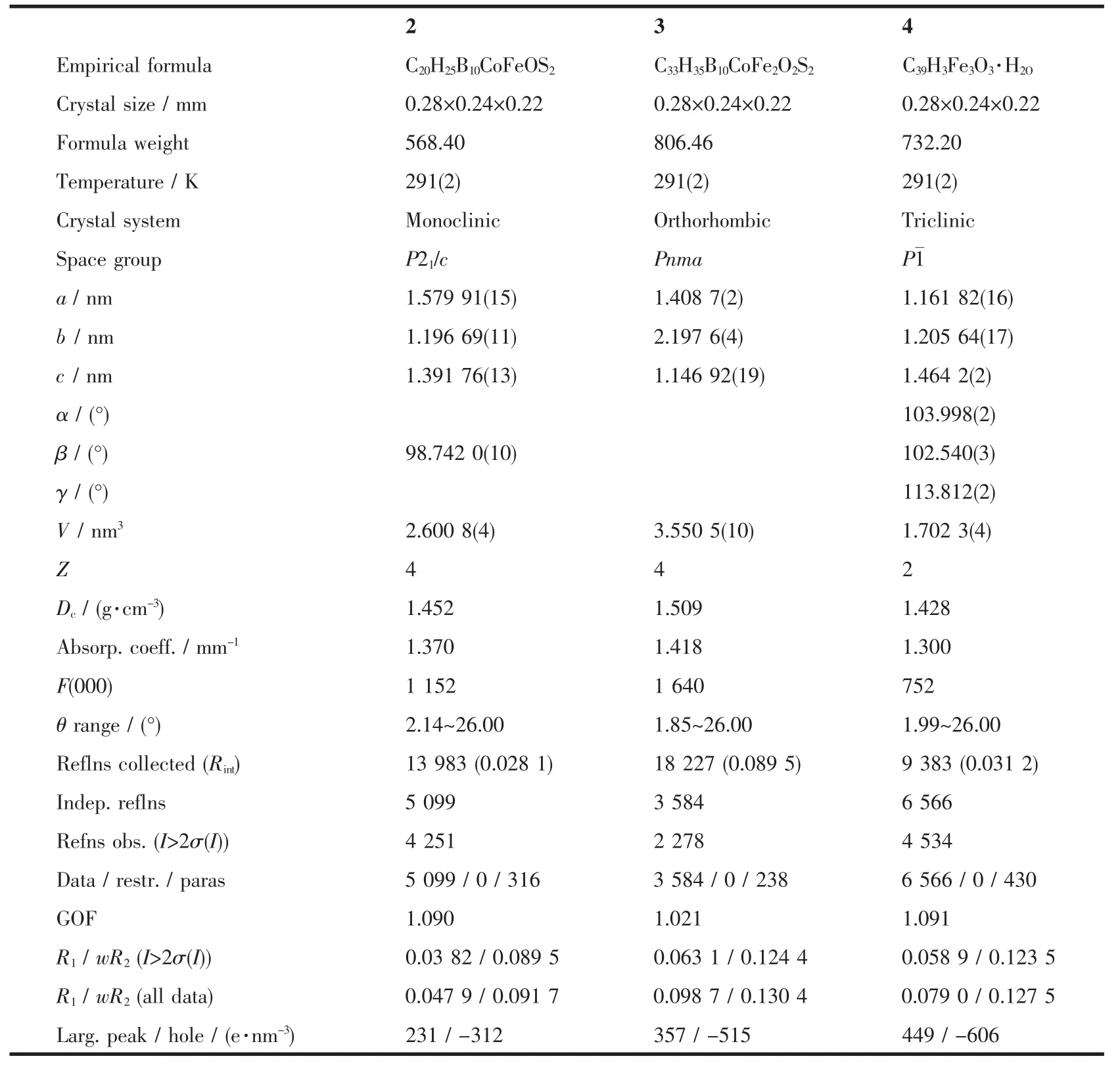
Table 1 Crystal and structure refinement data for complexes 2,3 and 4
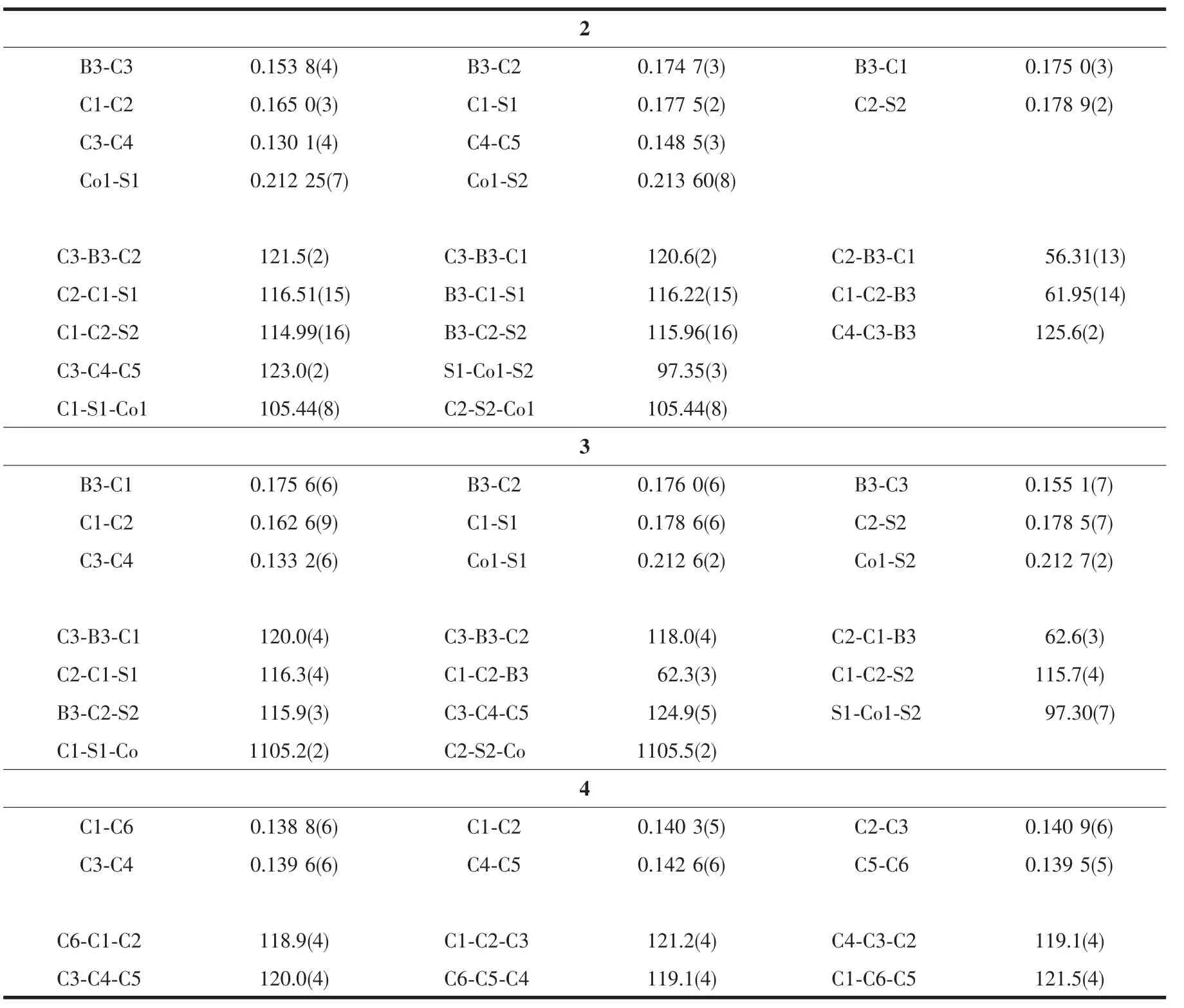
Table 2 Selected bond lengths(nm)and bond angles(°)for the complexes 2,3 and 4
2 Results and discussion
2.1 Synthesis of complexes
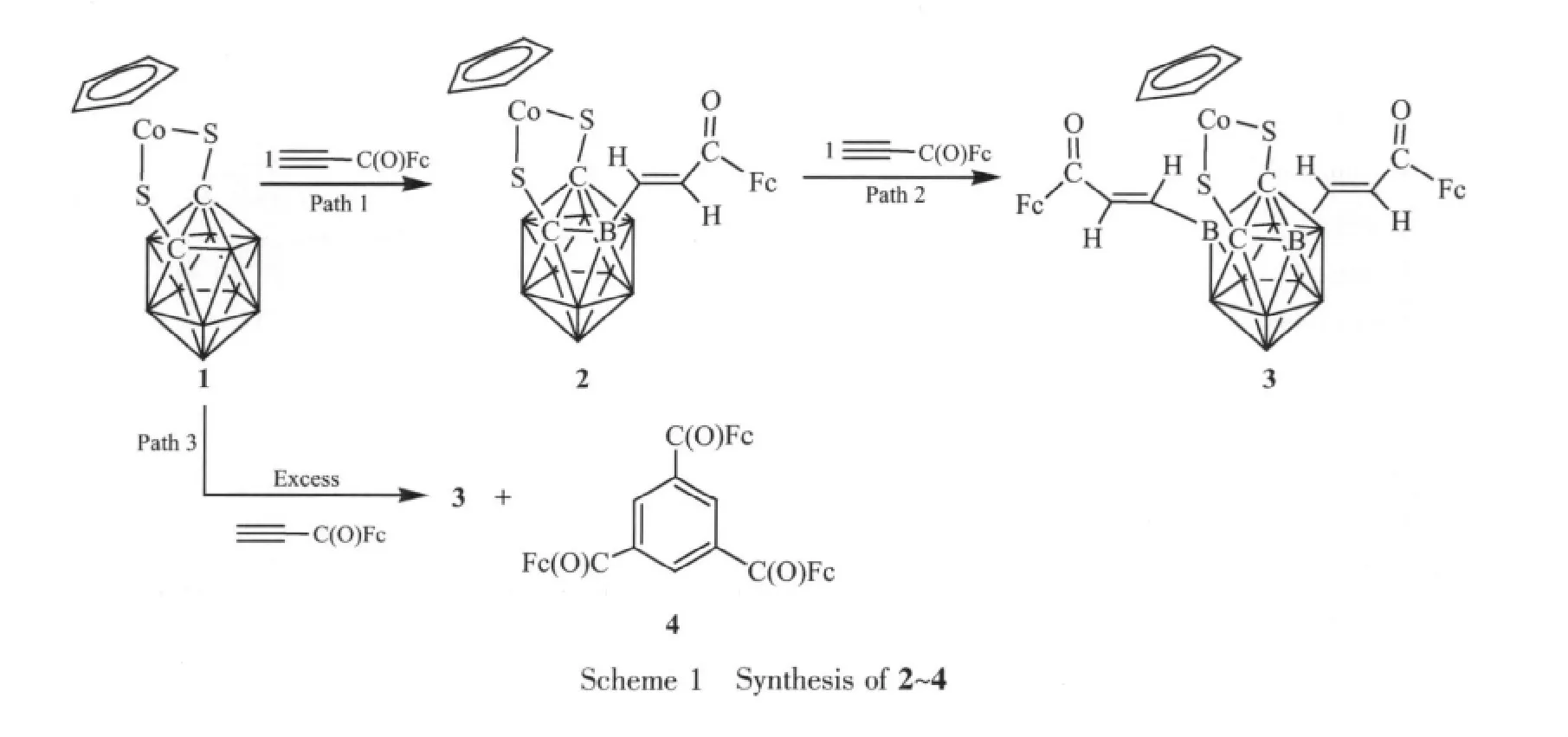
The reaction of 1 with HC≡CC(O)Fc in a ratio of 1∶1 in CH2Cl2at ambient temperature leads to 2 with a yield of 37%.2 could further react with the alkyne to generate 3 in a yield of 86%.Treatment of 1 with excess HC≡CC(O)Fc under the same reaction conditions affords 3 along with the alkyne trimerized compound 4 as a minor product in a yield of 5%(Scheme 1).
2.2 Structure of 2
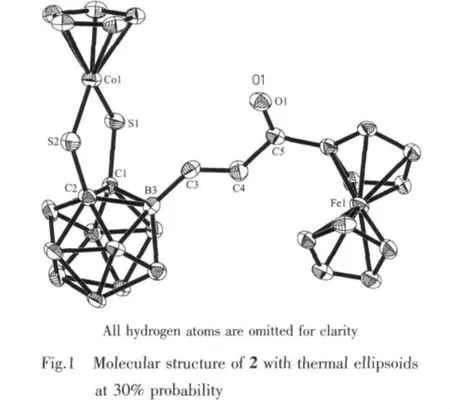
The single-crystal X-ray diffraction analysis shows that 2 crystallizes in P21/c space group.The molecular structure,as seen in Fig.1,shows that HC≡CC(O)Fc is added to the starting compound 1 at the B(3)site of the carborane cage.And the hydrogen atom of the BH bond has been transferred to the internal carbon of the alkyne to form the olefinic unit in an E configuration.As a result,the C≡C bond of the alkyne is reduced to a C=C bond(0.130 1 nm).The five-membered ring S(1)Co(1)S (2)C(2)C(1)is no longer planar which is slightly bent at the S(1)…S(2)vector with an angle of 175.0°owing to the alkyne addition.The1H NMR data demonstrate a B-monosubstituted product as well as reflected by the integrals.
2.3 Structure of 3
The single-crystal X-ray diffraction analysis shows that 3 crystallizes in Pnma space group.As shown in Fig.2,the second HC≡CC(O)Fc molecule is added to 2 at the B(6)site of carborane.The resulting two olefinic units take E/E configurations.3 is a symmetrical molecule,thus the five-membered ring S(1)Co(1)S(2)C (2)C(1)is planar.The1H NMR data show a B-disubstituted product as well,and both1H and13C data are parallel to those of the B-monosubstituted product 2.
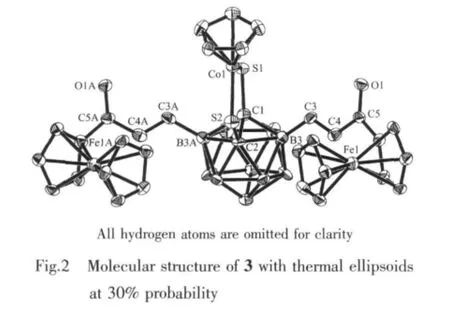
2.4 Structure of 4
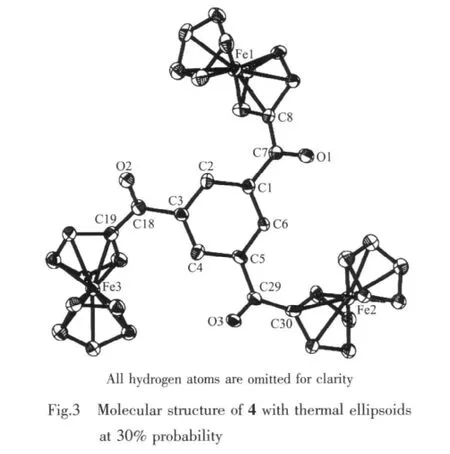
The single-crystalX-ray diffraction analysis indicates that 4 crystallizes in P1 space group.The molecular structure (Fig.3)shows that 2 is a 1,3,5-trisubstituted benzene derivative generated from the alkyne cyclotrimerization.Since 2 is symmetric,its1H NMR spectrum displays only one singlet at 8.84 ppm for the CH units in the benzene ring as well as one set of peaks from the ferrocenyl groups.The EI-MS measurement gives the M+peak at 714.1 with an abundance of 100%.
[1]Kim D H,Ko J,Park K,et al.Organometallics,1999,18:2738-2740
[2]Won J H,Kim D H,Kim B Y,et al.Organometallics,2002,21: 1443-1453
[3]Bae J Y,Lee Y J,Kim S J,et al.Organometallics,2000,19: 1514-1521
[4]Herberhold M,Jin G X,Yan H,et al.Eur.J.Inorg.Chem., 1999:873-875
[5]Meng X,Wang F,Jin G X.Coord.Chem.Rev.,2010,254: 1260-1272
[6]Herberhold M,Jin G X,Yan H,et al.J.Organomet.Chem., 1999,587:252-257
[7]Herberhold M,Yan H,Milius W.J.Organomet.Chem.,2000, 598:142-149
[8]Liu S,Zhang J S,Wang X,et al.Dalton Trans.,2006:5225-5230
[9]Wang J Q,Ren C X,Jin G X.Eur.J.Inorg.Chem.,2006:3274 -3282
[10]Wang J Q,Ren C X,Jin G X.Chem.Commun.,2005:4738-4740
[11]Han Y F,Zhang J S,Lin Y J,et al.J.Organomet.Chem., 2007,692:4545-4550
[12]Wang J Q,Ren C X,Weng L H,et al.Chem.Commun.,2006: 162-164
[13]Liu S,Wang G L,Jin G X.Dalton Trans.,2008:425-432
[14]Herberhold M,Yan H,Milius W,et al.Organometallics, 2000,19:4289-4294
[15]Herberhold M,Yan H,Milius W,et al.Chem.Eur.J.,2000,6: 3026-3032
[16]Herberhold M,Yan H,Milius W,et al.Z.Anorg.Allg.Chem., 2000,626:1627-1633
[17]Herberhold M,Yan H,Milius W,et al.J.Organomet.Chem., 2000,604:170-177
[18]Herberhold M,Yan H,Milius W,et al.Chem.Eur.J.,2002,8: 388-395
[19]Herberhold M,Yan H,Milius W,et al.Angew.Chem.,Int.Ed.,1999,38:3689-3691
[20]Herberhold M,Yan H,Milius W,et al.Dalton Trans.,2001: 1782-1789
[21]Herberhold M,Yan H,Milius W,et al.J.Organomet.Chem., 2001,623:149-152
[22]Xu B H,Wu D H,Li Y Z,et al.Organometallics,2007,26: 4344-4349
[23]Xu B H,Tao J C,Li Y Z,et al.Organometallics,2008,27:334-340
[24]Xu B H,Peng X Q,Li Y Z,et al.Chem.Eur.J.,2008,14: 9347-9356
[25]Wu D H,Wu C H,Li Y Z,et al.Dalton Trans.,2009:285-290 [26]Li Y G,Jiang Q B,Li Y Z,et al.Inorg.Chem.,2010,49:4-6
[27]Li Y G,Jiang Q B,Zhang X L,et al.Inorg.Chem.,2010,49: 3911-3917
[28]Li Y G,Ye H D,Guoyiqibayi G,et al.Sci.China Chem., 2010,53:2129-2138
[29]Ye H D,Ding G Y,Xie M S,et al.Dalton Trans.,2011,40: 2306-2313
[30]GULINSA Guoyiqibayi(古林莎·古依其巴依),ZHANG Rui (张锐),YAN Hong(燕红).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(5):733-743
[31]Barriga S,Marcos C F,Riant O,et al.Tetrahedron,2002,58: 9785-9792
[32]Bruker;SMART,Version5.0;SAINT,Version6;SHELXTL, Version6.1;SADABS,Version2.03.Bruker AXS Inc., Madison,WI,2000.
Selective Stepwise Substitution in B(3,6)Positions of 16e Half-Sandwich Complex CpCo(S2C2B10H10)
YE Hong-De1,2JIANG Qi-Bai1XIE Ming-Shi1DING Guan-Yu1LI Yi-Zhi1YAN Hong*,1
(1School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093,China)
(2School of Chemistry and Chemical Engineering,Shangrao Normal University,Shangrao,Jiangxi 334001,China)
The reaction of the 16e half-sandwich complex CpCo(S2C2B10H10)(Cp:cyclopentadienyl)(1)and the alkyne HC≡CC(O)Fc(Fc:ferrocenyl)in a molar ratio of 1∶1 leads to CpCo(S2C2B10H9)(CH=CHC(O)Fc)(2)in which thealkyneis coupled to theB(3)siteof the carborane cage thus resulting in the transferring hydrogen of B(3)-H to the internal carbon of the alkyne to form a olefinic substituent CH=CHC(O)Fc.2 could further react with a second HC≡CC(O)Fc to generate a B-disubstituted product CpCo(S2C2B10H8)(CH=CHC(O)Fc)2(3).3 is still a 16e compound with two CH=CHC(O)Fc olefinic substituents in E/E configurations at the B(3,6) sites.In the presence of excess alkyne,the reaction leads to 3 and the alkyne cyclotrimerization product 1,3,5-{HC=CC (O)Fc}3(4).Complexes 2,3,4 have been characterized by IR,NMR,elemental analysis,mass spectrometry and single-crystal X-ray diffraction analysis.CCDC:813827,2;813828,3;802195,4.
transition metal;carborane;B-H activation;crystal structure
O614.343
A
1001-4861(2011)08-1601-06
2011-03-08。收修改稿日期:2011-04-12。
国家自然科学基金(No.20925104、21021062),国家基础研究计划(No.2007CB925101、2010CB923303),江苏省自然科学基金(No.BK2010052),
教育部博士点基金(No.20090091110015)资助项目。
*通讯联系人。E-mail:hyan1965@nju.edu.cn,Tel:025-83686724
