基于双咪唑配体的一维银配位聚合物的合成、晶体结构和表征
2011-09-16赵丽娜陈李欣欣
赵丽娜陈 浩 李欣欣
(环境友好材料制备与应用省部共建教育部重点实验室,吉林师范大学化学学院,四平136000)
基于双咪唑配体的一维银配位聚合物的合成、晶体结构和表征
赵丽娜*陈 浩 李欣欣
(环境友好材料制备与应用省部共建教育部重点实验室,吉林师范大学化学学院,四平136000)
通过水热法合成了1个一维配位聚合物[Ag(1,2-bix)]·HL,并对该化合物进行了元素分析、红外和单晶X-射线表征(1,2-bix=1,2-(二亚甲基苯)二咪唑配体,HL=反式-4,4′-二羧基偶氮苯阴离子)。该化合物属于三斜晶系,空间群P1,晶胞参数a= 0.937 80(5)nm,b=1.119 91(16)nm,c=1.356 36(10)nm,α=102.923(8)°,β=91.751(5)°,γ=110.792(10)°,V=1.288 5(2)nm3,Z=2,C28H23AgN6O4,Mr=615.39,Dc=1.586 g·cm-3,F(000)=624,μ(Mo Kα)=0.829 mm-1,R=0.0407,wR=0.1020。在该化合物中,双咪唑配体连接着中心的Ag⑴原子形成了一维链状结构。二羧酸配体之间通过O-H…O键形成另一种一维链状结构,两种类型的链以相互垂直的方式延伸。此外,我们还仔细研究了该化合物的荧光性质。
配位聚合物;晶体结构;双咪唑配体;二羧酸
The design and synthesis of coordination networks are of considerable interest because of their potential application in the areas of catalysis,separation, sorption,sensors,and in electronic and magnetic devices[1-3].These coordination polymer networks can be specially designed by the careful selection of metal cations with preferred coordination geometries,the nature of the anions,the structure of the connecting ligands,and the reaction conditions[4-6].Among these, the selection of ligand is extremely important becausechanging their geometries can control the topologies of the resulting coordination frameworks.In this regard, carboxylate-based ligands have been successfully employed in the generation of many interesting systems[7].The work by Yaghi et al.has succeeded in highlighting the value of carboxylate-based systems in the generation of stable,highly porous,functionalized open networks[8].So far,aromatic polycarboxylate ligands,such as 1,4-benzenedicarboxylate,1,3-benzenedicarboxylate,1,3,5-benzenecarboxylate,and 1,2,4,5-benzenetetracarboxylate have been employed extensively in the construction of a variety of highdimensional structures during the last decade[8].The azo-based carboxylates represent one type of bridging aromatic carboxylate ligand employed in the generation of coordination networks.However,as far as we know, they are rarely used in the construction of coordination polymers[9].In this work,we selected 4,4′-azodibenzoic acid(H2L)as an carboxylate and 1,2-bis(imidazole-1-ylmethyl)benzene(1,2-bix)as a N-donor bridging ligand,generating a new one-dimensional coordination polymer,[Ag(1,2-bix)]·HL(1).
1 Experimental
1.1 Generals
The 1,2-bix ligand was synthesized according to the reported document[10]and all other materials were analytical reagent grade and used as received without further purification.Elemental analysis was carried out with a Perkin-Elmer 240C analyzer;IR spectra were obtained on a Perkin-Elmer 2400LSII spectrometer. The luminescent properties of K2L,1,2-bix and compound 1 were measured on a Perkin-Elmer LS55 spectrometer.
1.2 Synthesis and crystal grow th
The pH value of a mixture of AgNO3·2H2O(0.5 mmol),H2L(0.5 mmol),1,2-bix(0.5 mmol),and H2O (10 mL)was adjusted to between 5 and 6 by addition of triethylamine.The resultant solution was heated at 415 K in a Teflon-lined stainless steel autoclave for three days.The reaction system was then slowly cooled to room temperature.Yellow crystals of 1 suitable for single crystal X-ray diffraction analysis were collected from the final reaction system by filtration,washed several times with distilled water and dried in air at ambient temperature.Yield:29%based on Ag⑴.IR (KBr,cm-1):1 625m,1 587m,1 462m,1 423m,1 379m, 1 340m,1 309m,1 222w,1 091w,1 011w,873w,858w, 807w,790w,712w,621w,565w,507w,487w.Anal. Calcd.for C28H23AgN6O4(%):C,54.65;H,3.77;N, 13.66.Found(%):C,54.82;H,3.41;N,13.35.
1.3 X-ray structure determ ination
A single crystal with dimensions of 0.18 mm×0.17 mm×0.15 mm was selected and mounted on a Bruker Smart Apex CCD diffractometer equipped with a graphite-monochromatized Mo Kα(λ=0.071 073 nm) radiation by using an ω-2θ scanning method at a temperature of(20±2)℃.Out of the total 8 240 reflections collected in the 2.01°≤θ≤25.35°range, 4 701 were independent with Rint=0.038 0,of which 3 393 were considered to be observed(I>2σ(I))and used in the succeeding refinement.The structure was solved by Direct Method with SHELXS-97 program[11]and refined with SHELXL 97[12]by full-matrix leastsquares techniques on F2.All non-hydrogen atoms were refined anisotropically and hydrogen atoms isotropically.The final R=0.040 7 and wR=0.010 2(w= 1/[σ2(Fo2)+(0.063 7P)2],where P=(Fo2+2Fc2)/3).S=0.940, (Δρ)max=1.022,(Δρ)min=-0.646 e·nm-3and(Δ/σ)max= 0.001.Crystal data as well as details of the data collection and refinement for the title complex are summarized in Table 1.
CCDC:836464.
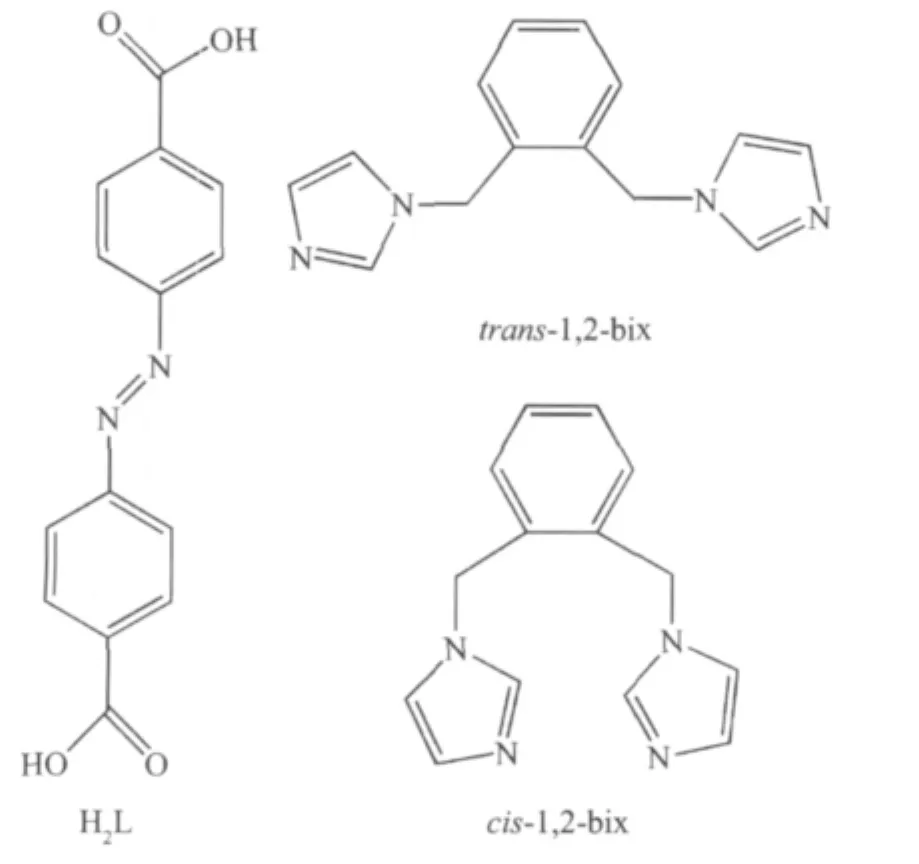

Table 1 Crystal data and the structure refinement for the title complex
2 Results and discussion
2.1 Description of crystal structure
As shown in Fig.1,there are one free HL-anion and one[Ag(1,2-bix)]+cation in the asymmetric unit of 1.The Ag⑴atom is two-coordinated by two N atoms from two different 1,2-bix ligands in a linear mode.The H2L ligand is partially deprotonated,and acts as a counter-anion.The Ag-N distance is 0.234 4(4)nm, which is near to the ones reported in{Ag2(H2MDIP)(1,2-bix)2·H2O}n(H4MDIP=methylenediisophthalic acid)[4]. The 1,2-bix ligands bridges two Ag⑴atoms in a bridging mode to form a one-dimensional chain with the Ag…Ag separation of 1.356 4 nm(Fig.2).Notably,the neighboring HL-anions form another type of onedimensional chain through O-H…O hydrogen bond (O(2)-H(2A)0.082 nm,H(2A)…O(3)iii0.164 nm,O(2)…O(3)iii0.245 2(4)nm,O(2)-H(2A)…O(3)iii=169.8°; symmetry code:iiix-1,y-1,z-1)(Fig.3).Interestingly, the two types of one-dimensional chains extend each other in vertical modes(Fig.4).So far,although a number of compounds with two types of chain structures have been reported,the ones containing two types of vertical chains are rarely observed[4-10].
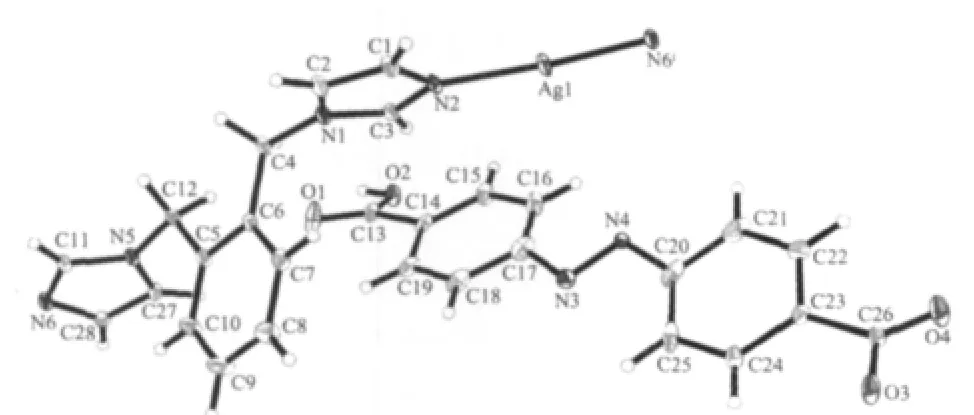
Fig.1 Coordination environment of Ag⑴atom in 1
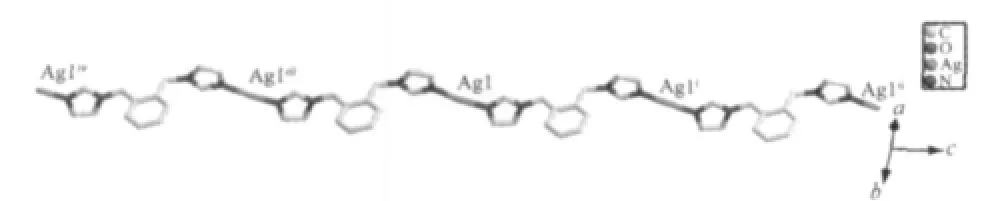
Fig.2 View of the one-dimensional chain constructed byAg⑴and 1,2-bix
It should be pointed out that the flexible 1,2-bix is capable of adopting either cis or anti conformations depending on the orientation of the two imidazole arms. As can be seen in complex 1,the 1,2-bix ligands bridge Ag⑴atoms exclusively through the anti conformation, whereas the cis conformation was observed in reported document[9].The anti conformation observed in compound 1 indicates that the conformation of the 1,2-bix is strongly dependent upon the organic anions used.The detailed investigation on the conformation of the 1,2-bix is under progress in our laboratory.
2.2 IR analysis

Fig.3 View of the one-dimensional chain constructedthrough interlayer O-H…O interactions
Fig.4 View of the two types of chains extended each other in vertical modes
In the IR spectrum of the compound 1,the characteristic absorption bonds associated with the azo-dibenzoate ligand(HL)appear at 1625 and 1587 cm-1for νassym(O-C-O),and 1340 and 1 379 cm-1for νsym(O-CO).The peaks at 565~487 cm-1are assigned to ν(Ag-N). Peak at 1462 cm-1could be assigned to ν(C=N) stretching vibration of the 1,2-bix ligand.The peaks around 1423 cm-1can be ascribed to the N=N stretch of azobenzoates.
2.3 Lum inescent properties
Luminescent properties of coordination polymers are very attractive due to their various applications in chemical sensors,photochemistry and electroluminescence[13-14].The solid-state luminescent properties of K2L and compound 1 have been investigated in the solid state at room temperature.Their emission and excitation peaks are shown in Fig.5.According to the reported document[5],the 1,2-bix ligand exhibits a fluorescent emission band at 468 nm,while the K2L shows an emission band at 490 nm(λex=365 nm).The emission bands for the 1,2-bix and K2L are attributable to the π*→n or π*→π transitions.The emission spectrum for compound 1 shows a main peak at 425 nm (λex=332 nm).However,the emissions arising from the 1,2-bix and K2L are not observable for the compound 1. The absence of ligand-based emission suggests energy transfer from the ligands to the Ag⑴center during photoluminescence.The result indicates that the luminescence of compound 1 is Ag-based emission.

Fig.5 Solid state photoluminescent spectra of K2L(a)and compound 1(b)at room temperature
[1]Batten S R,Robson R.Angew.Chem.Int.Ed.,1998,37:1460-1494
[2]Dinolfo P H,Hupp J T.Chem.Mater.,2001,13:3113-3125
[3]Pan L,Liu H,Lei X,et al.Angew.Chem.Int.Ed.,2003,42: 542-546
[4]Cheng X,Liu T,Duan X,et al.CrystEngComm,2011,13: 1314-1321
[5]Liu Y Y,Ma J F,Yang J,et al.Inorg.Chem.,2007,46:3027-3037
[6]Kong Z G,Ma X Y,Xu Z L.Z.Naturforsch.,2010,65b:1173-1176
[7]Qiao Q,Wu G Q,Tang T D,et al.Acta Crystallogr.,2009,C65: m146-m148
[8]Yaghi O M,Li G,Li H.Nature,1995,378:703-706
[9]Chen Z F,Zhang Z L,Tan Y H,et al.CrystEngComm,2008, 10:217-231
[10]Tan H Y,Zhang H X,Ou H D.Inorg.Chim.Acta,2004,357: 869-874
[11]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of Grttingen,Germany,1997.
[12]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Structure,University of Grttingen,Germany,1997.
[13]Liu G X,Xu Y Y,Nishihara S,et al.Russ.J.Coord.Chem., 2010,36:739-745
[14]HU Bin(胡斌),QU Zhi-Rong(瞿志荣).Chinese J.Inorg. Chem.(Wuji Huaxue Xuebao),2007,23(2):283-285
Synthesis,Crystal Structure and Characterization of a One-Dimensional Ag⑴Coordination Polymer Based on Bis(im idazole)Ligand
ZHAO Li-Na*CHEN Hao LI Xin-Xin
(Key Laboratory of Preparation and Applications of Environmental Friendly Materials, Department of Chemistry,Jilin Normal University,Siping,Jilin 136000,China)
A complex,[Ag(1,2-bix)]·HL(1)(H2L=4,4′-azodibenzoic acid and 1,2-bix=1,2-bis(imidazole-1-ylmethyl)benzene)has been hydrothermally synthesized and characterized by elemental analysis,IR and singlecrystal X-ray diffraction.It crystallizes in triclinic system,space group P1 with a=0.93780(5)nm,b=1.11991(16) nm,c=1.356 36(10)nm,α=102.923(8)°,β=91.751(5)°,γ=110.792(10)°,V=1.288 5(2)nm3,Z=2,C28H23AgN6O4, Mr=615.39,Dc=1.586 g·cm-3,F(000)=624,μ(Mo Kα)=0.829 mm-1,R=0.0407 and wR=0.1020.In this compound, the 1,2-bix ligands bridge the neighboring Ag⑴atoms to generate a one-dimensional chain structure with the Ag…Ag separation of 1.3564 nm.The neighboring HL ligands form another type of one-dimensional chain through O-H…O hydrogen bond.Interestingly,the two types of one-dimensional chains extend each other in vertical modes.In addition,the luminescent property of 1 has been studied in detail.CCDC:836464.
coordination polymer;crystal structure;bis(imidazole);dicarboxylic acid
O614.122
A
1001-4861(2011)11-2263-04
2011-03-22。收修改稿日期:2011-06-21。
环境友好材料制备与应用省部共建教育部重点实验室研究基金资助项目。*
。E-mail:zhaolina1975@yahoo.com.cn
