基于萘二甲酸及邻菲咯啉衍生物的铅配位聚合物的合成及结构
2011-09-16徐占林贺逄金慧孔治国
徐占林贺 宇 逄金慧 孔治国
(吉林师范大学化学学院,吉林师范大学环境友好材料制备与应用省部共建教育部重点实验室,四平 136000)
基于萘二甲酸及邻菲咯啉衍生物的铅配位聚合物的合成及结构
徐占林*贺 宇 逄金慧 孔治国
(吉林师范大学化学学院,吉林师范大学环境友好材料制备与应用省部共建教育部重点实验室,四平 136000)
利用水热合成法得到了二维配位聚合物[Pb2(L)(1,4-NDC)2]·0.5H2O(1),并对该化合物进行了元素分析、红外、热重和单X-射线表征,其中L为邻菲咯啉衍生物配体(1-(1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)naphthalen-2-ol),1,4-NDC为1,4-萘甲酸根。该化合物属于三斜晶系,空间群P1,晶胞参数a=0.95078(6)nm,b=1.26511(8)nm,c=1.785 91(12)nm,α=78.441(2)°,β 78.620(1)°,γ=77.124(1)°,V=2.0253(2)nm3。该化合物为二维层状结构,层与层之间又进一步地通过π-π相互作用形成二维双超分子结构。
配位聚合物;晶体结构;邻菲咯啉衍生物;1,4-萘二甲酸
0 Introduction
The design and synthesis of polymeric complexes has developed rapidly in recent years due to their potential applications in some fields,such as gas storage,size-and shape-selective catalysis,material science,medicine and magnetism,as well as the intriguing variety of architectures and topologies[1-[4].1,4-Naphthalenedicarboxylic acid(1,4H2NDC)is a versat ile ligand for designing new coordination polymeric architectures.Up to now,a number of complexes with 1,4-NDC have been found to display diverse structure types,but only a few coordination polymers with both 1,4-NDC and chelating N,N′-based ligands have been reported[5].
The preparation of ordered functional crystalline solids,which display a variety of well-defined supramolecular architectures mediated by supramolecular interactions,is currently of great interest[6].Up to now, 1,10-phenanthroline(phen)has been widely used to build supramolecular architectures because of its excellent coordinating ability and large conjugated system that can easily form π-π interactions[7].However, to the best of our knowledge,coordination polymers based on its derivative 1-(1H-imidazo[4,5-f][1,10] phenanthrolin-2-yl)naphthalen-2-ol(L),are rarely reported[8-15].In this work,we selected 1,4-NDC as an organic linker and L as a N-donor chelating ligand, generating a new two-dimensional coordination polymer,[Pb2(L)(1,4-NDC)2]·0.5H2O(1).
1 Experimental
1.1 Generals
The L ligand was synthesized according to the reported method[12]and all other materials were analytical reagent grade and used as received without further purification.Elemental analysis was carried out with a Perkin-Elmer 240C analyzer;IR spectra were obtained on a Perkin-Elmer 2400LSII spectrometer.Thermogravimetric analysis(TGA)was performed on a Perkin-Elmer TG-7 analyzer heated from 40 to 600℃under nitrogen gas.
1.2 Synthesis and crystal grow th
A mixture of Pb(NO3)2(0.5 mmol),1,4-H2NDC(0.5 mmol)and L(0.5 mmol)was dissolved in 12 mL distilled water.The pH value of the mixture was adjusted to between 5 and 6 by addition of triethylamine.The resultant solution was heated at 460 K in a Teflon-lined stainless steel autoclave for six days.The reaction system was then slowly cooled to room temperature.Pale yellow crystals of 1 suitable for single crystal X-ray diffraction analysis were collected from the final reaction system by filtration,washed several times with distilled water and dried in air at ambient temperature.Yield:42%based on Pb(Ⅱ).IR(KBr, cm-1):3034w,1612s,1 580m,1 541m,1 464m,1 377w, 1 340m,1 121w,845m,730w,713w,621w.Anal.Calcd. For C94H53N8O19Pb4(%):C,46.53;H,2.18;N,4.62. Found(%):C,46.70;H,2.28;N,4.36.
1.3 X-ray structure determ ination
A single crystal with dimensions of 0.22 mm×0.20 mm×0.15 mm was selected and mounted on a Bruker-AXS Smart CCD diffractometer equipped with a graphite-monochromatized Mo Kα(λ=0.071 073 nm) radiation by using an ω-2θ scanning method at a temperature of(20±2)℃.Out of the total 10558 reflections collected in the 1.67°≤θ≤26.05°range,7 784 were independent with Rint=0.019 5,of which 5 871 were considered to be observed(I>2σ(I))and used in the succeeding refinement.The structure was solved by Direct Method with SHELXS-97 program[16]and refined with SHELXL 97[17]by full-matrix least-squares techniques on F2.All non-hydrogen atoms were refined anisotropically and hydrogen atoms isotropically.The H atoms of water molecule were not located from difference Fourier map.The final R=0.035 8 and wR=0.0758 (w=1/[σ2(Fo2)+(0.042 1P)2],where P=(Fo2+2Fc2)/3).S= 0.986,(Δρ)max=1.234,(Δρ)min=-0.902 e·nm-3and(Δ/σ)max=0.001.
CCDC:833352.
2 Results and discussion
2.1 Description of crystal structure
The selected bond distances and angles are listed in Table 1.The asymmetric unit of 1 contains two unique Pb(Ⅱ)atoms,one L molecule,and two unique 1,4-NDC ligands(Fig.1).The Pb1 centre is sevencoordinated by two N atoms from one L ligand,and five carboxylate O atoms from three different 1,4-NDC ligands(Fig.1).However,the Pb2 centre is fivecoordinated by five carboxylate O atoms from three different 1,4-NDC ligands.The Pb-O distances range from 0.234 3(4)to 0.284 8(5)nm,which are near to the data reported for[Pb2(ndc)2(tcpp)](tcpp=4-(1H-1,3,7,8-tetraazacyclopenta[l]phenanthren-2-yl)phenol)[12].It isinteresting to note that the two types of 1,4-NDC ligands (1,4-NDC1 and 1,4-NDC2)show different coordination modes(Fig.2).Each carboxylate of the 1,4-NDC1 bridges two Pb(Ⅱ)atoms in a chelating-bridging tridentate mode,while the one of the 1,4-NDC2 connects one Pb(Ⅱ)atom in a chelating mode.As such, the 1,4-NDC1 ligands connect neighboring Pb(Ⅱ)atoms to form a two-dimensional layer(Fig.2).Notably,the L ligands are only attached on one side of the layer.The L ligands from neighboring layers are well matched, allowing the formation of the aromatic π-π stacking interactions.The π-π stacking interactions between the quinoline ring(N3/C15-C18 at(x,y,z))and naphthalene ring(C27iii-C30iiiat(-1-x,1-y,-z))of the L ligands(centroid-to-centroid distance of ca.0.360(4) nm,face-to-face distance of ca.0.342(6)nm,and dihedral angle of 1.111(2)°),extended the adjacent layers into a two-dimensional supramolecular double layer structure(Fig.3).Obviously,the strong aromatic π-π stacking interactions play an important role in stabilizing the supramolecular architecture of 1.Finally, the N-H…O hydrogen bond(d(N4-H4)=0.086,d(H4…O9)=0.195,d(N4…O9)=0.252 7(8)nm,N4H4O9= 123.7°)further stabilizes the structure of 1.It is noteworthy that the structure of 1 is entirely different from that of the related compound[Pb(L)2(1,4-NDC)][12]. In that reported compound,the 1,4-NDC ligands linked the Pb(Ⅱ)atoms to generate a one-dimensional chain structure.Theπ-πstacking interactions among neighboring chains extend the chains into a twodimensional supramolecular layer structure.
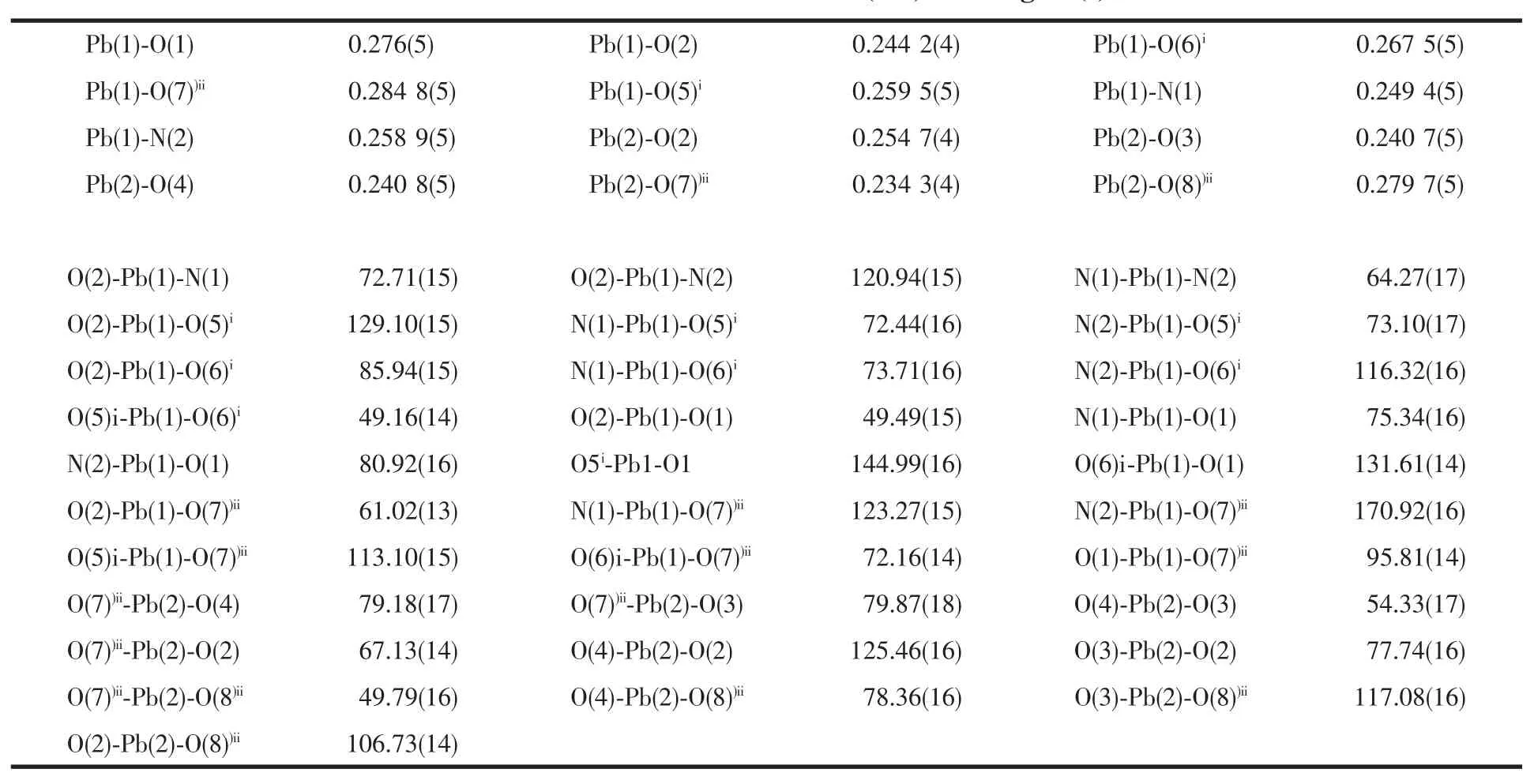
Table 1 Selected bond distances(nm)and angles(°)
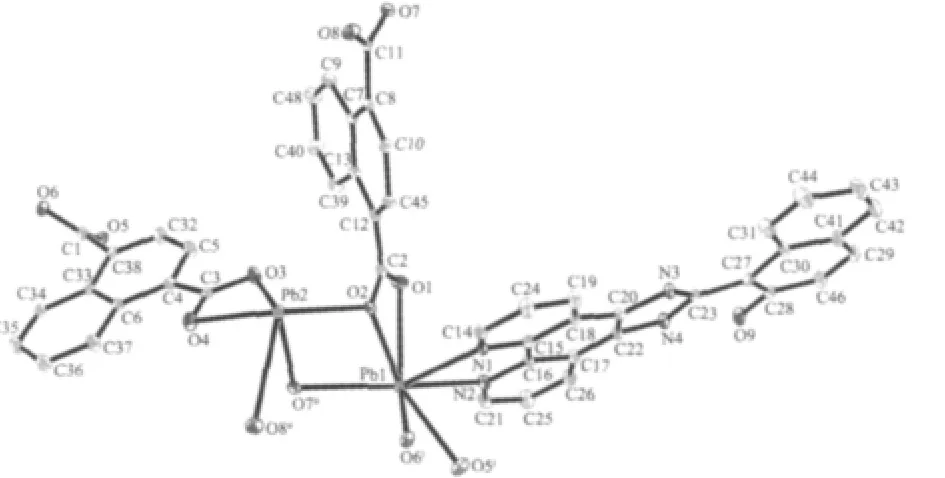
Fig.1 Coordination environments of Pb(Ⅱ)atoms in complex 1 with displacement ellipsoids at 30% probability level
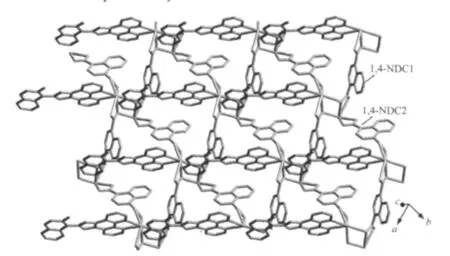
Fig.2 View of the layer structure of complex 1

Fig.3 View of the double layer architecture of 1 constructed through interlayer π-π interactions
2.2 IR analysis
The carboxylate group of the 1,4-NDC anion is coordinated with its asymmetric and symmetric stretching appearing at 1 612,1 580 cm-1(ν(OCO)assym) and 1340,1377 cm-1(ν(OCO)sym),respectively.Peak at 1 464 cm-1could be assigned toν(C=N)stretching vibration of the phen-like ligand L.The absence of characteristic bands at about 1 700 cm-1in the compound indicates the complete deprotonation of 1,4-NDC ligand upon reaction with Pb(Ⅱ)atoms.
2.3 Thermogravimetric analysis
In order to characterize the compounds more fully in terms of thermal stability,the thermal behavior of 1 was examined by TG.The experiments were performed on samples consisting of numerous single crystals under N2atmosphere with a heating rate of 10℃·min-1,in temperatures ranging from room temperature to 600℃(Fig.4).The weight loss corresponds to the release of water molecule in the range of 35~125℃(obsd.1.21%, calcd.0.74%).The host framework starts to be decomposed beyond 300℃,and end before 515℃.However, it is difficult to determine these weight losses accurately as these processes are overlapped with the weight losses due to the dissociation of the organic fractions.The remaining weight loss of 38.50%corresponds to the values for PbO(calcd.36.77%).This result is in good accordance with the composition of the complex.

Fig.4 TG curve of complex 1
[1]Ferey G.Chem.Mater.,2001,13:3084-3098
[2]Batten S R,Robson R.Angew.Chem.Int.Ed.,1998,37:1460-1494
[3]Dinolfo P H,Hupp J T.Chem.Mater.,2001,13:3113-3125
[4]Pan L,Liu H,Lei X,et al.Angew.Chem.Int.Ed.,2003,42: 542-546
[5]Blake A J,Champness N R,Hubberstey P,et al.Coord.Chem. Rev.,1999,183:117-138
[6]Wang X L,Chen Y Q,Gao Q,et al.Cryst.Growth Des.,2010, 10:2174-2184
[7]Kong Z G,Ma X Y,Xu Z L.Z.Naturforsch.,2010,65b:1173-1176
[8]Qiao Q,Wu G Q,Tang T D,et al.Acta Crystallogr.,2009,C65: m146-m148
[9]HU Bin(胡斌),QU Zhi-Rong(瞿志荣).Chinese J.Inorg. Chem.(Wuji Huaxue Xuebao),2007,23(2):283-285
[10]Zhang X M,Tong M L,Gong M L,et al.Eur.J.Inorg.Chem., 2003,1:138-142
[11]Chen X M,Liu G F.Chem.Eur.J.,2002,18:4811-4817
[12]Yang J,Li G D,Cao,J J,et al.Chem.Eur.J.,2007,13:3248-3261
[13]Yang J,Ma J F,Liu Y Y,et al.Cryst.Growth Des.,2009,9: 1894-1911
[14]Fan J,Sun W Y,Okamura T,et al.New J.Chem.,2002,2: 199-201
[15]Zhou Y F,Zhao Y J,Sun D F,et al.Polyhedron,2003,22: 1231-1235
[16]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of Grttingen,Germany,1997.
[17]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Structure,University of Grttingen,Germany,1997.
Synthesis and Structure of a Two-Dimensional Pb(Ⅱ)Coordination Polymer w ith Naphthalenedicarboxylic Acid and Phenanthroline Derivative Ligand
XU Zhan-Lin*HE Yu PANG Jin-Hui KONG Zhi-Guo
(Department of Chemistry,Jilin Normal University,Key Laboratory of Preparation and Applications of Environmental Friendly Materials(Jilin Normal University),Ministry of Education,Siping,Jilin 136000,China)
A coordination polymer,[Pb2(L)(1,4-NDC)2]·0.5H2O(1)(L=1-(1H-imidazo[4,5-f][1,10]phenanthrolin-2 yl)naphthalen-2-ol and 1,4-H2NDC=1,4-naphthalenedicarboxylic acid)has been hydrothermally synthesized an characterized by elemental analysis,IR,thermogravimetric analysis(TGA),and single-crystal X-ray diffraction. crystallizes in triclinic system,space group P1 with a=0.95078(6)nm,b=1.26511(8)nm,c=1.78591(12)nm,α 78.441(2)°,β=78.620(1)°,γ=77.124(1)°,V=2.025 3(2)nm3,In compound 1,the 1,4-NDC ligands bridged th neighboring Pb(Ⅱ)atoms to yield a two-dimensional layer structure.The π-π stacking interactions between ligands extended the adjacent layers into a two-dimensional supramolecular double layer.The N-H…O hydroge bond further stabilizes the structure of 1.CCDC:833352.
coordination polymer;crystal structure;1,10-phenanthroline derivative;1,4-naphthalenedicarboxylic acid
O614.43+3
A
1001-4861(2011)11-2279-04
2011-03-05。收修改稿日期:2011-06-21。
四平市研究基金和吉林师范大学研究生创新基金资助项目。
