Quiet rest ameliorates biochemical metabolism in the brain in a simple concussion rabbit model Evaluation of hydrogen proton magnetic resonance spectroscopy*☆
2011-07-27LinOuyangYuhuiXiaoCuiYueJunpengMaRongyueShiLinGaoYiheGuoJiarongMengQianxinJia
Lin Ouyang, Yuhui Xiao, Cui Yue, Junpeng Ma, Rongyue Shi, Lin Gao, Yihe Guo,Jiarong Meng, Qianxin Jia
1Department of Radiology, the 175 Hospital of Chinese PLA (Southeast Hospital Affiliated to Xiamen University), Zhangzhou 363000, Fujian Province, China
2Department of Pathology, the 175 Hospital of Chinese PLA (Southeast Hospital Affiliated to Xiamen University), Zhangzhou 363000, Fujian Province, China
INTRODUCTION
An increasing number of studies have shown that simple concussion induces long-term neurocognitive disorder and psychiatric sequelae, including a variety of physical discomforts (such as headaches, dizziness,and fatigue), cognitive impairment (such as attention difficulties, memory loss, decline in program and organizational capacity), and behavioral/emotional abnormalities (such as irritability, mood swings, and depression)[1-8].The previous studies have primarily focused on post-concussion neuropsychological and psychological changes[9-10], neurobehavioral changes[11-13], and rehabilitation of athletes[7-8].
Previous studies have not detected macroscopic neuropathological abnormalities followings simple concussion,but rather reversible changes in brain function.However, it is important to note that conventional computer tomography (CT)and magnetic resonance imaging (MRI)often do not detect neuropathological abnormalities and function changes in simple concussion[6-8,14].However,pathophysiological changes following simple concussion include changes in glucose metabolism, cerebral blood flow, and axonal function[7,15-16].
The present study utilized hydrogen proton magnetic resonance spectroscopy (H-MRS)to analyze cerebral biochemical metabolite levels of N-acetyl aspartate (NAA), choline(Cho), creatine (Cr) and lactate (Lac) in a simple concussion rabbit model following three different therapies, including quiet rest,hyperbaric oxygen therapy, and interference stimulation.
RESULTS
Quantitative analysis of experimental animals
A total of 21 adult rabbits were randomly assigned to three groups: quiet rest,hyperbaric oxygen, and interference stimulation.Therapeutic interventions were initiated after the simple concussion models were established.A total of 20 rabbits resulted in successful model establishment with a success rate of 95%.One rabbit model failed due to brain contusion, as detected by conventional CT and MRI examination.The 20 successful rabbit models underwent H-MRS and diffuse tensor imaging (DTI) detection, and four died from excessive anesthetic or long-term anesthesia.In total, 16 rabbits were included in the final analysis, which included six rabbits in the quiet rest group, five rabbits in the hyperbaric oxygen group, and five rabbits in the stimulation group.
Brain biochemical metabolites in simple concussion rabbits
H-MRS detection of brain metabolites revealed 2.02 ppm NAA, 3.03 ppm Cr, 3.20 ppm Cho, and 1.33 ppm Lac, respectively.Following simple concussion, peak values of NAA and Cho significantly decreased (P<0.05), and Cr peak values significantly increased (P<0.05) in brain tissues of rabbits.Following quiet rest or hyperbaric oxygen therapy, NAA and Cho peak values returned to normal levels (P> 0.05), but these changes were not detected following interference stimulation(P< 0.05).
A comparative analysis of NAA/Cr and Cho/Cr showed that NAA/Cr and Cho/Cr ratios increased to the normal levels in the quiet rest group and hyperbaric oxygen group, but the difference was not statistically significant(P> 0.05).In the interference stimulation group,NAA/Cr and Cho/Cr ratios remained unchanged (data not shown).H-MRS spectra are shown in Figure 1 and measurement results are shown in Table 1.
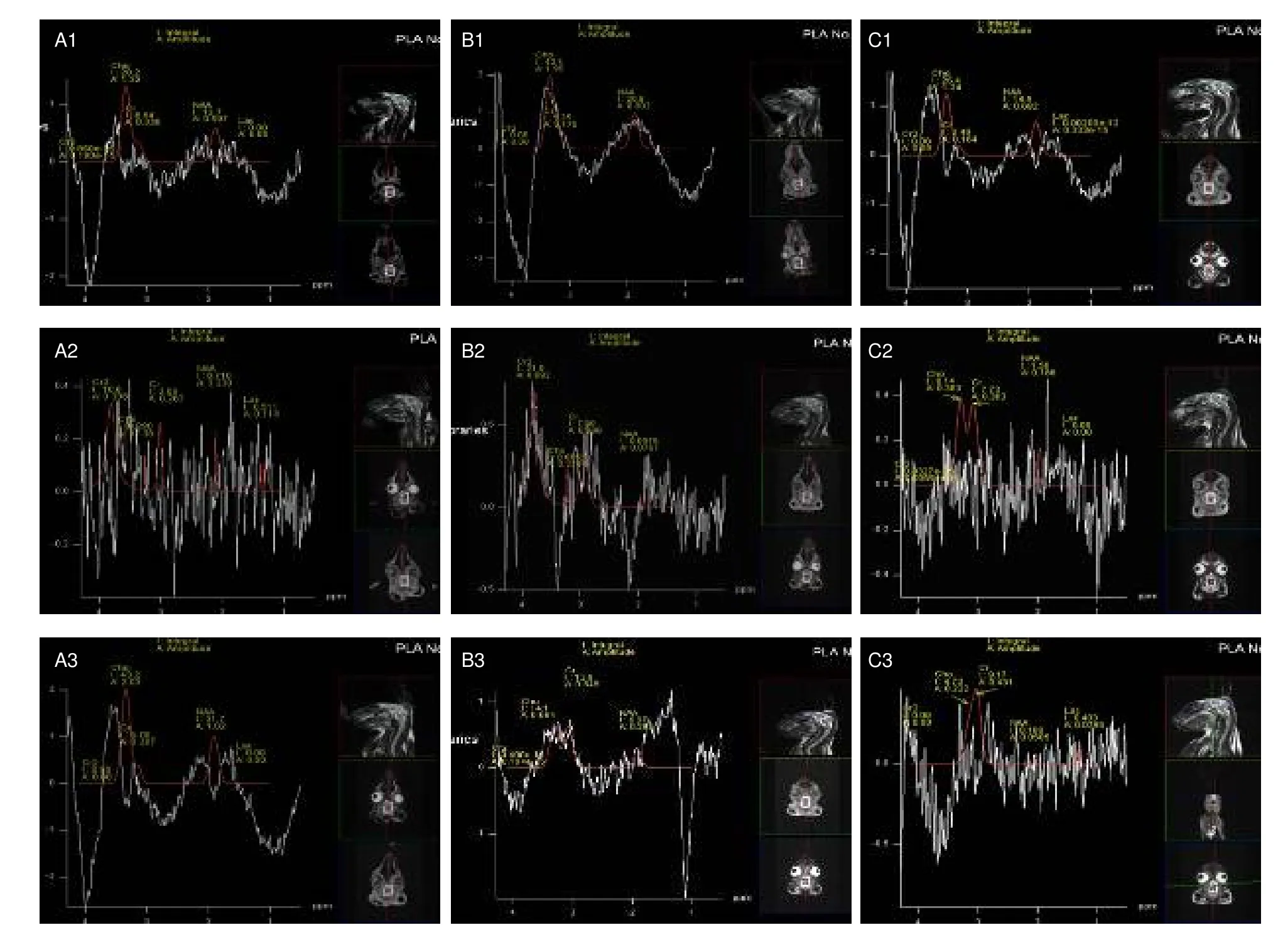
Figure 1 Hydrogen proton magnetic resonance spectroscopy detection of biochemical metabolites in brains of simple concussion rabbits.NAA: N-acetyl aspartate; Cho: choline; Cr: creatine; Lac: lactate.
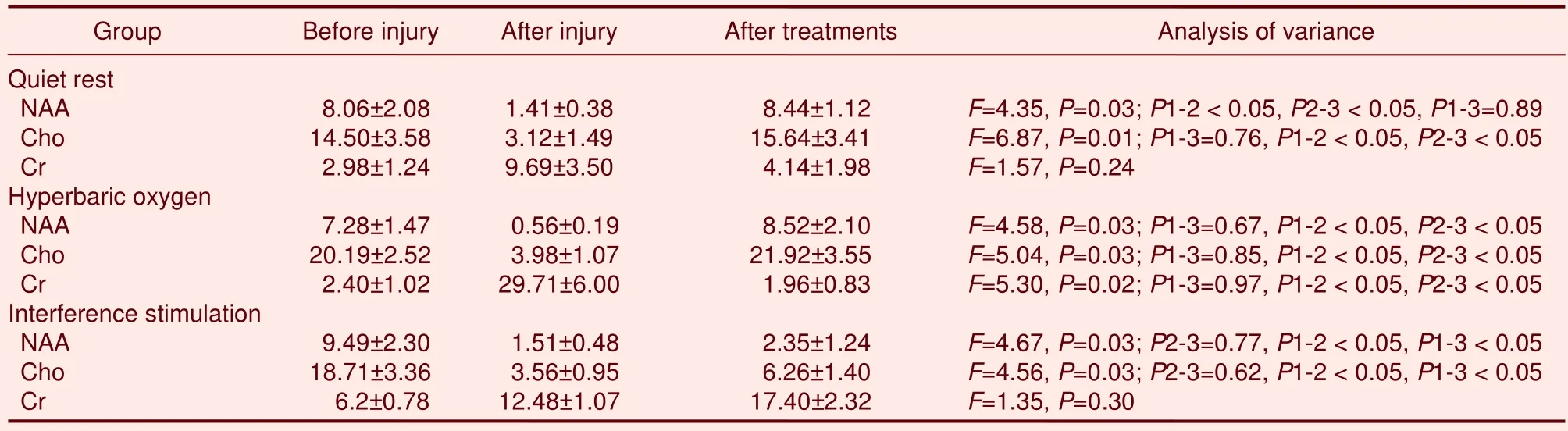
Table 1 Changes of biochemical metabolites (mmol)in brains of simple concussion rabbits before and after injury, as well as following treatment with quiet rest, hyperbaric oxygen, or interference stimulation
DISCUSSION
Results from the present study showed that: (1) levels of biochemical metabolites were immediately altered after simple concussion; (2) amelioration of abnormal changes was dependent on the treatment.A quiet rest environment proved to be conducive for recovering biochemical metabolism in the brain, while interference stimulations delayed or impeded recovery of these abnormal changes.
Based on a previously described impact/compression trauma model[17-18], models of simple concussion were established in the present study using a sliding slope,which was similar to a deceleration injury induced by a car accident, falling, or collision.Using this model, the shortcomings of deceleration injury models, which include limited stress to the skull, small areas of brain damage, and regional cerebral contusion, could be avoided.Initial results showed that impact at a > 40°slope gradient induced death in the experimental rabbits,while < 10° a slope gradient resulted in no significant difference of spectral content of NAA, Cho or Cr prior to and after modeling.Therefore, the present study model was established with a 20° slope gradient.As previously described[6-8], simple concussion diagnosis was assessed by cognitive performance, conventional imaging, and pathology.This model exhibited several advantages, such as ease of operation, large stress area to the skull, and injury similar to head deceleration injury.However, the degree of injury is difficult to control in this model.
Results from the present study revealed significantly decreased levels of NAA (NAA/Cr) and Cho (NAA/Cr) in brains of simple concussion rabbits compared with pre-injury levels, although Cr content significantly increased after injury.Previous studies have utilized magnetic resonance spectroscopy to detect decreased NAA values in brain injury patients[19-23].Cellular energy becomes depleted post-injury, because NAA synthesis is a consuming process of ATP energy.In addition,following injury, mitochondrial dysfunction induced multiple metabolic disorders and inhibited NAA synthesis and metabolism, which do not result in neuronal or axonal injury[19-23].Findings from the present study were consistent with the above-described studies.Cr levels increased following injury, which suggested energy depletion.However, Cho content results were not consistent with previous studies, which have reported increased Cho levels following brain injury[19-20,24].Maintenance of brain energy metabolism balance in crucial for the clinical treatment of brain injury[25-26].In the present study, both quiet rest and oxygen therapy resulted in satisfactory recovery effects.However,hyperbaric oxygen therapy is not convenient for treating at the site of concussion.Therefore, these results suggested that the best treatment for concussion on site is quiet rest[27].
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
Experiments were performed in the Center Laboratory,Southeast Hospital Affiliated to Xiamen University, China from August 2010 to May 2011.
Materials
A total of 21 six-month-old, healthy, New Zealand white rabbits, of either sex and weighing 2.5 kg, were provided by the Center Laboratory, Southeast Hospital Affiliated to Xiamen University, China, No.SYXK (Army)2007-032.Experimental procedures were in strict accordance with theGuidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[28].
Methods
Establishment of simple concussion models
Rabbits were anesthetizedviaa 0.1-0.2 mL/kg gluteal muscle injection of veterinary Sumianxin II (according to Chinese Veterinary Pharmacopoeia, license No.veterinary (2009) 070011582; Jilin Huamu Animal Health Product, Jilin Province, China).The rabbits were fixed onto a pulley, which was placed on the top of slope[17-18].Because of the small size and flat distribution of rabbit brains, the area below the roofing bone represented the maximum brain area, so the rabbit mouth was adducted and fixed to the chest, with the brain facing down.The skull region between the ears was placed opposite to the baffle at the slope bottom, and the pulley locations on the slope were regulated to ensure that a maximal area of rabbit roofing bone would strike the baffle.The slope gradient was set to 20° and the slope length was 60.0 cm.The pulley fixtures were loosened to allow for free sliding to produce a simple concussion.
Anesthetized rabbits that awoke by 20 minutes after Sumianxin injection were considered to be successful models of brain concussion.The successful models were returned to an unconsciousness state, but exhibited corneal reflex, pain response to acupuncture, and external auditory meatus response to stimulation disappearance.Routine CT scan and MRI (T1WI, T2* WI,SWI) were immediately performed to rule out the existence of intracranial hemorrhage or brain contusion.Rabbits were sacrificed under anesthesia following experiment completion.The brains were harvested and fixed in 5% formalin.Prior to pathological examination,general observation was conducted to detect edema and/or hemorrhage.The median maximal sagittal plane of the entire brain was then incised, and brain tissues were cut into 2-mm thick slices parallel to the median sagittal plane, followed by dehydration, transparency,de-waxing, embedding, and hematoxylin-eosin staining.Simple concussion was diagnosed as the appearance of no hemorrhage by general observation (Figure 2A).Brain parenchyma was observed under optical microscope (hematoxylin-eosin staining, × 200; BX50,Olympus, Japan), and cells exhibited mild swelling with no other abnormalities (Figure 2B).
Interventions immediately after simple concussion(acute stage)
Quiet rest: the rabbits were housed in separate cages in a dark, well-ventilated room with no sound or light stimulation for 60 minutes.
Hyperbaric oxygen therapy: the rabbits received hyperbaric oxygen therapy in a chamber (GYS-16B,Jiujiang Shipping Machinery Manufacturing Factory,Jiujiang City, China) with 0.2 MPa pressure and 93±3%concentration for 60 minutes.
Interference stimulation: the rabbits received interferences that simulated daily life conditions and emergent medical care without therapeutic measures for simple concussion.This was meant to simulate patients returning to work, sports, and life.The injured rabbits were placed in a noisy, constantly lit room, with 120 dB noise and 100 W light.The rabbits were forced to run within the room for 5 minutes, followed by 5 minutes of rest (two cycles around the room, total length was 20 ×2 m.Treatment lasted for 60 minutes.
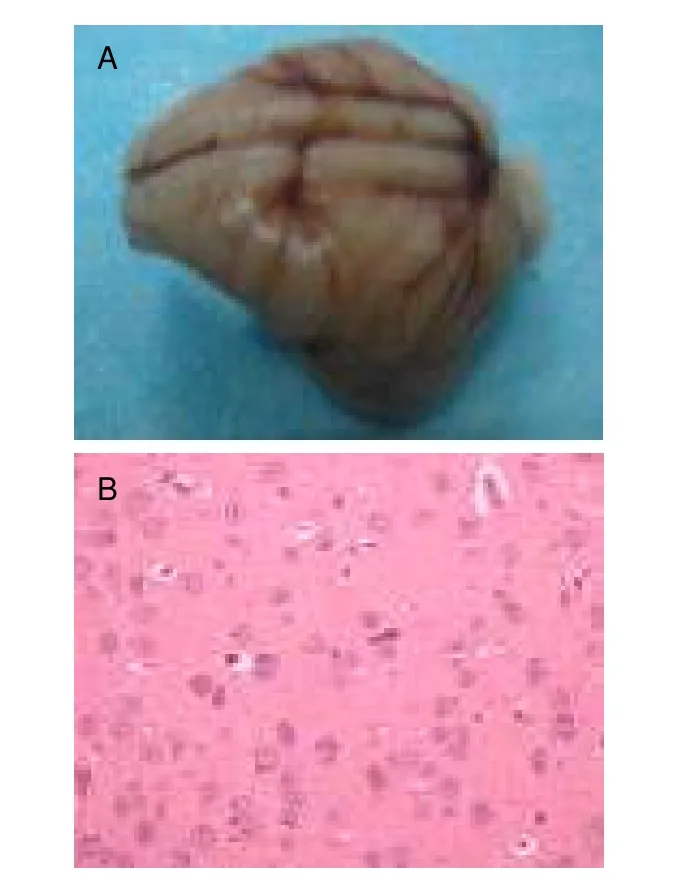
Figure 2 Pathological determination of simple concussion.
H-MRS examination
H-MRS was performed prior to injury, immediately after injury, and 1 hour after injury.Single voxel detection was used to avoid multi-voxel pollution of extracellular signals from the receptor voxel.The region of interest for spectral acquisition included the parenchyma under the skull (corresponding to the rabbit ears).Scanning parameters: REF positioning mode, 3: 44 acquisition time, 15 × 10 × 10 mm volume, 3.4 mm × 3.4 mm × 5 mm voxel, 26.27orotation, 1 700 ms/135 ms TR/TE, 128 average, 3 acquisition times, normalize filter pre-scan,advanced shim mode, adjusted water suppression frequency to 50 Hz, 1 200 Hz band width, and 1 H reference amplitude.
The spectral line of single voxel acquisition was processed using a 3.0T magnetic resonance instrument(Siemens, Trio, German), and collected data was used to automatically generate the spectral line.According to previously described studies[17-18], NAA, Cho, Cr, and Lac release are closely related to brain trauma and served as evaluation indices.The integral values were automatically calculated after MRS processing and values were compared as follows: before injury and after injury; after treatment and after injury; after treatment and before injury.
Statistical analysis
Data were analyzed using SPSS 17.0 statistical software and measurement data were expressed as mean±SD.MRS measurements of NAA (NAA/Cr), Cho (Cho/Cr), Cr,and Lac prior to injury, after injury, and after treatment were compared using two-way analysis of variance.A value ofP< 0.05 was considered statistically significant.
Author contributions:Ouyang Lin was responsible for study concept and design, data analysis, manuscript writing and validation, statistical analysis, and funding.Yuhui Xiao was responsible for MRI and MRS technical operations and data acquisition.Cui Yue was responsible for CT operations.Rongyue Shi was responsible for anesthesia and fixation,simple concussion injury, and animal sacrifice.Lin Gao participated in treatment after injury.Yihe Guo was responsible for craniotomy and brain harvesting.Jiarong Meng was responsible for staining, sectioning, and pathological diagnosis of injured rabbit brains.Qianxin Jia was involved in data integration, provided MRS technical support, and instructed the study.
Conflicts of interest:None declared.
Funding:This study was supported by the Military Medical Science and Technology Research During the Eleventh Five-Year Plan, No.06MA96.
Ethical approval:The experiment was approved by Animal Ethics Committee, Xiamen University School of Medicine,China.
Acknowledgements:We would like to thank Minfeng Zhang,director of the Pathology Department, for help in brain pathological diagnosis, as well as Yongshi Hu, director of Science and Education Office, and Jiming Zhang from the Animal Laboratory Center, Southeast Hospital Affiliated to Xiamen University, China.
[1] Kirsch NL, de Leon MB, Maio RF, et al.Characteristics of a mild head injury subgroup with extreme, persisting distress on the Rivermead Postconcussion Symptoms questionnaire.Arch Phys Med Rehabil.2010;91(1):35-42.
[2] Heitger MH, Jones RD, Macleod AD, et al.Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability.Brain.2009;132(Pt 10):2850-2870.
[3] Meares S, Shores EA, Taylor AJ, et al.Mild traumatic brain injury does not predict acute postconcussion syndrome.J Neurol Neurosurg Psychiatry.2008;79:300-306.
[4] Chen JK, Johnston KM, Petrides M, et al.Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms.Arch Gen Psychiatry.2008;65(1):81-89.
[5] Powell GE.Mild traumatic brain injury and postconcussion syndrome: the importance of base rates in diagnosis and clinical formulation.J Neurol Neurosurg Psychiatry.2008;79:237.
[6] DeMatteo CA, Hanna SE, Mahoney WJ, et al.“My Child Doesn’t Have a Brain Injury, He Only Has a Concussion”.Pediatrics.2010;125:327-334.
[7] Meehan III WP, Bachur RG.Sport-related concussion.Pediatrics.2009;123:114-123.
[8] Mayers L.Return-to-play criteria after athletic concussion: a need for revision.Arch Neurol.2008;65(9):1158-1161.
[9] Vaishnavi S, Rao V, Fann JR.Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic.Psychosomatics.2009;50(3):198-205.
[10]Bombardier CH, Fann JR, Temkin NR, et al.Rates of major depressive disorder and clinical outcomes following traumatic brain injury.JAMA.2010;303(19):1938-1945.
[11]Catena RD, van Donkelaar P, Chou LS.The effects of attention capacity on dynamic balance control following concussion.J Neuroeng Rehabil.2011;8:8.
[12]Bazarian JJ, Blyth B, Mookerjee S, et al.Sex differences in outcome after mild traumatic brain injury.J Neurotrauma.2010;27(3):527-539.
[13]Catena RD, van Donkelaar P, Chou LS.Different gait tasks distinguish immediate vs.long-term effects of concussion on balance control.J Neuroeng Rehabil.2009;6:25.
[14]Makdissi M.Sports related concussion - management in general practice.Aust Fam Physician.2010;39(1-2):12-17.
[15]Bazarian JJ, McClung J, Cheng YT, et al.Emergency department management of mild traumatic brain injury in the USA.Emerg Med J.2005;22:473-477.
[16]Pleacher MD, Dexter WW.Concussion management by primary care providers.Br J Sports Med.2006;40:e2.
[17]Foda MA, Marmarou A.A new model of diffuse brain injury in rats.Part II: Morphological characterization.J Neurosurg.1994;80(2):301-313.
[18]Marmarou A, Foda MA, Vanden Brink W, et al.A new model of diffuse brain injury in rats.Part I: Pathophysiology and biomechanics.J Neurosurg.1994;80(2):291-300.
[19]Yang ZH, Feng F, Wang XY.A Guide to Technique of Magnetic Resonance Imaging-Criterion of Examination Clinical Strategy and Application of New Techniques.Beijing: People’s Military Medical Press.2007.
[20]Brandao LA, Domingues RC.MR Spectroscopy of the Brain.Tianjin: Tianjin Technology Translation Publication Company.2005.
[21]Zhang Y, Li HT, Xie B, et al.Multivoxel 1H-MRS for contusion and laceration area of mild to moderate traumatic brain injury with in adults.Disan Junyi Daxue Xuebao.2009;31(2):172-174.
[22]Zhang Y, Li HT.Study and applicaton foreground of proton magnetic resonance spectroscopy in brain contussion and laceration.Xiandai Shengwu Yixue Jinzhan.2008;8(5):967-969
[23]Shu XJ, Liu W, Zhou HY, et al.Hippocampus neuronal lesion in postconcussion syndrome rat by patho-counting and H-magnetic resonance spectroscopy.Jianghan Daxue Xuebao: Ziran Kexue Ban.2010;38(1):74-78.
[24]Schuhmann MU, Stiller D, Skardlly M, et al.Long-time in vivo metabolic monitoring following experimental brain contusion using proton magnetic resonance spectroscopy.Acta Neurochir Suppl.2002;81:209-212.
[25]Sharma S, Zhuang Y, Ying Z, et al.Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma.Neuroscience.2009;161(4):1037-1044.
[26]Dietrich WD, Bramlett HM.The evidence for hypothermia as a neuroprotectant in traumatic brain injury.Neurotherapeutics.2010;7(1):43-54
[27]McCrory P, Meeuwisse W, Johnston K, et al.Consensus statement on concussion in sport-the 3rdInternational Conference on Concussion in Sport held in Zurich, November 2008.J Clin Neurosci.2009;16(6):755-763.
[28]The Ministry of Science and Technology of the People’s Republic of China.Guidance Suggestions for the Care and Use of Laboratory Animals.2006-09-30.
杂志排行
中国神经再生研究(英文版)的其它文章
- Effects of Fujian tablet on Nogo-A mRNA expression and plasticity of the corticospinal tract in a rat model of focal cerebral ischemia*☆
- Sidiming attenuates morphine withdrawal syndrome and nitric oxide (synthase) levels in morphine-dependent rats and rhesus monkeys*★
- Dynamic analysis of 10 components of the Chinese herbal compound Wuzhuyu-tang absorbed into rat plasma**☆
- Effects of Shuyusan on monoamine neurotransmitters expression in a rat model of chronic stress-induced depression*★
- Effects of electroacupuncture at Zusanli (ST 36) on neurons in the colonic myenteric plexus in rats with irritable bowel syndrome with constipation*★
- Magnetic resonance imaging characteristics of postoperative intracranial dissemination of recurrent gliomas*****☆
