Effects of Shuyusan on monoamine neurotransmitters expression in a rat model of chronic stress-induced depression*★
2011-07-27
Department of Neurology, South Building of Chinese PLA General Hospital, Beijing 100853, China
INTRODUCTION
Depression is a common disease that threatens human health.Approximately 200 in 100 000 people have the disease, and 10-15% commit suicide[1-2].An increasing use of traditional Chinese medicine for depression treatment has shown that traditional prescription drugs exhibit certain clinical efficacy, enhance efficacy, and reduce dose and side effects of common medicines in combination with otheranti-depressants.In addition, traditional Chinese medicine allows for a greater number of treatments for special types of depression[3].Therefore, the combination of traditional Chinese medicine and modern experimental research methods could benefit the development of depression therapeutics.
Most antidepressant treatment studies using Chinese medicine have focused on clinical observations and relevant animal studies have observed animal behavioral changes[4].Shuyusan, a traditional Chinese medicine,exhibits some therapeutic effects on depression[5].Previous animal studies have shown the anti-depressant effects ofShuyusanbased on behavioral improvement in rats with depression[6].In addition, studies have demonstrated that monoamine neurotransmitters and their metabolites play an important role in the molecular mechanisms of depression onset[7-9].Some studies have attempted to analyze anti-depressant treatment of Chinese medicine by measuring neurotransmitter expression changes in half of the brain or the entire brain[10-12].However, very little is known regarding neurotransmitter metabolite content or neurotransmitter expression levels in various brain regions related to depression,such as the hypothalamus, corpus striatum and hippocampus.The present study measured 5-hydroxytryptamine (5-HT),5-hydroxyindoleacetic acid (5-HIAA),noradrenaline (NE), 3-methoxy-4-hydroxyphenylglycol (MHPG), dopamine(DA), and homovanillic acid (HVA) levels in the hypothalamus, corpus straitum, and hippocampus to determine the effects ofShuyusanon monoamine neurotransmitter and metabolite expression in a rat model of chronic stress-induced depression.
RESULTS
Quantitative analysis of experimental animals
A total of 40 rats were equally and randomly assigned to model, control, high-doseShuyusan(5.0 g/mL), low-doseShuyusan(0.5 g/mL), and Amitriptyline groups.The model group was fed normally and subjected to stress stimulation for 21 days,while the control group was normally fed without any treatment.The high- and lowdoseShuyusangroups were administered 4 mLShuyusan, which contained 5.0 and 0.5 g/mL crude drug, respectively,viagastric perfusion once per day[13].The Amitriptyline group was perfused with 10 mg/kg Amitriptyline dissolved in 2 mL normal saline following model establishment.The rats were treated at 22 days after model establishment for a total of 22 days.All 40 rats were included in the final analysis.
Effects of Shuyusan on a rat model of chronic stress
At 21 days post-stress stimulation, compared with the control group, all other rats exhibited obvious praxiological changes, manifested by significantly reduced horizontal and vertical activity scores in the open field test (P< 0.01).At 21 days post-drug treatment,horizontal and vertical activity scores significantly increased in the high- and low-doseShuyusangroups,as well as the Amitriptyline group, compared with the model group (P< 0.01; supplementary Table 1 online).At 21 days post-drug treatment, swimming frequency significantly increased in the high- and low-doseShuyusanand Amitriptyline groups compared with the model group (P< 0.01; supplementary Table 2 online).
Effects of Shuyusan on monoamine neurotransmitter expression in a rat model of chronic stress
At 21 days post-drug treatment, high-performance liquid chromatography methodology was used for quantitative determination of monoamine neurotransmitters 5-HT and its metabolite 5-HIAA, NE and its metabolite MHPG, and dopamine and its metabolite HVA in the hypothalamus,corpus striatum, and hippocampus, respectively.
Measurements of monoamine neurotransmitters and their metabolites in the hypothalamus
Compared with the model group, 5-HT levels increased(P< 0.05) and 5-HIAA levels significantly increased (P<0.01) in the hypothalamus in rats from the high-doseShuyusan, low-doseShuyusan, and Amitriptyline groups,but levels remained similar to the control group.Compared with the model group, 5-hydrotryptophon levels significantly increased in the hypothalamus in the high-doseShuyusan(P< 0.01), Amitriptyline (P< 0.01),and low-doseShuyusan(P< 0.05) groups, but remained similar to the control group (P> 0.05).
There was no significant difference in DA, NE, or MHPG levels in the hypothalamus of rats in the high- and low-doseShuyusangroups compared with the model group (P> 0.05; Table 1).
Determination of monoamine neurotransmitter and their metabolite levels in the corpus striatum
Compared with the model group, 5-HT levels in the corpus striatum significantly increased in the high-doseShuyusan, low-doseShuyusan, and Amitriptyline groups(P< 0.01), but remained similar to the control group.5-HIAA levels in the corpus striatum significantly increased in the high-doseShuyusangroup compared with the model group (P< 0.01), but remained similar to the control group.In the high-doseShuyusangroup,5-hydrotryptophon levels in the corpus striatum increased compared with the model group (P< 0.05), but remained similar to the control group (P> 0.05).
Compared with the model group, there was no statistical difference in DA, homovanillic acid and dihydroxyphenyl acetic acid (DOPAC), HVA, NE, and MHPG levels in the corpus striatum in bothShuyusangroups (P> 0.05;Table 2).
Determination of monoamine neurotransmitter and their metabolite levels in the hippocampus
Compared with the model group, 5-HT levels in the hippocampus significantly increased in the high-doseShuyusan, low-doseShuyusan, and Amitriptyline groups (P< 0.01), with no difference between the groups.In the high-doseShuyusangroup, 5-HIAA levels significantly increased in the hippocampus compared with the model group (P< 0.01).In the highand low-doseShuyusangroups, 5-hydrotryptophon levels significantly increased in the hippocampus compared with the model group (P< 0.05), but there was no significant difference between the high-doseShuyusanand control groups (P> 0.05).
Compared with the model group, there was no significant difference in the content of DA, DOPAC, HVA,NE and MHPG in the hippocampus of high and low-doseShuyusangroups (P> 0.05; Table 3).
Compared with the model group, 5-HT synthesis in the hippocampus increased in the high-doseShuyusangroup (P< 0.05), but there was no difference between model and low-doseShuyusangroups.Although 5-HT degradation decreased, there was no statistical difference (P> 0.05; Table 4).
Effects of Shuyusan on pathological changes in a rat model of chronic stress
Hematoxylin-eosin staining of the frontal cortex and hippocampal CA1 and CA3 regions following Shuyusan treatment
The cells were slightly disordered in theShuyusanand Amitriptyline groups compared with the model group.The number of shrunken cells significantly decreased compared with the model group (Figures 1, 2).
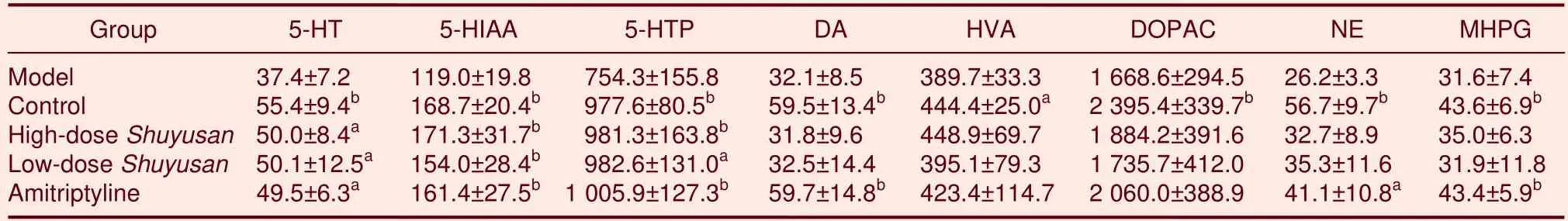
Table 1 Content of monoamine neurotransmitters and metabolites (ng/g)in the hypothalamus
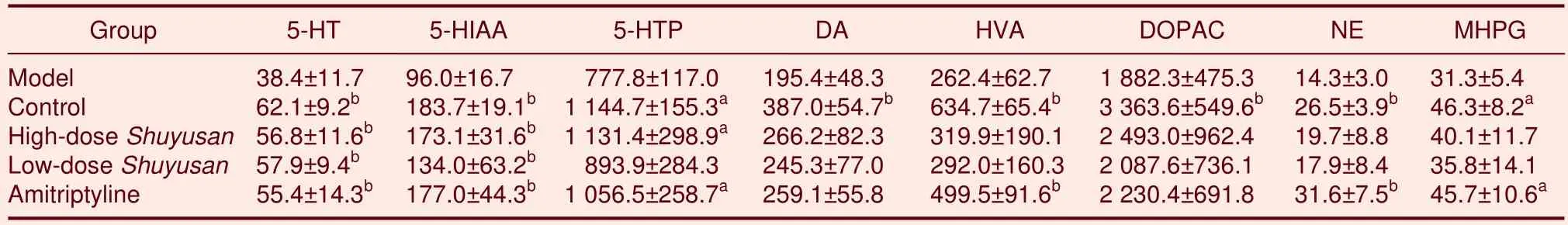
Table 2 Content of monoamine neurotransmitters and metabolites (ng/g)in the corpus striatum
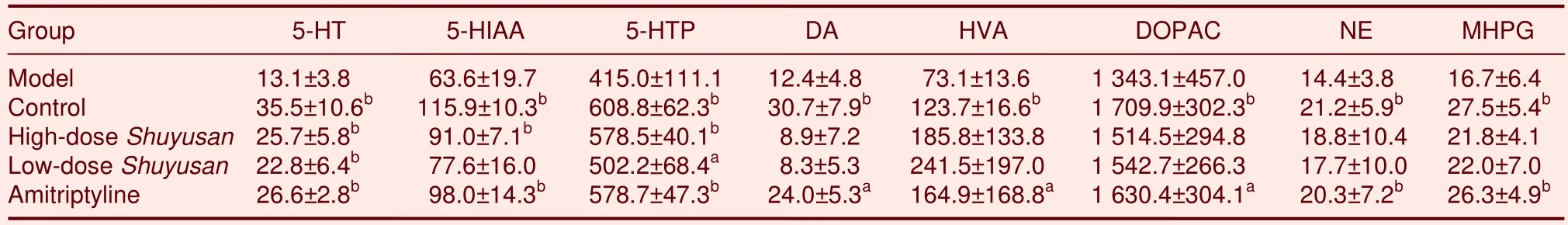
Table 3 Content of monoamine neurotransmitters and metabolites(ng/g)in the hippocampus
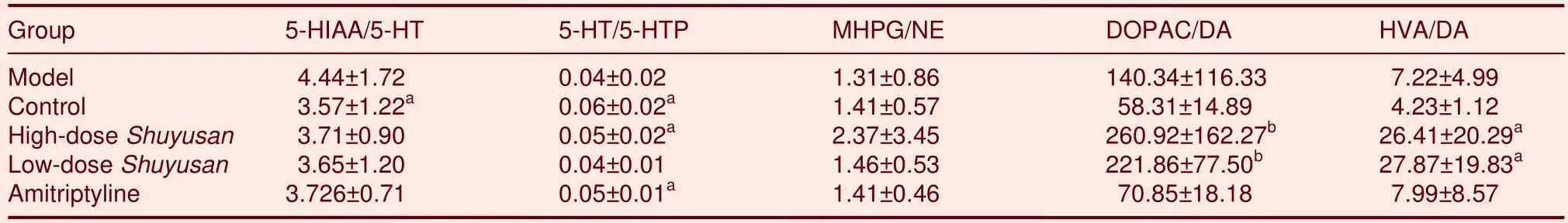
Table 4 Quantitative ratio between monoamine neurotransmitters and metabolites or precursors in the hippocampus
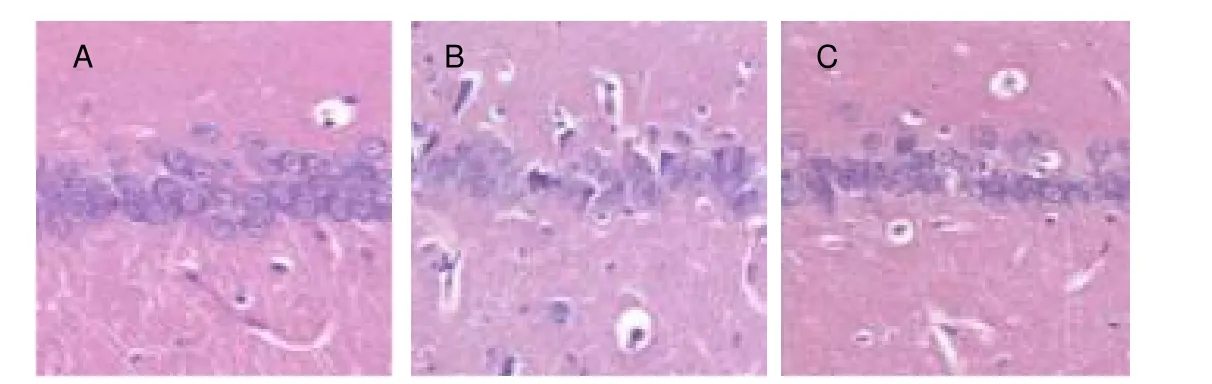
Figure 1 Pathological changes in the rat hippocampal CA1 region (hematoxylin-eosin staining, × 400).
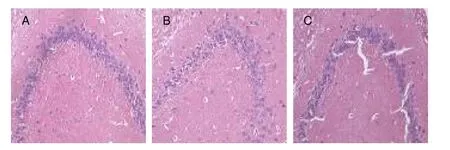
Figure 2 Pathological changes in the rat hippocampal CA3 region (hematoxylin-eosin staining, × 400).
Immunohistochemical results of 5-HT expression in hippocampal CA1 and CA3 regions following Shuyusan treatment
In the model group, the number of 5-HT-positive cells decreased in hippocampal CA1 and CA3 regions.5-HT expression in these areas significantly increased following treatment withShuyusancompared with the model group(P< 0.05 orP< 0.01; Figures 3, 4 and Table 5).

Figure 3 5-hydroxytryptamine (5-HT) expression in the rat hippocampal CA1 region (immunohistochemistry, × 400).Arrows indicate 5-HT positive neurons.

Figure 4 Changes in 5-hydroxytryptamine (5-HT) expression in the rat hippocampal CA3 region (immunohistochemistry, ×400).Arrows indicate 5-HT-positive neurons.
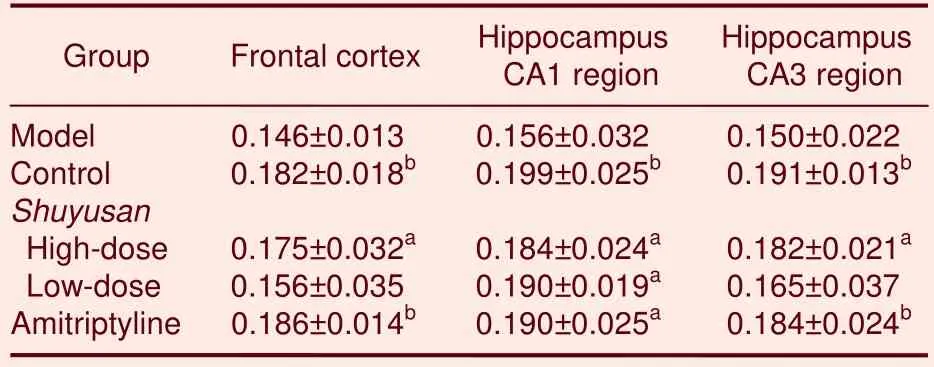
Table 5 Absorbance of 5-hydroxytryptamine-positive expression (/400- fold visual field) in the frontal cortex and hippocampus
DISCUSSION
In traditional Chinese medicine, depression or mental illness is considered to be a type of melancholy, which is commonly caused by emotional stress and injury as well as failure of liver catharsis function and stagnation of liverQi, energy flow, or life source.Illness is initially due to liver malfunctions and gradually affects heart and spleen functions as it worsens with disharmonized connection ofQibetween the organs[14].Differentiated treatments for depression have been based on subtypes of the illness.Treatments focus on the liver to smoothQiand releaseQistagnation if it is categorized asShi-zheng, while therapeutic efforts have been dedicated to alleviate deficiency and restore normal heart and spleen functions if depression is diagnosed asXu-zheng[15].Some commonly used and well-known prescriptions of traditional Chinese medicine for the treatment of depression include Bupleurum plus keel oyster soup,Xiaoyaosan,Banxiahoupusoup,YuejuPill,Bu Xin Tang, and Happy Powder.In addition, some physicians prescribe specific and customized medications based on personal knowledge and experiences.
Based on the above-mentioned traditional Chinese medicine theories, as well as achievements from modern scientific studies and clinical trials, the present study developed the prescriptionShuyusanto treat depression.Shuyusanexhibits therapeutic effects on depression by purifying the heart, resolving phlegm, regulatingQi, and alleviating melancholy associated with depression[5].Previous animal studies have revealed the anti-depressant effects ofShuyusanbased on behavioral improvement in rat models of depression[6].In addition,the rat model of depression induced by chronic,unpredictable, mild stress exhibits symptoms of depression, accompanied by other praxiological changes,including reduced exploratory behavior and spontaneous activities, as well as disordered sleeping patterns[16-19].The chronic stress-induced depression model is an effective model for studying depression and has been widely utilized in basic research and drug screening for depression[20].
The substrate tryptophan is used to synthesize 5-HT after hydroxylation, which is followed by decarboxylation by 5-hydroxytryptophon decarboxylase.5-HT does not easily traverse across the blood-brain barrier; therefore, 5-HT in the central nervous system is synthesized by tryptophan transported in bloodviathe blood-brain barrier.Under normal circumstances, the amount of tryptophan transported into serotonergic neurons determines the rate of 5-HT synthesis[21].In a stress state,hypothalamic-pituitary-adrenal axis function becomes hyperactive and 5-HT synthesis significantly decreases as a result of insufficient tryptophan transported into the central nervous system.Insufficient tryptophan is due to tryptophan degradation in plasma by liver tryptophan pyrrolase, which is induced by increased glucocorticoids.Previous results have shown that 5-HT synthesis is due to a reduced proportion of tryptophan and other neutral amino acids in the blood, as well as decreased tryptophan transported from the blood to the brain in patients with depression[22].Results from the present study demonstrated thatShuyusanincreased expression of the 5-HT precursor in the hypothalamus, corpus striatum, and hippocampus in a rat model of depression.Shuyusanincreased 5-HT synthesis in the hippocampus, thereby improving depressive symptoms in the rats.Results suggested that the mechanisms of action ofShuyusanwere due to increased 5-hydrotryptophon content, which further led to increased 5-HT synthesis.Nevertheless,further studies are needed to clarify these mechanisms.Following oxidative deamination by monoamine oxidase,5-HT becomes 5-hydroxyindole acetic aldehyde, which is then quickly oxidized into 5-HIAA by aldehyde dehydrogenase.Results have reported that brain 5-HIAA levels in individuals who died from depression-related suicide were significantly less that in people who died without depression[23].Results from the present study demonstrated thatShuyusanincreased 5-HT expression,as well as its metabolite, in the hypothalamus, corpus striatum, and hippocampus, respectively, in a rat depression model.However,Shuyusandid not exhibit significant effects on 5-HIAA in the hippocampus or the degradation ratio of 5-HT.
Under chronic stress, body homeostasis becomes disrupted.Because a series of neurochemical changes could cause organic damage to the brain, frontal cortices and the limbic system, which are involved in emotion control, as well as the hippocampal formation, are more vulnerable to damage.The CA3 is a subregion of the hippocampus that is sensitive to stress, and previous studies have shown that long-term, chronic stress leads to reduced cell numbers, morphological changes,expansion of intercellular space, and loose and irregular arrangement of pyramidal cells in the CA3 subregion[24-25].Apical dendrites of pyramidal cells in the CA3 subregion have been shown to shrink following chronic restraint stress stimulation or chronic multiple stress stimulation,as determined by a significantly decreased number of branches in apical dendrites and total length reduction[26].The present study showed thatShuyusanexhibited neuroprotection.In theShuyusangroup, the number of shrunken cells significantly decreased and cell injuries were alleviated compared with the model group.Altered 5-HT levels and receptor functions are thought to be involved in functional regulation of serotonergic neurons[27].Central expression of 5-HT plays various functional roles, such as maintaining mental and emotional stability, promoting sleep, improving pain threshold, reducing appetite, and preventing aggressive behavior.Previous studies have shown that 5-HT expression in the synaptic cleft decreases and the 5-HT receptor (5-HTR) becomes dysfunctional during depression[28-29].Results from the present study showed thatShuyusanincreased 5-HT precursor levels, thereby improving depressive symptoms.There are seven types of 5-HTRs, among which 5-HT1AR and 5-HT2AR are closely related to depression.During depression,5-HT1AR-specific binding in the hippocampus is significantly reduced, while 5-HT2AR binding in the cerebral cortex significantly increases.These changes alter the balance and result in increased cytoplasmic Ca2+concentration, followed by neuronal degeneration and apoptosis[27].Previous results have shown that Amitriptyline inhibits presynaptic membranes, thereby increasing 5-HT levels sufficiently to boost binding of 5-HT to 5-HT1AR; subsequently, Ca2+influx is abated and chronic stress-induced functional damage to the hippocampus is mitigated[28].
In conclusion, the number of 5-HT-positive neurons in the cerebral cortex and CA1 and CA3 hippocampal regions increased following treatment with Amitriptyline orShuyusan.In addition, quantitative analysis of monoamine neurotransmitters in the central nervous system showed that Amitriptyline andShuyusanimproved 5-HT and metabolite expression in the hippocampus of rats with depression, suggesting a possible pharmacological mechanism forShuyusan.These results suggested that 5-HT expression in the rat frontal cortex and hippocampus was facilitated by inhibiting the presynaptic membrane to recover 5-HT and increase central 5-HT levels, thereby alleviating chronic stress reactions.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
This study was performed at the Medical Experiment Center and Institute of Geriatrics in the General Hospital of Chinese PLA, China from November 2009 to January 2011.
Materials
Animals
A total of 40 adult, specific pathogen-free, male, Wistar rats, weighing 180–220 g (License No.SCXK (Jing)2005-0013), were provided by the Medical Experimental Animal Center of the Chinese PLA General Hospital.Protocols were conducted in accordance with theGuidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[29].
Drugs
Shuyusanwas provided by the Traditional Chinese Medicine Department of the Chinese PLA General Hospital and was identified by director Ping Liu at the Pharmacy of Traditional Chinese Medicine, where the drug was supplied.Shuyusancomprised 10 g bupleurum,15 g radix curcumae, 6 g mint, 10 g cape jasmine fruit,10 g poria cocos, 10 g radix polygalae, 10 g calamus,15 g spine date seed, and 10 g flower of silktree albizzia.After soaking, boiling, filtration, and concentration in a thermostatic water bath at 80°C, liquid containing 5 g/mL herbs was obtained and stored at 4°C.The solution was diluted with distilled water prior to use.
Methods
Grouping
An open-field method was used to conduct praxiological scoring in 40 Wistar rats.The open-field device was made of opaque materials with a 76 cm × 76 cm square located on the bottom, which was equally divided into 25 equilateral squares.This was surrounded by a 42-cm-high wall.Each rat was placed in the central square; the number of squares that the rat crossed during 2 minutes (only squares entered on four feet were quantified as a score of horizontal activity) and the number of times standing on hind limbs (two forepaws rising or clinging to the wall represented a score of vertical activity) were observed.Each rat was measured once for two minutes, and scoring was performed by two observers to calculate an average value[30].
A total of 40 rats with similar scores were randomly assigned to the model, control, high-doseShuyusan,low-doseShuyusan, and Amitriptyline groups, with 8 rats in each group.The model group was fed normally after 21-day stress stimulation[31].The control group was normally fed without any treatment.The high- and low-doseShuyusangroups were administered 4 mLShuyusancontaining 5.0 or 0.5 g/mL crude drugs,respectively, by gastric perfusion once per day[13].The Amitriptyline group was treated with 10 mg/kg Amitriptyline (Hunan Dongting Pharm., China; batch number: H43020561) dissolved in 2 mL normal saline.The rats were treated at 22 days post-model establishment for 22 days.Praxiological changes were observed every week using the Open-Field and Forced Swimming Tests.
Sample processingFollowing successful model establishment, the rats were sacrificed and brains were dissected.The hypothalamus,unilateral hippocampus, and corpus striatum were isolated according to a previously described method[32];tissues were placed into a tissue cryovial following weighing.During homogenization, pre-cooled 0.4 mol/L perchloric acid solution was added to the hypothalamus,unilateral corpus striatum, and unilateral hippocampus,respectively, at a ratio of 0.1 g/mL.The homogenate was then placed on ice for 30 seconds (200 r/min), and the samples were centrifuged at 12 000 r/min at 4°C.An Agilent 1100 series of liquid chromatography workstation(Chengdu CHIFFO Electronics Group, Chengdu, China)was used to measure 5-HT, 5-HIAA, 5-hydrotryptophon,NE, MHPG, DA, and DOPAC levels in the brain homogenates.Neurotransmitter levels per gram brain tissue were calculated.
Each hemisphere, containing cortex and hippocampus,was harvested and sliced into 7-µm thick paraffin sections.Sections from the hippocampal CA1 region,CA3 region, and cerebral cortex were subjected to hematoxylin-eosin staining.
Hematoxylin-eosin staining
Sections from the hippocampal CA1 and CA3 region were subjected to hematoxylin-eosin staining[33]and were observed and photographed under a light microscope (Olympus, Tokyo, Japan).
Immunohistochemistry
Endogenous peroxidase was inactivated with 3% H2O2for 5 minutes.Sections were blocked in 10% normal goat serum at 37°C for 30 minutes, followed by incubation in rabbit anti-rat 5-HT polyclonal antibody (1: 500;Advanced Targeting Systems, San Diego, CA, USA) at 4°C overnight, followed by goat anti-rabbit antibody(ready-to-use)[34].The sections were then washed in PBS.Average absorbance of stained cells was analyzed using Image-ProPlus Image analyzing software (Media Cybernetic, Bethesda, MD, USA).Three slices from each group were selected for analysis.For each slice, three images from three different areas, layers 4–5 of hippocampal region CA1 (located 600 µm from starting point of middle line of A1 area), as well as hippocampal region CA3, were evaluated under a 200 × objective lens.
Statistical analysis
SPSS 12.0 software package (SPSS, Chicago, IL, USA)was adopted and data were expressed as mean±SD.Variance analysis was used to compare between groups.P< 0.05 was considered statistically significant.
Author contributions:The study was designed under the
guidance of Jianjun Jia and Liping Chen, conducted by Yuanyuan Zhang and Zhitao Han, and assessed by Yulan Zhao,Honghong Zhang, and Yazhuo Hu.Yuanyuan Zhang participated in study concept and design, data analysis, and wrote the manuscript.Zhitao Han participated in experimental animal breeding and disposal, and provided data support.Jianjun Jia participated in study concept and design, article authorization, and study instruction.Liping Chen was in charge of funds.
Conflicts of interest:None declared.
Funding:This study was sponsored by a grant from Science and Technology Bureau of Beijing, No.Z090507017709030.
Supplementary information:Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol.6, No.33, 2011 after selecting the “NRR Current Issue” button on the page.
[1]Duman RS, Kehne JH.Depression.CNS Neurol Disord Drug Targets.2007;6(3):161-162.
[2]Chapman DP, Perry GS.Depression as a major component of public health for older adults.J Prev Chronic Dis 2008;5(1):1-9.
[3]Cao H, Liu J, Lewith GT.Traditional Chinese Medicine for treatment of fibromyalgia: a systematic review of ramdomized controlled trials.J Altern Complement Med.2010;16(4):397-409.
[4]Liu P.Development and basic research overview of antidepressant drugs.Zhongguo Yaowu Yingyong yu Jiance.2004;1(3):1-3.
[5]Chen LP, Wang FW, Herbert W.Effect of the combination of acupuncture and shuyusan in the treatment of depression.Zhongguo Linchuang Kangfu.2004;8(21):420.
[6]Chen LP, Li W, Lin MX, et al.The eftect of suyu decoction on rat behavior in stress induced depressive model.Zhongguo Yaowu Yingyong yu Jiance.2007;4(6):19-20.
[7]Jokinen J, Nordstrom AL, Nordstrom P.The relationship between CSF HVA/5-HIAA ratio and suicide intent in suicide attempters.Arch Suicide Res.2007;11(2):187-192.
[8]Roger J, Martin LJ.Genetics of monoamine metabolites in baboons: overlapping sets of genes influence levels of 5-hydroxyindolacetic acid, 3-hydroxy-4-methoxyphenylglycol, and homovanillic acid.Biol Psychiatry.2004;55(7):739-744.
[9]Mitoma M, Yoshimura R, Sugita A, et al.Stress at work alters serum brain-derived neurotrophic factor (BDNF) levels and plasma 3-methoxy-4-hydroxyphenylglycol (MHPG) levels in healthy volunteers: BDNF and MHPG as possible biological markers of mental stress.Prog Neuropsychopharmacol Biol Psychiatry.2008;32(3):679-85.
[10]Yan XY, Zhang XL.Effect of baoshentang on monoamine neurotransmitters and monoamine oxidase in brain of chronic stress induced depression model rats.Zhongguo Zhongyiyao Xinxi Zazhi.2007;14(5):31-33.
[11]Zhou BH, Li XJ, Feng Q.Antidepressant activity of ethanolic extracts of Gastrodia elata in mice.Zhongguo Yiyuan Yaoxue Zazhi.2007;27(11):1525-1528.
[12]Li Z, Liu GQ.Effects of hypericum perforatum Linn.extracts on monoaminoxidase and monoamine neurotransmitters.Zhongguo Yaoke Daxue Xuebao.2002;33(2):138-141.
[13]Chen Q.Survey on Pharmacological Methodology of Sedative Chinese Materia Medica.Beijing: People's Medical Publishing House.1996:1103-1105.
[14]Wang YY, Lu ZL.Chinese Internal Medicine.Beijing: People's Medical Publishing House.1999.
[15]Zhang LD.Traditional Chinese Medical Classification and Treatment in Affective Disorders.Shanghai: Shanghai Archives of Psychiatry.1990.
[16]Romeas T, Morissette MC, Mnie-Filali O, et al.Simultaneous anhedonia and exaggerated locomotor activation in an animal model of depression.Psychopharmacology (Berl).2009;205(2):293-303.
[17]Yan HC, Cao X, Das M, et al.Behavioral animal models of depression.Neurosci Bull.2010;26(4):327-337.
[18]Duman CH.Models of depression.Vitam Horm.2010;82:1-21.
[19]Chaviaras S, Mak P, Ralph D, et al.Assessing the antidepressant-like effects of carbetocin, an oxytocin agonist,using a modification of the forced swimming test.Psychopharmacology (Berl).2010;210(1):35-43.
[20]Li XQ, Xu J.Progresses on animal models of depression study.Chin J Psychiatry.2002;35(3):184-186.
[21]Popova NK, Kulikov AV.Targeting tryptophan hydroxylase 2 in affective disorder.Expert Opin Ther Targets.2010;14(11):1259-1271.
[22]Oxenkrug GF.Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: the serotonin hypothesis revisited 40 years later.Isr J Psychiatry Relat Sci.2010;47(1):56-63.
[23]Anderson GM.Assessing the assessment of brain serotonin turnover.Arch Gen Psychiatry.2008;65(10):1223-1224.
[24]Maletic V, Robinson M, Oakes T, et al.Neurobiology of depression:an integrated view of key findings.Int J Clin Pract.2007;61(12):2030-2040.
[25]Sheline YI, Gado MH, Kraemer HC, et al.Untreated depression and hippocampal volume loss.Am J Psychiatry.2003;168(8):1516-1518.
[26]Magarinos AM.Does stress damage the brain.Neuroscience.1995;69(1):83-88.
[27]Liu XW, Xu J, Li XQ, et al.Changes of 5-HT-(1A) receptor and 5-HT-(2A) receptors in the brain of rat with stress-induced depression.Jichu Yixue yu Linchuang.2004;24(2):174-178.
[28]Graeff FG, Guimaraes FS, Telma GCS, et al.Role of 5-HT in stress, anxiety, and depression.Pharmacol Biochem Behav.1996;54(1):129-141.
[29]The Ministry of Science and Technology of the People’s Republic of China.Guidance Suggestions for the Care and Use of Laboratory Animals.2006-09-30.
[30]Archer J.Tests for emotionality in rats and mice: a review.Anim Behav.1973;21(2):205-235.
[31]Winner P, Towell A, Sampson D, et al.Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic anti-depressant.Psychopharmacology(Berl).1987;93(3):358-364.
[32]Glowinski J, Iversen LL.Regional studies of catecholamines in the rat brain: the disposition of norepinephrine, dopamin and dopa in various regions of the brain.J Neurochem.1966;13(8):655-669.
[33]Fischer AH, Jacobson K, Rose J, et al.Hematoxylin and eosin staining of tissue and cell sections.CSH Protoc.2008;2008:pdb.prot 4986.
[34]Moukhles H, Bosler O, Bolam JP, et al.Quantitative and morphometric data indicate precise cellular interactions between sertonin terminals and postsynaptic targets in rat substantia nigra.Neuroscience.1997;76(4):1159-1171.
杂志排行
中国神经再生研究(英文版)的其它文章
- Effects of Fujian tablet on Nogo-A mRNA expression and plasticity of the corticospinal tract in a rat model of focal cerebral ischemia*☆
- Sidiming attenuates morphine withdrawal syndrome and nitric oxide (synthase) levels in morphine-dependent rats and rhesus monkeys*★
- Dynamic analysis of 10 components of the Chinese herbal compound Wuzhuyu-tang absorbed into rat plasma**☆
- Quiet rest ameliorates biochemical metabolism in the brain in a simple concussion rabbit model Evaluation of hydrogen proton magnetic resonance spectroscopy*☆
- Effects of electroacupuncture at Zusanli (ST 36) on neurons in the colonic myenteric plexus in rats with irritable bowel syndrome with constipation*★
- Magnetic resonance imaging characteristics of postoperative intracranial dissemination of recurrent gliomas*****☆
