Effects of Fujian tablet on Nogo-A mRNA expression and plasticity of the corticospinal tract in a rat model of focal cerebral ischemia*☆
2011-07-27WeiLiuYonghongZhouQingJiaBingbingHanGuoliZhang
Wei Liu, Yonghong Zhou, Qing Jia, Bingbing Han, Guoli Zhang
1Department of Encephalopathy, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250011, Shandong Province, China
2Department of Traditional Chinese Medicine, Medical College of Qingdao University, Qingdao 266071, Shandong Province, China
3Institute of Basic Medicine, Shandong Academy of Medical Science, Jinan 250062, Shandong Province, China
4College of Basic Medical Sciences, Jinan 250355, Shandong Province, China
5Department of Radiation Oncology, Shandong Tumor Hospital & Institute, Jinan 250117, Shandong Province, China
INTRODUCTION
Concurrent with the challenges of translating neuroprotective strategies that appear to be effective in animal models into therapies for use in human patients, much attention has been focused on other possible strategies to increase functional recovery after stroke by enhancing brain plasticity[1].Recent studies[2-9]have demonstrated that remodeling of the corticospinal tract (CST)can aid in motor function recovery after cerebral ischemia, especially in those with an extensive unilateral infarct.CST is the main pathway for control of precision voluntary movement, which originates from the pyramidal neurons in the cerebral cortex and connects with motor neurons in the spinal cord directly or indirectly.It has been found that the CST originating from the contralesional intact cerebral hemisphere can cross the midline into the denervated side of the spinal cord after cerebral ischemia to promote motor recovery[10].Further studies have demonstrated that to improve impaired motor function, CST remodeling can be promoted by such interventions as anti-Nogo-A antibody infusion[5], intracerebroventricular administration of a function-blocking NgR fragment or erythropoietin[6,9], and tail vein injection of bone marrow stromal cells[10].But it is a long distance from the laboratory to clinical application, and no certain drug has been proved effective to promote CST plasticity.
Fujiantablet (FJT) is a Chinese experiential prescription.It is mainly composed of preparedHeshouwu,YinyanghuoandJuemingzi, and can regulate and nourish the liver and kidney to facilitate functional recovery ofsuiin traditional Chinese medicine, including the brain and spinal cord.A previous study has shown that FJT can improve impaired motor function induced by ischemic stroke[11].The mechanism involves enhancing the expression of vascular endothelial growth factor and growth associated protein 43, increasing regional cerebral blood flow and inhibiting Nogo-A mRNA expression in the brain tissues surrounding ischemia[12-14].The present study was designed to investigate the effect of FJT on CST remodeling and the correlation between CST plasticity and Nogo-A expression.
RESULTS
Quantitative analysis of experimental animals
Sixteen of 24 male Wistar rats were used to establish models of middle cerebral artery occlusion (MCAO)[15].The model rats were further assigned to model (MCAO +normal saline) and FJT (MCAO + FJT treatment) groups,with eight animals in each group.The other eight rats that served as the sham-surgery group were subjected to surgery, but did not undergo electric artery coagulation.A total of 24 rats were involved in the final analysis.
Effects of FJT on biotinylated dextran amine (BDA)expression in the denervated side (left) of thecervical cord (C4-6)
BDA was injected at the end of 3 weeks following model establishment.At 5 weeks, positive expression of BDA was present in the CST and the cytoplasm of motor neurons in the denervated side of the spinal cord (Figure 1).
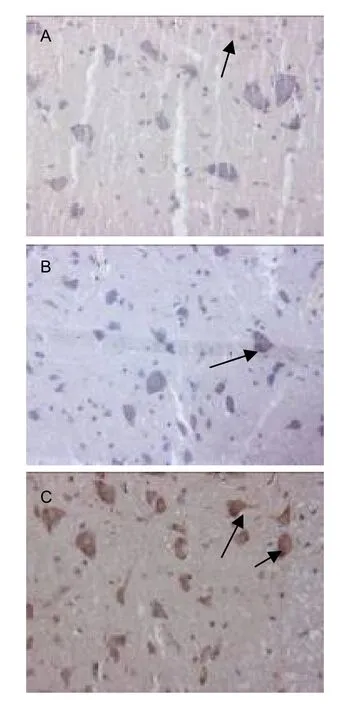
Figure 1 Expression of biotinylated dextran amine(BDA)in the left cervical cord of rats in different groups(immunohistochemical staining, × 200; arrows: BDA positive expression).In the sham-surgery group, little BDA positive staining was observed in the cytoplasm of spinal motoneurons (A).After stroke, BDA expression was increased in the cytoplasm of the denervated spinal motoneurons (B).BDA expression was increased further in the cytoplasm of the denervated spinal motoneurons after Fujian tablet treatment (C).
The mean gray values of BDA expression in the cervical cord were significantly lower in the model group compared with the sham-surgery group (P<0.01; higher gray value indicates less BDA expression).And the mean gray values of BDA expression were lower in the FJT group compared with the model group (P<0.05).These results demonstrated that the CST originating from the intact cerebral cortex could cross the midline to enhance the innervation of stroke-impaired peripheral tissues, and that FJT could promote the remodeling of CST.
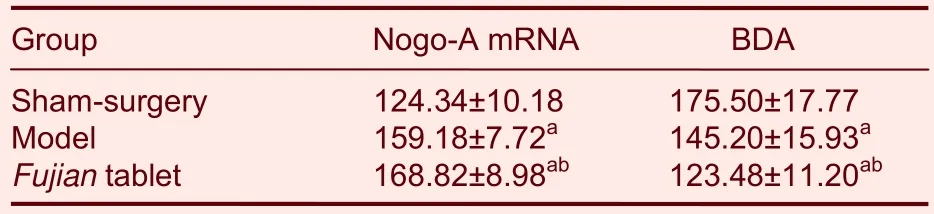
Table 1 Mean gray values of biotinylated dextran amine(BDA) and Nogo-A mRNA expression in the denervated side of the cervical cord (C4-6) in each group
Effects of FJT on Nogo-A mRNA expression in the denervated side (left) of the cervical cord (C4-6)
Nogo-A mRNA-positive cells were stained brown mainly in the cytoplasm (Figure 2).
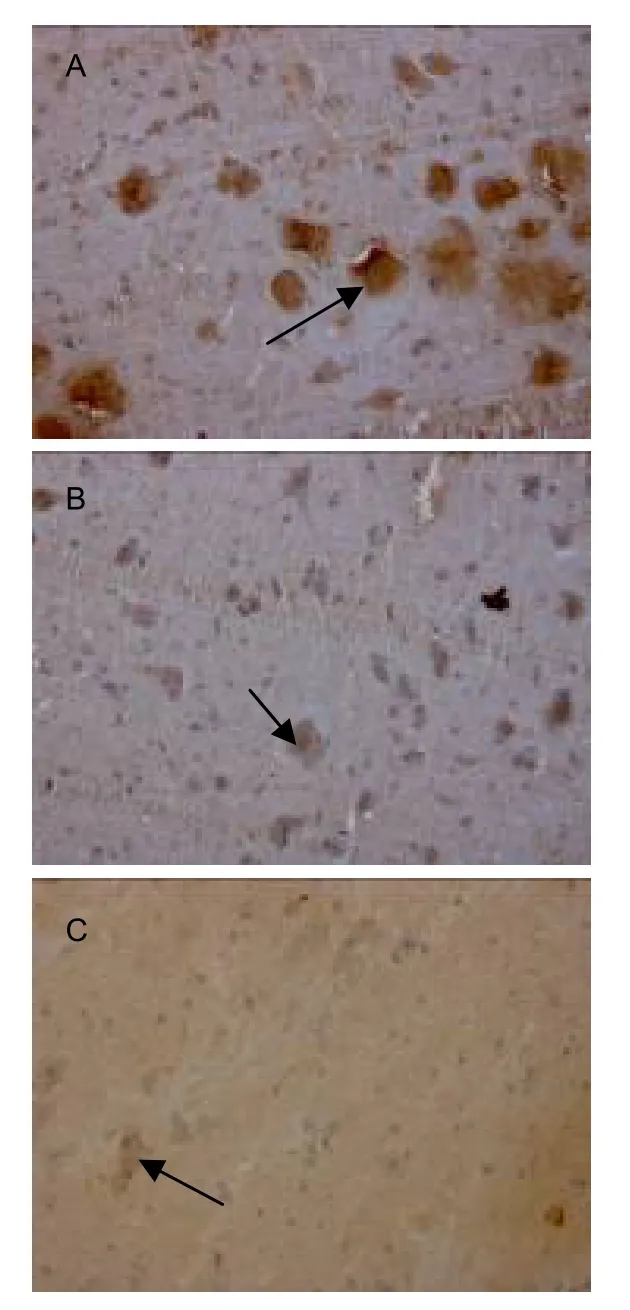
Figure 2 Nogo-A mRNA expression in the left cervical cord of rats in different groups (in situ hybridization, × 200;arrows: Nogo-A mRNA expression).In the sham-surgery group, Nogo-A mRNA positive staining was observed in the cytoplasm of spinal neurons (A).After stroke, Nogo-A mRNA expression was reduced in the cytoplasm of the left spinal neurons (B).Nogo-A mRNA expression was reduced further in the cytoplasm of the left spinal neurons after Fujian tablet treatment (C).
Results ofin situhybridization at the end of 5 weeks after model establishment showed that, compared with the sham-surgery group, mean gray values of Nogo-A mRNA expression in cervical cord were increased significantly in the FJT and model groups (P<0.01).Furthermore, mean gray values of Nogo-A mRNA expression were higher in the FJT group compared with the model group (P<0.05; Table 1).These results demonstrated that at 5 weeks following cerebral ischemia, Nogo-A mRNA expression was decreased and FJT could further reduce the expression.
Correlation between BDA and Nogo-A mRNA expression in the denervated side of the cervical cord (C4-6)
Pearson’s correlation coefficients between the expression of BDA and Nogo-A mRNA were equal to-0.744, which indicates that BDA expression is negatively related to that of Nogo-A mRNA.
Effects of FJT on motor function of MCAO rats
At the end of 1 week after MCAO, the scores of beam walking tests were decreased significantly in the model and FJT groups compared with the sham-surgery group(P<0.01).At 5 weeks, the scores of beam walking tests were increased in the model group, but remained lower than in the sham-surgery group (P<0.01); the scores in the FJT group were increased and were similar to those in the sham-surgery group (P> 0.05; Table 2).

Table 2 Scores of beam walking test in each group at different time points
DISCUSSION
Consistent with the results of our previous study[11], in the present study, FJT treatment significantly improved functional recovery in adult rats after experimental ischemic stroke.The present study utilized the anterograde neuronal tracer BDA to label the CST.Compared with horseradish peroxidase and other neuronal tracers, BDA has the advantages of being stable and transferred for long distances, which can provide a better display of the CST remodeling[16].Results show that by the end of 5 weeks following focal cerebral ischemia, the CST originating from the intact cerebral cortex crossed the midline to enhance the innervation of stroke-impaired peripheral tissues, and that FJT could promote the plasticity to contribute to motor function recovery.However, it remains unclear whether the mechanism of FJT to improve neurological function involves other neuronal pathways, such as ipsilateral ventral CST, corticorubral tract, or other brainstem-spinal systems.
As a well-known myelin-associated neurite outgrowth inhibitory protein in the central nervous system, Nogo-A has been proven to be related to CST plasticity[5].
Wiessneret al[5]found that purified monoclonal anti-Nogo-A antibody (7B12) contributed to a significant increase of midline crossing by corticospinal fibers originating in the unlesioned sensorimotor cortex after photothrombotic cortical injury, which facilitated the improvement of forelimb motor function.Leeet al[6]reported that after a stroke, an increasing number of axons emanating from the undamaged cortex crossed the midline to innervate the ipsilateral cervical spinal cord,which was enhanced in Nogo-AB-/-mice.One previous study demonstrated that FJT could inhibit Nogo-A mRNA expression in the cervical cord following focal cerebral ischemia[17].The present study further shows that in the FJT group, Nogo-A mRNA expression in the denervated side of the cervical cord (C4-6) was significantly reduced,which was negatively related to BDA expression,indicating that the mechanism of FJT to promote CST plasticity may involve an inhibitory effect of FJT on Nogo-A mRNA expression in the cervical cord.In addition, for the first time, the present study provides a promising therapeutic intervention for addressing CST plasticity, which may aid in the development of a new method for investigating the mechanism by which Chinese herbs promote neurological function recovery after focal cerebral ischemia.
MATERIALS AND METHODS
Design
A randomized controlled animal trial.
Time and setting
The experiment was performed at the Department of Encephalopathy, Affiliated Hospital of Shandong University of Traditional Chinese Medicine and Institute of Basic Medicine in Shandong Academy of Medical Science, China, from January to July 2010.
Materials
Animals
A total of 24 healthy adult specific-pathogen-free male Wistar rats, aged 6-8 weeks and weighing 260±10 g,were provided by the Quality Supervision Center of Shandong Lukang Pharmaceutical Co., Ltd., China(SCXK (Lu) 20080002).All rats were allowed free access to food and water before and after surgery and were housed at 24-25°C, with humidity of 40-70%, under natural illumination.Protocols were followed in accordance with theGuidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[18].All animals in this study had similar physiological values(rectal temperature and mean arterial blood pressure)before, during and after MCAO among groups.No abnormal behavior, depression of respiration or hypothermia were observed in any group.
Drugs
Decoction of FJT (Batch No: 20091104) was prepared by the College of Pharmacology, Shandong University of Traditional Chinese Medicine, China.FJT was mainly composed ofHeshouwu, YinyanghuoandJuemingzi.A milliliter of the decoction contained 1.2 g of crude Chinese herbs.
FJT preparation
Crude Chinese herbs were extracted by reflux of 70%alcohol as six times the volume of crude drugs twice, and each extraction lasted for 1.5 hours.Then the fluid was extracted by reflux of alcohol as five times the volume of drugs once for 1 hour.All the extracted fluid was mixed and concentrated to a thick paste with a relative density of 1.15 (60°C) after alcohol recovery.The thick paste was then dried under spray drying conditions: liquid with relative density of 1.10±0.01 (60°C), inflow wind at 160±5°C and outflow wind at 80-85°C.
Methods
Establishment of MCAO model
Rats in the model and FJT groups were subjected to electrocoagulation and transaction of the right MCA proximal to the inferior cerebral vein according to the modified method described by Tamuraet al[15].In the sham-surgery group, the cranium was opened but the MCA was not occluded.Successful models were demonstrated by rats exhibiting the following traits: loss of the contraction of left limbs as a normal reaction to pain; easily falling to the left or circling to the left; and being unable to pull left upper limbs ahead when suspended by the tail[15].
Drug intervention
Rats in the FJT group were intragastrically perfused with the decoction of FJT at a dose of 9 g crude drug/kg[13],while the others were administered equal doses of normal saline, once a day for 4 weeks.Treatment started at 7 days after surgery and rats were allowed free access to food and water.
Neurological functional test
A series of beam walking tests[19]were performed at the end of 1 week and 5 weeks after MCAO to evaluate functional recovery.The rating scale of beam walking test ranged from 1 to 7.
Anterograde CST tracing
At the end of 3 weeks after surgery, 10% solution of BDA(10 kDa; Molecular Probes, Invitrogen, CA, USA) in PBS was injected through a finely drawn glass capillary into four points in the left frontal motor cortex (200 nL per injection; stereotaxic coordinates: 1 and 2 mm rostral to the bregma, 3.5 and 4.5 mm lateral to the midline[20]) to anterogradely label the CST originating from this area.The micropipette remained in place for 4 minutes after completion of the injection.
Tissue preparation
Eight rats from each group were anesthetized using ketamine at 5 weeks after MCAO.The cervical cords(C4-6) were harvested, immersed in 4%paraformaldehyde overnight, embedded in paraffin wax and cut into coronal sections 20-μm thick.
Detection of Nogo-A mRNA expression using in situ hybridization
Sections were dewaxed and rehydrated routinely, mixed with 30% H2O2in distilled water at a ratio of 1: 10 for 8 minutes at room temperature to block endogenous enzyme activity and washed with distilled water three times for 10 minutes.The segment of mRNA was exposed.The mixture of 3% citric acid with fresh diluted gastric protease (1 mL 3% citric acid: two drops concentrated gastric protease) was added to the sections to digest for 20 minutes at 37°C, the sections were then washed with PBS three times for 5 minutes,followed by a wash with distilled water once.Postfixation:sections were fixed with 1% paraformaldehyde/0.1 mol/L PBS (pH 7.2-7.6) containing 1/1 000 diethylpyrocarbonate at room temperature for 10 minutes and rinsed with distilled water three times.
Prehybridization: 20% glycerol was added to the bottom of the kit, mixed with 20 μL of prehybridization liquid(Wuhan Boster Biotechnology Co.Ltd, Wuhan, China),added to each slice and incubated at 37°C in the thermostat for three hours.Additional liquid was removed.Hybridization: 20 μL of hybridization mix containing oligonucleotide probes (Shanghai Boshang Biotechnology Co., Ltd., Shanghai, China) was added to each section.These slices were covered with coverglasses specially made forin situhybridization with their protective membrane recovered.Hybridization occurred overnight in the thermostat at 37°C.The coverglasses were removed, the slices were rinsed three times for 5 minutes with pre-warmed (37°C) 2 × saline sodium citrate (Wuhan Boster Biotechnology), washed once with pre-warmed 0.5 × saline sodium citrate for 15 minutes at 37°C, followed by a wash with pre-warmed 0.2 × saline sodium citrate for 15 minutes at 37°C.Each section was blocked with a blocking agent for 30 minutes at 37°C.Additional liquid was removed.Sections were incubated with rat anti-digoxin biotin (Wuhan Boster Biotechnology) for 60 minutes at 37°C, washed three times with PBS for 5 minutes, incubated with streptavidin-biotin peroxidase complex (Wuhan Boster Biotechnology) for 20 minutes at 37°C, washed three times with PBS for 5 minutes, incubated with biotin-peroxidase (Wuhan Boster Biotechnology) for 20 minutes at 37°C, washed four times with PBS for 5 minutes, colored with diaminobenzidine color solution(Beijing Zhongshan Goldenbridge Biotechnology, Beijing,China) for 30 minutes, dehydrated with alcohol,hyalinized with dimethylbenzene and sealed.Six photographs of each slice were taken randomly in a 200-fold field of view with a light microscope Leica DM4000B (Leica, Solms, Germany).The hot zone was selected and the mean gray values were measured by Leica QWinV3 image analysis system.
The sequence of the probe of Nogo-A mRNA is as follows:
5’-GCT CTT CCT GCT GCA TCT GAG CCT GTG ATA-3’;5’-TTC AGA ATT AGA ATA CTC AGA AAT GGG ATC-3’;5’-GCA GAT AGA TCA TTA TCT AGG ACT TGC AAA-3’.
Detection of CST tracing using BDA
The cervical cord segments of C4-6were sliced into traverse sections (75 µm) using a vibratome.The sections were incubated in 0.5% H2O2for 20 minutes followed by an incubation with avidinbiotin-peroxidase complex (Wuhan Boster Biotechnology) at 4°C for 48 hours.BDA-labeled axons or neurons were visualized using 3, 3’-diaminobenzidine-nickel (Beijing Zhongshan Goldenbridge Biotechnology) for light microscopy examination.For each animal, the mean gray values of BDA-labeled CST or motor neurons in the denervated side of the spinal cord at C4-6levels were quantified from 2 sections (five random 200-fold fields of view from each section).These sections were observed with Leica QwinV3 image analysis system and the mean dyeing gray value was measured.
Statistical analysis
Data were expressed as mean±SD.The behavior outcomes were compared among groups with two-way analysis of variance.Two-samplet-test was used to analyze the difference in the mean gray value of Nogo-A mRNA in cervical cords and that of BDA between groups.A value ofP<0.05 was regarded as significant.Pearson’s correlation coefficients were calculated between mean gray values of the expression of BDA and Nogo-A mRNA in the FJT group.
Author contributions:Wei Liu designed this study.Wei Liu,Guoli Zhang, Qing Jia and Bingbing Han conducted experiments.Yonghong Zhou evaluated the data using a double-blind method.
Conflicts of interest:None declared.
Funding:This work was supported by the National Natural Science Foundation of China, No.30801470.
Ethical approval:The experiments were approved by the Animal Ethics Committee of Shandong University of Traditional Chinese Medicine, China.
[1]Cheatwood JL, Emerick AJ, Kartje GL.Neuronal plasticity and functional recovery after ischemic stroke.Top Stroke Rehabil.2008;15(1):42-50.
[2]Cramer SC, Crafton KR.Somatotopy and movement representation sites following cortical stroke.Exp Brain Res.2006;168(1-2):25-32.
[3]Gerloff C, Bushara K, Sailer A, et al.Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke.Brain.2006;129(Pt 3):791-808.
[4]Chen P, Goldberg DE, Kolb B, et al.Inosine induces axonal rewiring and improves behavioral outcome after stroke.Proc Natl Acad Sci U S A.2002;99(13):9031-9036.
[5]Wiessner C, Bareyre FM, Allegrini PR, et al.Anti-Nogo-a antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats.J Cereb Blood Flow Metab.2003;23(2):154-165.
[6]Lee JK, Kim JE, Sivula M, et al.Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity.J Neurosci.2004;24(27):6209-6217.
[7]Liu Z, Li Y, Qu R, et al.Axonal sprouting into the denervated spinal cord and synaptic and postsynaptic protein expression in the spinal cord after transplantation of bone marrow stromal cell in stroke rats.Brain Res.2007;1149:172-180.
[8]Chen MS, Huber AB, van der Haar ME, et al.Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1.Nature.2000;403(6768):434-439.
[9]Reitmeir R, Kilic E, Kilic U, et al.Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity.Brain.2011;134(Pt 1):84-99.
[10]Liu Z, Li Y, Zhang X, et al.Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment.Stroke.2008;39(9):2571-2577.
[11]Wang XL, Wang ZL, Zhou YH.The mechanism of traditional chinese drug in activating the cerebral area of functional down-regulation.Zhongyiyao Xuekan.2004;22(8):1365-1367.
[12]Zhou YH.Effects of Fujian tablets on expression of bFGF, VEGF and PDGF in the brain of MCAO rats.Zhongyiyao Xuekan.2003;21(1):70-72.
[13]Liu W, Zhou YH, Fu XJ, et al.Effects of Fujian tablets on expression of GFAP and GAP-43 in brain tissue of ischemic stroke sequela model rats.Zhongyi Zazhi.2006;47(5):378-380.
[14]Zhou YH, Wang XL, Hu HQ.Effect of Chinese herb for tonifying the liver and kidney on the expression of Nogo protein-A in the brain of rats with middle cerebral artery occlusion.Zhongguo Linchuang Kangfu.2006;10(23):43-45.
[15]Tamura A, Graham DI, McCulloch J, et al.Focal cerebral ischemia in the rat:1.Description of technique and early neuropathological consequences following middle cerebral artery occlusion.J Cerb Blood Flow Metab.1981;1(1):53-60.
[16]GrandPre T, Li S, Strittmatter SM.Nogo-66 receptor antagonist peptide promotes axonal regeneration.Nature.2002;417(6888):547-551.
[17]Liu W, Zhang GL.Effect of Fujian tablet on the expression of Nogo-A mRNA in cervical spinal cord of middle cerebral artery occlusion model rats.Neural Regen Res.2007;2(10):599-602.
[18]The Ministry of Science and Technology of the People’s Republic of China.Guidance Suggestions for the Care and Use of Laboratory Animals.2006-09-30.
[19]Feeney DM, Gonzalez A, Law WA.Amphetamine, haloperidol and experience interact to affect rate of recovery after motor cortex injury.Science.1982;217(4562):855-857.
[20]Paxions G, Watson C.The Rat Brain in Stereotaxic Coordinates.Beijing: People’s Medical Publishing House.2005.
杂志排行
中国神经再生研究(英文版)的其它文章
- Magnetic resonance imaging characteristics of postoperative intracranial dissemination of recurrent gliomas*****☆
- Effects of electroacupuncture at Zusanli (ST 36) on neurons in the colonic myenteric plexus in rats with irritable bowel syndrome with constipation*★
- Effects of Shuyusan on monoamine neurotransmitters expression in a rat model of chronic stress-induced depression*★
- Quiet rest ameliorates biochemical metabolism in the brain in a simple concussion rabbit model Evaluation of hydrogen proton magnetic resonance spectroscopy*☆
- Dynamic analysis of 10 components of the Chinese herbal compound Wuzhuyu-tang absorbed into rat plasma**☆
- Sidiming attenuates morphine withdrawal syndrome and nitric oxide (synthase) levels in morphine-dependent rats and rhesus monkeys*★
