Establishment of a beagle dog model of oculomotor nerve injury**★
2011-07-19WenxiangZhongXuhuiWangWenchuanZhangShitingLiMinYang
Wenxiang Zhong, Xuhui Wang, Wenchuan Zhang, Shiting Li, Min Yang
Department of Neurosurgery, Xinhua Hospital, Medical School of Shanghai Jiao Tong University, Shanghai 200092, China
lNTRODUCTlON
End-to-end anastomosis after nerve injuries,a method for direct reconstruction, is an optimal approach for cranial nerve reconstruction[1-8]. Previous studies have established oculomotor nerve cut-off models in experimental rats and cats, but end-to-end anastomosis of oculomotor nerves has usually failed because of the depth of the nerve and the narrow manipulation space[9-11]. Relatively large beagle dogs can satisfy the requirements of microsurgery and stimulating electrode insertion. After electrode insertion, all established models can be utilized repetitively.
This study established beagle dog models of oculomotor nerve injury (ONI) and investigated the changes in physiological electrical stimulus parameters of oculomotor nerves.
RESULTS
Quantitative analysis of experimental animals
Satisfactory bioelectrical signals with sound repeatability and stability were acquired from all 10 dogs. The survival rate was 80%. One animal died due to imperfect hemostasis after rupture of the cavernous sinus, while another died after losing consciousness for 2 days on account of damage to the brain stem caused by stretching during operation.
The surviving eight animals gradually recovered, and no obvious complications,such as intracranial infection, infection of incisional wound, intracranial hemorrhage post operation, or epilepsy, were identified.
Experimental data were collected before the two animals died, so no more animals were added to the experiment.
Compound muscle action potentials(CMAPs) before and after ONl
CMAP bioelectric signal changes were detected before and after ONI. After cutting the oculomotor nerve, the pupil on the damage side enlarged immediately, and direct and indirect light reflexes disappeared.
The eyeball was abducted, the convergency reflex disappeared, and the upper eyelid could not elevate. Electromyogram results revealed that CMAP amplitude was lower after ONI (0.077±0.036 mV) compared with that before ONI (0.573±0.280 mV) (P < 0.05;supplementary Table 1 online), suggesting a successfully established animal model(Figure 1).
DlSCUSSlON
Oculomotor nerves in beagle dogs were used in this experiment. Having relatively large bodies, beagle dogs meet the requirements of microsurgical manipulation during the experiment and placement of a stimulating electrode. The animal’s age should be in the range of 2-3 years old, and those animals not within this range were excluded from preparatory experiments.
Animals over 3 years could hardly tolerate the operation because of deterioration of their organ functions, while young animals usually had poor immunity.

During experiments, animals’ head positions, the position and size of incisions, bony window, and control over intracranial pressure could all have an effect on the exposure of oculomotor nerves. The operation approach adopted in this experiment ensured sufficient manipulating space, whilst stimulating electrodes could easily reach the free oculomotor nerves in the posterior cavernous sinus and the cut-off nerve could easily be sewn up with 11-0 non-traumatic sutures.
Electrical stimulation promoted regeneration of peripheral nerves[12-13]. There have no reports to date,however, of promotion of regeneration of cranial nerves,nor have there been any electrophysiological studies in animal models of ONI. Many reports of monitoring oculomotor nerves through intercranial stimulation revealed oculomotor nerve functions through recording electromyograms of extraocular muscles[14-16].
Appropriate nervous stimulation parameters have always been one of the problems in monitoring electrophysiology of peripheral nerves. Different electrical stimulation parameters are suitable for varied peripheral nerves and they could produce different effects on nerve regeneration. Since the present Power Lab Biological Signal Processing System only provides constant pressure stimulation, repetitive rectangular pulse stimulation with varied parameters was used in this study. The strength of the electrical stimulation was 0.3-2.0 V, which could provide a wide range of changes;as a result, it could be conductive to selecting proper stimulation parameters for oculomotor nerves.
To acquire accurate bioelectrical signals, laboratories should be equipped with sound earth connection conditions. It is of vital importance for experimental animals to be earthed and efforts should be made to avoid any impact from external interferences and stimulations. A piece of small cotton was placed between the free oculomotor nerves and skull. Blood or cerebrospinal fluids surrounding the nerves were constantly sucked away by a capitular suction apparatus(diameter 1.0 mm). When measuring electrical signals,operations such as using high-frequency electrocoagulators and washing surgical sites are forbidden to avoid distortion in recording electrical signals. Furthermore, there are certain special requirements for electrodes (Figure 2). The stimulating electrode used in this experiment was a platinum filament hook-like shield electrode (diameter, 0.3 mm;interval between two filaments, 1.5 mm). Results from pilot experiments showed that its performance was superior to that of electrodes of other materials or with other shapes. At the end of this stimulating electrode(shield electrode), there exists a protective “small bend”,which prevented any effects on the functions of oculomotor nerves during hitching and dropping off of oculomotor nerve stems during stimulation. The inserted length of the pin-shaped muscle leading electrode(diameter 0.35 mm) should not be less than 0.7 mm, with the interval between two pointed ends 1 mm. The pointed end must be very sharp to minimize muscle pain.
Its remote end should be located at a high level, to ensure that it would not short-circuit when muscles ooze blood, which could be achieved by proper adjustment of eyeball direction. After multi-directional single-point grounding and selection of appropriate electrodes, ideal bioelectrical signals were collected for all animals by adopting 5 000 × magnification and using the recording mode with an upper limiting frequency of 10 000 Hz and lower limiting frequency of 80 Hz. All acquired graphics were processed using a Power Lab Biological Signal Processing System (segment measurement,out-of-window frequency integral) and CMAPs of the interior oblique muscle of oculomotor nerves stimulated with varied voltage strength were yielded. The results from this study indicated that the peak value of CMAPs of interior oblique muscles was gradually increased following a steady increase in electrical stimulation strength. The appropriate stimulation strength for the oculomotor nerve stem was 0.9-1.6 V, which enabled the peak value of CMAPs of interior oblique muscles to reach about 500 μV. After that, there were no explicit changes in action potentials when increasing stimulation strength.
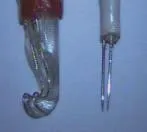
Figure 2 Stimulating electrode (left) and leading electrode (right). The stimulating electrode is a platinum filament hook-like shield electrode (diameter 0.3 mm, the interval between the two filaments is 1.5 mm).
Significance of the animal model: the model can be used to simulate determination of each parameter for physiologic electrostimulation of oculomotor nerves,study electrophysiological changes pre- and post-oculomotor nerve injuries, probe relationships between electrophysiological changes and appraisals of nervous functions, give guidance to functional reconstruction after ONI, and study the role played by electrical stimulation in promoting functional repair of oculomotor nerves.
In summary, the animal model described here has laid a foundation for monitoring the electrophysiology of oculomotor nerves during operations. The results of the present study could lead to reductions in the numbers of injuries to both oculomotor nerves and their functions,reduce the possibilities of operational complications, and contribute to development of operational technologies in neurosurgery.
MATERlALS AND METHODS
Design
A paired design, animal experiment.
Time and setting
The experiment was performed at the Department of Laboratory Animal Science of Medical School of Shanghai Jiao Tong University in China from January 2008 to June 2008.
Materials
Animals: a total of 10 adult, healthy, female, beagle dogs,aged 2-3 years, weighing 12-18 kg, with body length of 80-90 cm, were provided by Shanghai Xingang Experimental Animal Plant, with the license No. SCXK 2002-0014. They were raised in a closed environment under general breeding conditions for experimental dogs.
The protocols were conducted in conformity with the Guidance Suggestions for the Care and Use of Laboratory Animals, enacted by the Ministry of Science and Technology of the People’s Republic of China[17].
Instruments: The Power Lab system (produced by AD Instruments, Sydney, Australia) is a computerized data collection and analysis system running on a Windows operation platform. The system is an external instrument independent of computers, which consists of two parts,namely, signal processing and functional enhancement components. After connecting with computers, the signal processing component is able to do data transformation for various instrument signals less than 10 mV, and make multiple analyses and processing. Major appliances of the experiment, such as the platinum filament stimulating electrode (shield electrode), pin-shaped muscle leading electrode, digital computers, surgical microscope,high-speed abrasive drilling (diameter 0.3 cm),instruments of routine craniotomy, and microsurgery instruments, were produced by ourselves.
Methods
Anesthetization of animals and matters needing attention before and after operation
All experimental animals were intraperitoneally anaesthetized with Napental (30 mg/kg). The laboratory temperature was maintained at about 25°C. Attention was paid to keep animals warm during the operation to prevent amyostasia interference. Before the operation,retroauricular veins were opened up and intravenous drip of 10% glucose 500 mL was slowly administered. If necessary, drugs such as 20% mannite were added.
Intramuscular injections of 0.3 mg atropine were given to reduce respiratory tract secretions and ensure an unimpeded air flow. No muscle relaxants were used before or during the operation for fear of an effect on the nerve and muscle electrical signal measurements.
Exposure of oculomotor nerves and interior oblique muscle
Anaesthetized dogs were laid on their sides and their heads were laterally rotated 15-20° after being fixed using a lathe head. After trichomadesis, routine sterilization, and placement of drapes, a 4-5-cm straight incision perpendicular to the zygoma direction was cut along the anterior auricular border. After incision of the skin, subcutaneous tissue and temporalis, layer by layer,and precise hemostasis by bipolar diathermocoagulation,the temporalis was separated using a periosteum elevator. Integumentary musculature was spread outwardly toward the mouth-ear connecting line by retractors. A hole was bored at the center of the exposure field by high-speed abrasive drilling, while the bony “window” was enlarged to about 3 cm × 3 cm using bone rongeurs. Two needles were suspended from the duramater beneath the skull. The duramater was carefully cut open and sutured to the temporalis. Using an unclear Sylvian fissure identifiable by the naked eye as a mark, the oculomotor nerve, which was located in the posterior cavernous sinus and absent from the tentorium cerebelli was identified by lightly uplifting the lobus pyriformis with a narrow cerebellar spatula under the microscope. The entire oculomotor nerve was shown for about 1 hour (Figure 3). The bulbar conjunctiva was gently opened using a pointed knife. The conjunctiva was softly separated toward the rear of the eyeballs until the far end of the interior oblique muscle was found and attached to the sclera region. Myofacial incision was not necessary. A pin-shaped electrode was inserted along the ordinate axis of the interior oblique muscle by pushing the surrounding conjunctiva and the electrode was properly fixed with snakelike retractors. Being the bulkiest of all of the extraocular muscles, the interior oblique muscle was easy to expose. As a result, this is an ideal method to induce extraocular muscles to discharge electricity.

Figure 3 Exposure of the oculomotor nerve of a beagle dog. Using an unobvious sylvian fissure, which could be identified by the naked eye as a mark, the oculomotor nerve was located in the posterior cavernous sinus.
Recording of electricity discharges by interior oblique muscles
The oculomotor nerve stem was hitched using a platinum filament stimulating electrode (shield electrode) at the tentorium cerebelli, and then lifted slightly. Efforts were made to avoid an increase in nervous tension. The electrode was properly fixed on a special bracket. The oculomotor nerve stem was given repetitive rectangular pulse stimulation with varied parameters and CMAPs of the inferior oblique muscle were collected by a pin-shaped leading electrode. During measurements,warm paraffin oil was used to protect nerves.
ONI
After completion of bioelectrical signal measurement, the stimulating electrode was carefully removed and the free oculomotor nerve stem was traced. A quick cut-off of the oculomotor nerve was made in the posterior cavernous sinus region and an end-to-end connection was immediately made. Using two needles, point-to-point 180° original position sutures were made with 11-0 non-traumatic sutures. CMAPs were recorded again, and a significant difference between CMAPs collected before and after ONI showed that the animal model was successfully established.
Statistical analysis
All data were analyzed and processed using SAS 8.0(SAS, Miami, Florida, USA) statistical software package.
The differences of the measurement data (mean±SD)were compared using the paired t test (P < 0.05).
Author contributions:Wenxiang Zhong participated in the study conception. Xuhui Wang provided and integrated the data.Wenchuan Zhang designed the study. Shiting Li examined and approved the article. Min Yang participated in the statistical analysis.
Conflicts of interest:None declared.
Funding:This study was supported by the National Natural Science Foundation of China, No. 30571907*; the Shanghai Natural Science Foundation, No. 05QMH1409.
Ethical approval:This study was approved by the Animal Ethics Committee, Shanghai Jiao Tong University in China.
Supplementary information:Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 6 No. 21, 2011 after selecting the “NRR Current Issue” button on the page.
[1]Ruiz-de-Río N, Arbizu-Duralde A, Miranda-Lloret P, et al. Bilateral sixth nerve and left third nerve palsy after head trauma. Arch Soc Esp Oftalmol. 2006;81(1):41-44.
[2]Coello AF, Canals AG, Gonzalez JM, et al. Cranial nerve injury after minor head trauma. J Neurosurg. 2010;113(3):547-555.
[3]Mwanza JC, Ngweme GB, Kayembe DL. Ocular motor nerve palsy: a clinical and etiological study. Indian J Ophthalmol. 2006;54(3):173-175
[4]Li S, Pan Q, Liu N, et al. Neurotization of oculomotor, trochlear and abducent nerves in skull base surgery. Chin Med J (Engl).2003;116(3):410-414.
[5]Fernandez E, Di Rocco F, Lauretti L, et al. Reinnervation of extraocular muscles by facial-to-oculomotor nerve anastomosis in rats: anatomic nuclear changes. Neurosurgery. 2003;53(2):414-415.
[6]Fernandez E, Pallini R, Lauretti L, et al. Motonucler changes after cranial nerve injury and regeneration. Arch Ital Biol. 1997;135(4):343-351.
[7]Fernandez E, Pallini R, Marchese E, et al. Reconstruction of peripheral nerves:the phenomenon of bilateral reinnervation of muscle originally innervated by unilateral motoneurons.Neurosurgery. 1992;30(3):364-369.
[8]Menovsky T, van der Bergh Weerman M, Kubista OL, et al.End-to-end versus peripheral nerve graft repair of the oculomotor nerve in rats: A comparative histological and morphometric study.Microsurgery. 1999;19(8):392-400.
[9]Fernandez E, Pallini R, Gangitano C, et al. Oculomotor nerve regeneration in rats. Functional, histological, and neuroanatomical studies. J Neurosurg. 1987;67(3):428-437.
[10]Seifert V, Stolke D. Laser-assisted reconstruction of the oculomotor nerve: experimental study on the feasibility of cranial nerve repair. Neurosurgery. 1989;25(4):579-582.
[11]Sekhar LN, Lanzino G, Sen CN, et al. Reconstruction of the third through sixth cranial nerves during cavernous sinus surgery. J Neurosurg. 1992;76(6):935-943.
[12]Currier DP, Ray JM, Nyland J, et al. Effects of electrical and electromagnetic stimulation after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 1993;17(4):177-184.
[13]Geremia NM, Gordon T, Brushart TM, et al. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205(2):347-359.
[14]Sekiya T, Hatayama T, Iwabuchi T, et al. Intraoperative recordings of evoked extraocular muscle activities to monitor ocular motor nerve function. Neurosurgery. 1993;32(2):227-235.
[15]Schlake HP, Goldbrunner R, Siebert M, et al. Intra-Operative electromyographic monitoring of extra-ocular motor nerves (Nn. III,VI) in skull base surgery. Acta Neurochir (wien). 2001;143(3):251-261.
[16]Sekula RF Jr, Bhatia S, Frederickson AM, et al. Utility of intraoperative electromyography in microvascular decompression for hemifacial spasm: a meta-analysis. Neurosurg Focus. 2009;27(4):E10.
[17]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
杂志排行
中国神经再生研究(英文版)的其它文章
- Expression of cyclinD1 in a rat model of oxygeninduced retinopathy☆
- Correlation between heart rate variability and pupillary reflex in healthy adult subjects under the influence of alcohol*☆
- Effect of Zhutan Tongluo Tang on fibrinolytic activity following intracerebral hemorrhage in rats*★
- Xuefuzhuyu decoction and astragalus prevent hypoxic-ischemic brain injury*★
- Effect of Yiqi Bushen prescription on hippocampal neuronal apoptosis in diabetic rats***☆
- Triptolide protects astrocytes from hypoxia/reoxygenation injury**★
