Expression of cyclinD1 in a rat model of oxygeninduced retinopathy☆
2011-07-19YouLiYuqiangZhangShaoyangShiXiaolongChen
You Li, Yuqiang Zhang, Shaoyang Shi, Xiaolong Chen
1Department of Ophthalmology, Shengjing Hospital, China Medical University, Shenyang 110004, Liaoning Province, China
2Department of Orthopedics, First Affiliated Hospital, China Medical University, Shenyang 110001, Liaoning Province, China
INTRODUCTION
An increasing number of studies indicate that retinal function is affected in retinopathy of prematurity[1-8]. Some mediators, such as vascular endothelial growth factor[9-11]and insulin-like growth factor-1[12-15], are known to regulate angiogenesis in eyes with retinopathy of prematurity. However, there are many other molecules involved in the complex process of angiogenesis, and detailed data are not available on the expression time course of these factors involved in angiogenesis.
CyclinD1 is a member of the G1 cyclins and is a major positive regulator of the cell cycle G1 restriction point. Overexpression of cyclinD1 renders the growth of normal cells less dependent on growth factors and accelerates passage through the G1 phase of the cell cycle, suggesting that increased expression may lead to the loss of normal regulatory constraints, and confer a growth advantage[16-17]. However, the relationship between cyclinD1 overexpression and prognosis is unclear.
We hypothesized that the emergence of neovascularization (NV) in oxygen-induced retinopathy (OIR) is associated with an increased expression of cyclinD1.
Therefore, this study sought to elucidate the time course of NV emergence, and to evaluate the expression of cyclinD1 in an OIR model.
RESULTS
Quantitative analysis of experimental animals
A total of 120 Sprague-Dawley rats aged 7 days were included in this study, and equally divided into an experimental group and a control group. In the experimental group, OIR was induced in 60 rats by placing postnatal day 7 (P7) pups in 75% oxygen for 5 days. An additional 60 rats were raised in ambient room air, and served as age-matched controls. Twelve rats were observed at days 12, 14, 17, 22, and 26(P12, P14, P17, P22, P26). Their left eyes were used for adenosine diphosphatase(ADPase) staining and cyclinD1 immunodetection, while their right eyes were used for the western blot assay. Two stretched preparations (6.67%) of the 30 ADPase-stained retina showed failure of the OIR model and therefore were excluded from the final analysis.
Retinal NV in OIR model rats
Retinal ADPase staining showed that the retinal vessels in the controlled rats grew well, and pre-retinal NV was not observed(Figure 1A). NV was seen in the OIR rats at the age of 12 days (Figure 1B). The retinal microvascular density increased in OIR rats(Figure 1C), exhibiting vasodilatation and tortuosity. Increasing NV was evident and reached a peak on P17. After 14 days of room air recovery (at the age of 26 days),the NV completely resolved (Figure 1D).
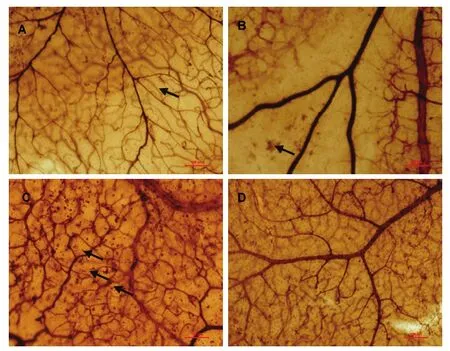
Figure 1 Adenosine diphosphatase staining of retina in oxygen-induced retinopathy rats. Scale bars: 100 μm.
CyclinD1 was expressed in retina of OIR rats
The expression of cyclinD1 protein in the retina of normal and OIR rats was determined with immunohistochemistry.
The experimental results showed that cyclinD1 protein was present at a low level in the normal retina (Figure 2A). In the OIR rats, cyclinD1-positive cells were scattered sparsely at P12, and highly dense cells with intense immunoreactivity were observed at P14, P17,and P22 (Figure 2B). The staining for cyclinD1 at P26 decreased to levels shown at P14 in the OIR retina. CyclinD1-positive cells were located at the ganglion cell/nerve fiber layer and in the superficial and deep retinal vessels in the retina (Figure 2, supplementary Figure 1 online).

Figure 2 Immunohistochemistry of the cyclinD1 protein in the retina of normal and oxygen-induced retinopathy rats.The yellow-brown or brown cyclinD1-positive cells are evident mainly at the ganglion cell/nerve fiber layer, and superficial and deep retinal vessels. Scale bars: 100 μm.
To confirm the immunohistochemical results, immunofluorescence staining was used to demonstrate the immunoreactivity of cyclinD1 protein in the retina. The immunofluorescence staining results showed that the green cyclinD1 protein signals were primarily confined to the cell nucleus (Figure 3). In addition, the cyclinD1 protein was expressed in the retina of OIR rats, apparently at the ganglion cell/nerve fiber layer and superficial and deep retinal vessels.
Changes in cyclinD1 protein expression in the retina of OIR rats
Western blot analysis was employed to test the expression of cyclinD1 protein in the retina of normal and OIR rats. The experimental findings showed that the cyclinD1 protein was present at low levels in the normal retina. In the retina of OIR rats, cyclinD1 protein expression time-dependently increased (Figure 4). The expression level of cyclinD1 protein decreased in the OIR rats at P12 compared with the control group (P <0.01), and began to increase at P14 compared with the control group (P < 0.05). CyclinD1 protein in the OIR group at P14, P17, and P22 was significantly higher than in the control group (P < 0.01). The signal decreased at P26, but was still higher than that of the normal control group (P < 0.01; Figure 5).
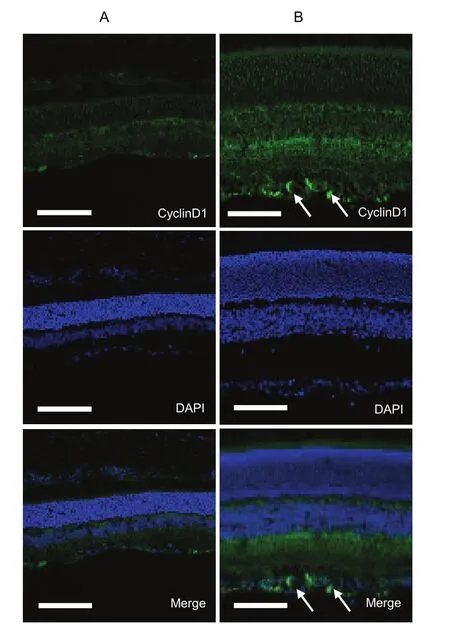
Figure 3 Immunofluorescence of the cyclinD1 protein in the retina of normal and oxygen-induced retinopathy rats.Scale bars: 100 μm. DAPI: 4’, 6-diamidino-2-phenylindole.

Figure 4 Western blot analysis of cyclinD1 protein in the retina of normal rats and oxygen-induced retinopathy rats.Lane 1: control group; lanes 2-6: postnatal 12, 14, 17, 22,and 26 days in the experimental group.
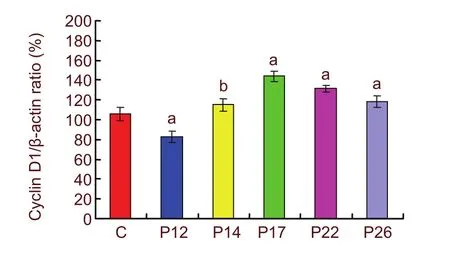
Figure 5 Western blot assay for the expression level of cyclinD1 protein in the oxygen-induced retinopathy rats.aP < 0.01, bP < 0.05, vs. control group. To quantify the temporal change in cyclinD1 protein, five eyes were evaluated at each survival time. C: Control; P12, 14, 17,22, and 26: postnatal 12, 14, 17, 22, and 26 days. Data are presented as mean ± SD.
DISCUSSION
Rat retinal development follows a pattern similar to that of the human. As in the human fetus, the rat retina is one of the last tissues to be vascularized. The retinal vasculature derives from mesenchymal precursor cells of the hyaloid artery, and vascularization proceeds in a wave-like fashion, beginning at the optic disk and growing outwards until the vessels reach the retinal periphery. In the human, retinal vascularization is usually complete at the time of birth; in the rat, the process is completed at about P15[18-19]. The retinal vasculature of a newborn rat pup, therefore, resembles that of a preterm infant; largely avascular and highly susceptible to retinopathy[20-21].
The emergence of retinal vascularization prior to room air recovery in the OIR animals is a phenomenon that has not previously been well characterized. In previous studies, investigators have typically studied NV after a period of room air recovery[22-24]. The present study also confirmed that NV emerges after 2 days of room air recovery and is maximal after 5 days of room air recovery in OIR. After 14 days of room air recovery (P26), the NV completely recedes.
In this study, the NV was associated with an increased expression of retinal cyclinD1. The retinal vessel was obliterated at P12. At the same time, the expression of cyclinD1 was down-regulated in OIR rats. At P14, NV emerged and cyclinD1 protein increased. We observed that both the incidence and severity of NV, and cyclinD1 protein expression reached a peak at P17. NV receded and cyclinD1 decreased at P26. Our data shows that retinal cyclinD1 is down-regulated and subsequently up-regulated prior to maximal NV in OIR, but retinal cyclinD1 is not increased at any time compared with room air control animals.
In summary, we have characterized the time course of NV in rats subjected to OIR. These data are useful when designing experiments to investigate the relationship of angiogenic factors to the presence and severity of NV in rat models of retinopathy of prematurity. In addition, we have correlated the temporal emergence and resolution of NV with cyclinD1 expression in the OIR model.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed at the Laboratory of Shengjing Hospital, China Medical University, China,between May 2010 and January 2011.
Materials
A total of 120 newborn Sprague-Dawley rats, of equal gender, aged 7 days were housed in an environmentally controlled room temperature 20 ± 1°C with a 12-hour light-dark cycle (light on 7:00-19:00). The animals had free access to food and water. Rats were obtained from the Laboratory Animal Center, Shengjing Hospital, China Medical University, China (license No. SYXK(Liao)2003-0019). The animal protocol complied with the Guidance Suggestions for the Care and Use of Laboratory Animals,issued by the Ministry of Science and Technology of China in 2006[25].
Methods
Establishment of OIR models in rats
Sixty 7-day-old rats in the experimental group were exposed to hyperoxia (75% O2) for 5 days (P7-P12), and then returned to room air to induce retinopathy[24,26-28].
Another 60 rats in the control group were kept at constant normoxia.
Retina tissue preparation of OIR and control rats
All rats were assigned to be sacrificed at specific time points (P12, P14, P17, P22, and P26), using deep anesthesia with excessive chloral hydrate (3 g/kg) administered by intraperitoneal injection. To evaluate vascular morphology and immunofluorescence staining, left eyes were removed and fixed with 4% neutral buffered formalin for 24 hours at 4°C. The retina of the right eyes was cryopreserved at -80°C for western blot analysis to measure cyclinD1 protein levels.
Retinal NV analysis of OIR and control rats detected by ADPase staining
The cornea, lens, and vitreous from the left eyes were surgically removed, and the retina was dissected and flat mounted. Retinas were processed for magnesium- activated ADPase staining, as described in previous studies[29]. ADPase-stained retina was flat mounted on microscope slides with a coverslip. NV was defined as clumps, sheets, or tufts of endothelial cells morphologically distinct from the normal vasculature, arising at the junction of the vascular and avascular retina[30]. Photographs were taken with an optical microscope (Olympus,Yokohama, Japan).
CyclinD1 expression of OIR and control rats detected by immunohistochemistry and immunofluorescence staining
Eyes were cryoprotected in sequential sucrose solution gradients (10%, 20%, and 30%, respectively, in PBS for 30 minutes, 1 hour, and overnight, respectively) at 4°C.
Eye cups were subsequently embedded in optimal cutting temperature compound and were frozen in a bath containing 2-methylbutane/liquid nitrogen. Eight micrometer-thick cryosections were collected on polylysine-S-coated slides for immunohistochemical analysis.
To block any nonspecific binding of the antibodies,retinal sections were incubated in 3% normal goat serum, 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO,USA) and 0.1 mol/L PBS (pH 7.4) for 30 minutes at room temperature. The sections were then incubated overnight at 4°C with the following rabbit anti-cyclinD1 polyclonal antibody (1: 100; Santa Cruz Biotechnology,Santa Cruz, CA, USA).
The next day, sections incubated with 0.1 mol/L PBS were used as negative controls, and the lack of immunostaining confirmed the specificity of the antibody. After the sections were rinsed with PBS/0.1% Tween 20, 200 μL of goat anti-rabbit IgG (1: 200; Wuhan Boster Bioengineering Co., Ltd., China) in PBS/0.1% Tween 20 was applied to the sections for 2 hours at room temperature.
Subsequently, the sections were washed with PBS/0.1%Tween 20 and incubated in ABC solution (Wuhan Boster Bioengineering Co., Ltd., China) at room temperature for 0.5 hour. The reaction was stopped by washing the tissue sections with PBS. Sections were air dried overnight,dehydrated and cover-slipped.
For immunofluorescence staining, sections were incubated with appropriate secondary antibody, goat anti-rabbit fluorescein isothiocyanate (1: 50; Wuhan Boster Bioengineering Co., Ltd.) for 1.5 hours at room temperature in dark, followed by PBS washing and DAPI(1: 200) staining for 5 minutes. Sections were rinsed,mounted, cover slipped and stored at -20°C in the dark.
Images were captured using a laser scanning confocal microscope (TCS SP2; Leica Microsystems Inc., Wetzlar,Germany).
Western blot analysis of cyclinD1 protein expression in OIR and control rats
Western blot analysis was performed to investigate the expression level of cyclinD1 protein in right eye retinas of experimental and control groups at each time point.
Protein homogenates from chloral hydrate euthanized rats were prepared by rapid homogenization in 10 volumes of lysis buffer (2 mmol/L EDTA, 10 mmol/L EGTA,0.4% NaF, 20 mmol/L Tris-HCl, pH 7.5). Samples were centrifuged at 17 000 × g, 4°C for 1 hour, and the protein concentration of soluble proteins was determined by the Coomassie G250 binding method[31]. The prepared protein lysates (12 μg/lane for each sample) were separated on 12% SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose membranes (Santa Cruz Biotechnology). Then these blots were incubated with rabbit anti-cyclinD1 polyclonal antibody (1: 500; Santa Cruz Biotechnology). The cyclinD1 protein bands on the immunoblots were visualized using the enhanced chemiluminescence kit (Santa Cruz Biotechnology). CyclinD1 protein bands and β-actin bands were scanned using Chemi Imager 5500 V2.03 software (AlPha InnCh,Miami, FL, USA), and IDVs were calculated by Fluor Chen 2.0 software (Olympus, Tokyo, Japan) and normalized against β-actin.
Statistical analysis
Data were presented as mean ± SD. One-way analysis of variance was used to compare differences among groups in the measurement of cyclinD1 by SPSS 13.0 software (SPSS, Chicago, IL, USA). Dunnett’s post hoc test was applied to compare specific group difference if one-way analysis of variance revealed a significant difference. A level of P < 0.05 was considered statistically significant.
Author contributions:You Li designed the experiments, wrote the paper, provided the reagents, materials, and the analysis tools. Yuqiang Zhang participated in experimental animal breeding and disposal, and provided data support. Shaoyang Shi was responsible for the study analysis and statistical analysis. Xiaolong Chen participated in study concept and design,article authorization and study instruction.
Ethical approval:The experimental protocol was approved by the Animal Ethics Committee of China Medical University,China.
Supplementary information:Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 6, No. 21, 2011 after selecting the “NRR Current Issue” button on the page.
[1]Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis.2007;10(2):133-140.
[2]Fleck BW, McIntosh N. Pathogenesis of retinopathy of prematurity and possible preventive strategies. Early Hum Dev. 2008;84(2):83-88.
[3]Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010;29(6):500-519.
[4]Akula JD, Favazza TL, Mocko JA, et al. The anatomy of the rat eye with oxygen-induced retinopathy. Doc Ophthalmol. 2010;120(1):41-50.
[5]Fletcher EL, Downie LE, Hatzopoulos K, et al. The significance of neuronal and glial cell changes in the rat retina during oxygeninduced retinopathy. Doc Ophthalmol. 2010;120(1):67-86.
[6]van Wijngaarden P, Brereton HM, Coster DJ, et al. Hereditary influences in oxygen-induced retinopathy in the rat. Doc Ophthalmol. 2010;120(1):87-97.
[7]Nakamura H, Yamazaki M, Ohyama T, et al. Role of angiotensin II type 1 receptor on retinal vascular leakage in a rat oxygeninduced retinopathy model. Ophthalmic Res. 2009;41(4):210-215.
[8]Byfield G, Budd S, Hartnett ME. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Invest Ophthalmol Vis Sci. 2009;50(7):3360-3365.
[9]Mechoulam H, Pierce EA. Retinopathy of prematurity: molecular pathology and therapeutic strategies. Am J Pharmacogenomics.2003;3(4):261-277.
[10]Werdich XQ, McCollum GW, Rajaratnam VS, et al. Variable oxygen and retinal VEGF levels: correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy.Exp Eye Res. 2004;79(5):623-630.
[11]Budd SJ, Thompson H, Hartnett ME. Association of retinal vascular endothelial growth factor with avascular retina in a rat model of retinopathy of prematurity. Arch Ophthalmol. 2010;128(8):1014-1021.
[12]Machalińska A, Modrzejewska M, Dziedziejko V, et al. Evaluation of VEGF and IGF-1 plasma levels in preterm infants--potential correlation with retinopathy of prematurity, clinical implications.Klin Oczna. 2009;111(10-12):302-306.
[13]Coleman RJ, Beharry KD, Brock RS, et al. Effects of brief,clustered versus dispersed hypoxic episodes on systemic and ocular growth factors in a rat model of oxygen-induced retinopathy.Pediatr Res. 2008;64(1):50-55.
[14]Kant S, Usha, Bhatia, et al. Pattern of retinopathy of prematurity and its correlation with insulin-like growth factor-1 (IGF-1). Ann Ophthalmol (Skokie). 2009;41(3-4):157-161.
[15]Pérez-Muñuzuri A, Fernández-Lorenzo JR, Couce-Pico ML, et al.Serum levels of IGF1 are a useful predictor of retinopathy of prematurity. Acta Paediatr. 2010;99(4):519-525.
[16]Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5(3):320-331.
[17]Das D, Pintucci G, Stern A. MAPK-dependent expression of p21(WAF) and p27(kip1) in PMA-induced differentiation of HL60 cells. FEBS Lett. 2000;472(1):50-52.
[18]Barnett JM, Yanni SE, Penn JS. The development of the rat model of retinopathy of prematurity. Doc Ophthalmol. 2010;120(1):3-12.
[19]Akula JD, Favazza TL, Mocko JA, et al. The anatomy of the rat eye with oxygen-induced retinopathy. Doc Ophthalmol. 2010;120(1):41-50.
[20]Dorfman AL, Polosa A, Joly S, et al. Functional and structural changes resulting from strain differences in the rat model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2009;50(5):2436-2450.
[21]Fulton A, Lachapelle P. Editorial for special issue: rat models of ROP. Doc Ophthalmol. 2010;120(1):1-2.
[22]Jiang JC, Hansen RM, Reynaud X, et al. Background adaptation in a rat model of retinopathy of prematurity. Doc Ophthalmol. 2002;104(1):97-105.
[23]Akula JD, Mocko JA, Benador IY, et al. The neurovascular relation in oxygen-induced retinopathy. Mol Vis. 2008;14:2499-2508.
[24]Liu K, Akula JD, Falk C, et al. The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity.Invest Ophthalmol Vis Sci. 2006;47(6):2639-2647.
[25]The Ministry of Science and Technology of the people’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[26]Zhang SX, Sima J, Shao C, et al. Plasminogen kringle 5 reduces vascular leakage in the retina in rat models of oxygen-induced retinopathy and diabetes. Diabetologia. 2004;47(1):124-131.
[27]Nakamura H, Yamazaki M, Ohyama T, et al. Role of angiotensin II type 1 receptor on retinal vascular leakage in a rat oxygeninduced retinopathy model. Ophthalmic Res. 2009;41(4):210-215.
[28]Langer S, Heiss C, Paulus N, et al. Functional and structural response of arterialized femoral veins in a rodent AV fistula model.Nephrol Dial Transplant. 2009;24(7):2201-2206.
[29]Leske DA, Wu J, Fautsch MP, et al. The role of VEGF and IGF-1 in a hypercarbic oxygen-induced retinopathy rat model of ROP.Mol Vis. 2004;10:43-50.
[30]Fulton AB, Hansen RM, Moskowitz A, et al. The neurovascular retina in retinopathy of prematurity. Prog Retin Eye Res. 2009;28(6):452-482.
[31]Wang SP, Xing SL. Determination of protein guantitation using the method of coomassie brilliant blue. Tianjin Huagong. 2009;23(3):40-42.
杂志排行
中国神经再生研究(英文版)的其它文章
- Establishment of a beagle dog model of oculomotor nerve injury**★
- Correlation between heart rate variability and pupillary reflex in healthy adult subjects under the influence of alcohol*☆
- Effect of Zhutan Tongluo Tang on fibrinolytic activity following intracerebral hemorrhage in rats*★
- Xuefuzhuyu decoction and astragalus prevent hypoxic-ischemic brain injury*★
- Effect of Yiqi Bushen prescription on hippocampal neuronal apoptosis in diabetic rats***☆
- Triptolide protects astrocytes from hypoxia/reoxygenation injury**★
