Mobilization of Peripheral Blood Stem Cells Using Regimen Combining Docetaxel with Granulocyte Colony-stimulating Factor in Breast Cancer Patients
2011-07-10JingYuJunRenLijunDiGuohongSongYulinZhuJieZhangXuLiangLiCheHanfangJiangJunJiaChunrongZhang
Jing Yu, Jun Ren*, Li-jun Di, Guo-hong Song, Yu-lin Zhu, Jie Zhang, Xu Liang, Li Che, Han-fang Jiang, Jun Jia, Chun-rong Zhang
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education),1Department of Breast Oncology,2Department of Transfusion, Peking University School of Oncology, Beijing Cancer Hospital & Institute, Beijing 100142, China
Mobilization of Peripheral Blood Stem Cells Using Regimen Combining Docetaxel with Granulocyte Colony-stimulating Factor in Breast Cancer Patients
Jing Yu1, Jun Ren1*, Li-jun Di1, Guo-hong Song1, Yu-lin Zhu1, Jie Zhang1, Xu Liang1, Li Che1, Han-fang Jiang1, Jun Jia1, Chun-rong Zhang2
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education),1Department of Breast Oncology,2Department of Transfusion, Peking University School of Oncology, Beijing Cancer Hospital & Institute, Beijing 100142, China
Objective:To evaluate the effectiveness and safety of the mobilization of peripheral blood hematopoietic stem cells by combining docetaxel with granulocyte colony-stimulating factor (G-CSF) in breast cancer patients.
Methods:A total of 57 breast cancer patients were treated with docetaxel 120 mg/m2. When the white blood cell (WBC) count decreased to 1.0×109/L, patients were given G-CSF 5 μg/kg daily by subcutaneous injection until the end of apheresis. Peripheral blood mononuclear cells (MNC) were isolated by Cobe Spectra Apheresis System. The percentage of CD34+cell was assayed by flow cytometry.
Results:At a median 6 of days (range 3-8) after the administration of docetaxel, the median WBC count decreased to 1.08×109/L (range 0.20-2.31). The median duration of G-CSF mobilization was 3 days (range 2-7). The MNC collection was conducted 8-12 days (median 10 days) after docetaxel treatment. The median MNC was 5.35×108/kg (range 0.59-14.07), the median CD34+cell count was 2.43×106/kg (range 0.16-16.69). The CD34+cell count was higher than 1.00×106/kg in 47 of 57 cases (82.46%) and higher than 2.00×106/kg in 36 cases (63.16%). The CD34+cell count was higher than 2.00×106/kg in 27 collections (23.68%). The MNC count and the CD34+cell count were correlated with the bottom of WBC after docetaxel chemotherapy (r=0.364, 0.502,P=0.005, 0.000). The CD34+cell count was correlated with the MNC count (r=0.597,P=0.000). The mobilization and apheresis were well tolerated in all patients. Mild perioral numbness and numbness of hand or feet were observed in 3 cases. No serious adverse events were reported.
Conclusion:Mobilization of peripheral blood hematopoietic stem cell by combining docetaxel with G-CSF was effective and safety in breast cancer patients.
Docetaxel; Granulocyte colony-stimulating factor (G-CSF); Mobilization; Stem cell
INTRODUCTION
High-dose chemotherapy (HDC) with hematopoietic stem cells support is regarded as an effective method to improve survival in some human malignant diseases such as leukemia, osteosarcoma, and germinal tumors. For breast cancer, some randomized clinical trails revealed that high-risk subgroups such as those with more than 10 positive lymph nodes, younger or premenopause and those with triple negative (hormone receptor (ER, PR) negative and Her2 negative) diseases, could benefit from high-dose chemotherapy with improved progression–free survival (PFS) and overall survival (OS)[1-3].
The Cancer and Leukemia Group B Trial 9741 demonstrated that the dosed-dense regimens significantly prolonged both disease-free survival (DFS) and OS without increasing toxicity[4]. Multiple high-dose chemotherapy is theoretically homologous as dose-dense chemotherapy but with a much higher peak and a relative prolonged intervention. Therefore we had developed a protocol utilizing multiple cycles of HDC with the support of peripheral blood hematopoietic stem cells (PBHSCs) for patients with metastatic breast cancer (MBC).
It has been documented that the recovery of bone marrow after chemotherapy-induced myelosuppression typically produces an abundant release of progenitor cells into the peripheral blood, and the magnitude of the increase in circulating progenitor cells appears to correlate with the intensity of the myelosuppression induced by chemotherapy[5]. Chemotherapeutic agents utilized for peripheral blood stem cell (PBSC) mobilization ideally produce short-term marked myelosuppression whilesparing stem cells from severe toxicity. An optimal chemotherapeutic mobilizing agent ideally should have known antitumor activity with manageable toxicities. Taxane including docetaxel has been shown to be an effective PBSC mobilizer as a single agent or in combination with cyclophosphamide, etoposide, and epirubicin[6,7]. Docetaxel has been reported to have significant activity against advanced breast cancer including anthracyclineresistant breast cancer[8-10]. The dose-limiting toxicity of docetaxel is grade 4 neutropenia, and the nonmyelocyte toxicity is mild. Considering the facts that cyclophosphamide, epirubicin, and paclitaxel have been widely used in adjuvant chemotherapy and docetaxel is superior to cyclophosphamide and other cytotoxic agents as PBSC mobilizer, docetaxel is to be incorporated into our subsequent high-dose regimen.
Because of these reasons above, in the study, we investigated the effectiveness and safety of docetaxel and granulocyte colony-stimulating factor (G-CSF) to mobilize PBSC for MBC patients who were supposed to receive multiple HDC.
MATERIALS AND METHODS
Patients Selection
Eligible patients were 18-70 years old with histologically and/or cytologically proven breast cancer. Patients were required to have ECOG performance status of 2 or lower and a life expectancy of over 6 month. Patients had received chemotherapy, radiation therapy and surgery were also eligible provided that the interventions at least 4 weeks before PBSC mobilization. Adequate bone marrow (Bloodtest:4.0×109/L ≤ white blood cell (WBC) count ≤10.0×109/L, 95 g/L ≤ hemoglobin count ≤165 g/L, 75×109/L ≤ platelet count ≤300×109/L), cardiac, pulmonary, renal, and hepatic function were required. Patients at pregnant and lactating were excluded. Patients with a history of myocardial infarction, peripheral neuropathy, life-threatening hypersensitivity to previous taxane, dysfunction of liver or kidney, and impaired marrow were also excluded. All patients provided written informed consents.
Protocol of Hematopoietic Stem Cell Mobilization
The mononuclear cells (MNC) were mobilized by chemotherapy and G-CSF. Docetaxel (QILU Pharmaceutical Co. Ltd. China) of 120 mg/m2was administered with premedicated dexamethasone 7.5 mg p.o. every 12 h ×6 doses starting 12 h prior to chemotherapy. When the WBC count of peripheral blood decreased to about 1.0×109/L, filgrastim (G-CSF, Chugai Pharmaceutical Co. Ltd. Japan) at a dose of 5 µg·kg-1·d-1were given subcutaneously until leukapheresis was completed.
Patients had a routine complete blood count daily following mobilization chemotherapy. Apheresis was initiated when the WBC count increased to ≥8.0×109/L using the COBE Spectra cell separator (COBE BCT, Lakewood, Co., USA). Control vein catheterization with double-lumen tube was inserted into the femoral vein. The circulating blood volume was set at 8-12 L, dexamethasone 10 mg was given 2 h before cell separation. 10% calcium gluconate 10 ml was intravenously given before and during cell separation.
All collections were mixed with culture medium containing DMSO (5%), human albumin (4%), and hydroxyethyl starch (3%) (final concentration) and cryopreserved at –80°C[11].
Assay of CD34+Cells in MNC
The MNC count was tested routinely. The CD34+cell percentage was assayed by flow cytometry according to standard ISHAGE program. CD34-PE/CD45-PC5 and isotype IG1-PE/IG1-PC5 antibodies were purchased from Immunotech Co. France.
Statistical Analysis
Statistical analyses were carried out using SPSS software Version 15.0. Date expressed by median and range of relevant data collected cells. Linear relationships between patients’ age, the WBC count at the bottom after docetaxel treatment, the WBC count and the ratio of lymphocytes plus monocytes (L+M) cells at the first collection day and the total number of collected MNC and CD34+cells were assessed using Spearman correlation. The nonparametric Mann-Whitney U test was used to compare the data with or without visceral metastasis, bone metastasis, previous chemotherapy cycles, radiotherapy and hormonal treatment on acquisition of MNC and CD34+cells. APvalue of less than 0.05 was regarded as statistically significant.
RESULTS
Patients Characteristics
A total of 57 patients with histologically and/or cytologically proven breast cancer were enrolled from April 2005 to May 2010. All patients agreed to accept high-dose chemotherapy with the support of peripheral blood stem cells and written consents were signed. All patients were female. The median age was 48 years (range 28 to 67 years). The pathological diagnoses were infiltrating ductal carcinoma for 49 cases, invasive lobular carcinoma for 3 cases, simple carcinoma for 3 cases, medullary carcinoma for one case and one case by fine needle aspiration cytology diagnosis of breast cancer.
The high-dose chemotherapy was administrated as adjuvant chemotherapy for 3 patients and relapse treatment for the other 54 patients. The mobilization chemotherapy, docetaxel treatment was used as first-line treatment in 23 cases, second-line treatment in 15 cases, third line treatment in 8 cases, and more than third line treatment in 10 cases. A total of 35 cases received previous endocrine therapy and 33 patients received previous radiation treatment.
Quality of Apheresis
In 57 breast cancer patients, the median dose of docetaxel was 200 mg (range 160-200 mg). At a median of 6 days (range 3-8), the mean white blood cells count was decreased to median 1.08×109/L (range 0.20-2.31). The median duration of G-CSF was 3 days (range 2-7) and the peripheral blood stem cell collection was started on median 10 days (range 8-12) after docetaxel mobilization. Totalled114 collections were performed in 57 patients. Before the first time blood cell separation, the median WBC count was 13.33×109/L (range 5.27-37.80), the median L+M ratio was 27.4% (range 3.10-88.00) and the median L+M count was 3.28×109/L (range 0.60-13.90).
The median MNC count was 5.35×108/kg (range 0.59-14.07) and the median CD34+cells count was 2.43×106/kg (range 0.16-16.69) in 57 patients (Table 1). The CD34+cell counts were less than 1.00×106/kg (median 0.68×106/kg) in 10 cases (17.54%) and more than 1.00×106/kg in the other 47 patients (82.46%) among which CD34+cell counts were more than 2.00×106/kg in 36 cases (63.16%). The CD34+cells counts were more than 2.00×106/kg at single collection in 27 collections (23.68%) (Table 2).
Factors Affecting Apheresis
The factors that could affect the quality of apheresis were analyzed and summarized in Table 3. Patients’ age, WBC count and the ratio of L+M cells at the first collection day had no relation with the number of collected MNC and CD34+cells. The total number of collected MNC and CD34+cells was correlated with the WBC count at the bottom after docetaxel treatment (correlation coefficient, r=0.364, 0.502,P=0.005, 0.000), meanwhile the total number of collected CD34+cells also had relation with the amount of collected MNC (r=0.597,P=0.000) and was positively correlated.
According to the clinical characteristics of patients before mobilization, compared the data with or without visceral metastasis, bone metastasis, previous cycles of chemotherapy, radiation therapy and hormonal treatmenton acquisition of MNC and CD34+cells, the results were not statistically significant. The results are shown in Table 4.

Table 1.Collection results of 57 breast cancer patients

Table 2.Collected CD34+cells results of 57 breast cancer patients
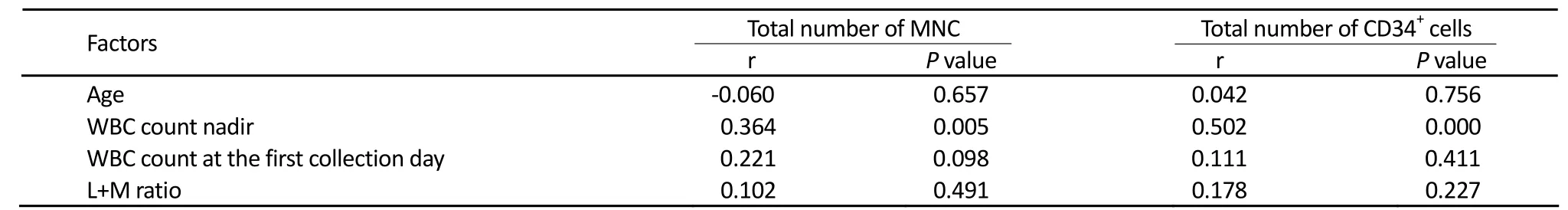
Table 3.Factors affecting apheresis
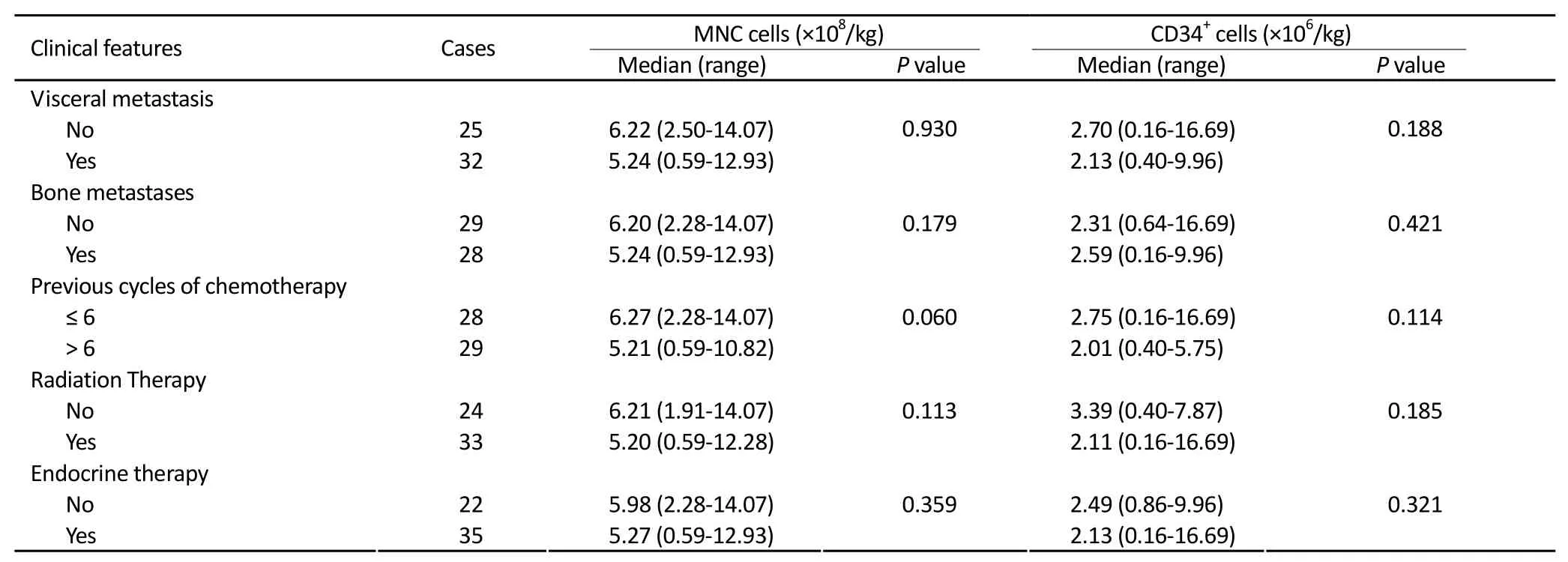
Table 4.The impact of clinical features of 57 cases of breast cancer patients on the acquisition of MNC and CD34+cells
Adverse Events
The G-CSF mobilization and cell separation of peripheral blood stem cells were well tolerated. During and after the cell collection, no body weight loss, painful venous catheter, bleeding, and thrombosis were observed. Three patients had experienced mild perioral numbness and numbness of hand and foot. The symptom was slight and disappeared after treatment with calcium gluconate.
DISCUSSION
Hematopoietic stem cells consist of 0.1%-1% bone marrow and CD34+is popularly used as cell marker to identify these subsets from other blood cells. The percentage of CD34+cells is 0.01%-0.1% in peripheral blood mononuclear cells. To fulfill successful peripheral derived hematopoietic stem cell transplantation requires administrating effective mobilizor to increase the percentage of CD34+cells in peripheral blood. Combining chemotherapy and G-CSF is currently being considered the best program for HSC mobilization[12].
G-CSF is a popular used cytokine in clinic to treat neutropenia and leucopenia caused by chemotherapy. It has been proved that G-CSF administration for 4 to 5 days could dramatically increase the number of peripheral blood progenitor cells to 40 to 80 times[13,14]. Imamura et al.[15]found that G-CSF could induce CD34+CD10+CD19-Lin-cells and CD34+CD10+CD19+Lin-cells to differentiate into B cells and natural killer cells. G-CSF could not only stimulate the proliferation of colony-forming unit in bone marrow but also mobilize mature cells releasing from bone marrow to peripheral blood. For the myelosuppression induced by chemotherapy, G-CSF could promote the release of large number of progenitor cells from bone marrow into circulation and effectively increase the number of the peripheral blood stem/progenitor cells[16]. The effects of G-CSF are dose-dependent and at dosage of up to 10-16 μg·kg-1·d-1the effects reach to maximum[17]. G-CSF could synergize with chemotherapy in mobilizing hematopoietic stem cells. The yield of CD34+cells is 10 to 30 times of that without mobilization[5].
An ideal mobilization chemotherapy regimen for metastatic breast cancer patients who previously received adjuvant chemotherapy should not only mobilize large numbers of CD34+cells into the circulation but also had antitumor activity to control tumor growth during this phase. Therefore, the selection of an appropriate mobilization agent for MBC patients should take into consideration both the following conditioning regiment and prior drug exposure. With the increased use of cyclophosphamide and anthracycline-containing regimens in the adjuvant treatment, the choice of mobilization regimen for patients supposed to receive multiple HDC with PBSC support for MBC is somewhat limited.
Docetaxel demonstrates significant antitumor activity with limited toxicity in breast cancer patients including heavily pretreated patients[8-10]. Similar to paclitaxel, docetaxel does not cause significant hematological toxicity and is not known to be damaging to stem cells[6,18]. A number of clinical trails has suggested that as second-line treatment, docetaxel has a clinical response rate of 27% to 48% and a median progression-free time of 4 to 6 months for MBC patients[8-10,19]. The dose-limiting toxicity of docetaxel is grade 4 neutropenia and the non-myeloid toxicity such as congestive heart-failure,hepatitis, peripheral neuritis etc are tolerable. The myelosuppression of docetaxel is short and recovers rapidly[6,18].
Docetaxel either single or in combination with cyclophosphamide or anthracycline has been reported to successfully mobilize CD34+cells from peripheral blood[20-22]. Prince et al.[20]prospectively evaluated docetaxel (100 mg/m2) with G-CSF (10 μg/kg S.C., daily) for mobilization efficiency in 26 patients with breast cancer. The median PBSC CD34+cell content ranged from 1.2 to 5.9×106/kg per day during days 7 to 11 with a median CD34+content of 3.4×106/kg (range 0.07–15.6) for total 72 PBSC collections. Fifteen patients obtained a peripheral blood progenitor cell (PBPC) collection exceeding 5×106/kg on a single day of collection. Twenty-two patients subsequently received repetitive high-dose therapy (HDT) and PBSC transplantation with 57 cycles of HDT having been delivered. The median time to reach absolute neutrophil count (ANC) at >0.5×109/L and 1.0×109/L was 10 days and 11 days respectively. The median time for platelets to reach greater than 20×109/L, 50×109/L and 100×109/L was 13 days, 17 days and 23 days respectively. Laport et al.[21]explored docetaxel as a peripheral blood stem cell mobilizing agent in 33 women with stage III-IV ovarian carcinoma (n=10) or stage II-IV breast cancer (n=23) who were in preparation for high-dose chemotherapy. The median number of prior regimens received before mobilization was two (range 1–3). The docetaxel was administered followed by G-CSF (10 μg/kg/day) starting 4 days after docetaxel administration. Thirty two (97%) patients began leukapheresis within 7–9 days after receiving docetaxel and 27 (82%) ≥2.0×106/kg CD34+cells were collected. None of the patients experienced neutropenic fever or required blood or platelet transfusion support.
In our study, PBSCs were mobilized by docetaxel at 120 mg/m2followed by 5 μg/kg/day G-CSF subcutaneous injection when the white blood cell count decreased to 1.0×109/L in 57 metastatic breast cancer patients. At a median 6 days (range 3-8) after the administration of docetaxel, the G-CSF was given and the median duration was 3 days (range 2-7) and the peripheral blood stem cell collection was started on median 10 days (range 8-12) after docetaxel mobilization. The median collected MNC count was 5.35×108/kg (range 0.59-14.07) and the median CD34+cell count was 2.43×106/kg (range 0.16-16.69). The CD34+cell counts were more than 1.00×106/kg in the other 47 patients (82.46%) among whom CD34+cell counts were more than 2.00×106/kg in 36 cases (63.16%). The CD34+cell counts were more than 2.00×106/kg at single collection in 27 collections (23.68%). These results are similar to previous reports[20-22]. The total number of collected MNC and CD34+cells had correlated with the WBC count at the bottom after docetaxel treatment (P=0.005, 0.000). This suggests thatchemotherapy mobilization plan should be performed for bone marrow suppression, it helps to collect more cells. According to the clinical characteristics of patients before mobilization, the acquisitions of MNC and CD34+cells with or without visceral metastasis, bone metastasis, previous cycles of chemotherapy, radiation therapy and hormonal treatment on were compared. Though the results were not statistically significant, we still have to take into account the decreases of bone marrow function in advanced breast cancer patients who experienced multiple courses of treatment, so the number of collected CD34+cells will be affected. We should choose the right time and mobilization program, to collect more CD34+cells, in order to facilitate patient’s follow-up treatment. The mobilization was well tolerated with no serious adverse events.
Our data demonstrate that docetaxel is an appropriate choice to mobilize PBSCs for patients with breast cancer who have been previously exposed to anthracyclines or cyclophosphamide. Notably, no patient experienced febrile neutropenia or required platelet transfusions. We conclude that docetaxel with G-CSF could effectively mobilize PBSC without serious adverse events.
REFERENCES
1. Gluz O, Nitz UA, Harbeck N, et al. Triple-negative high-risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: results of WSG AM-01 trial. Ann Oncol 2008; 19:861-70.
2. Zander AR, Schmoor C, Kröger N, et al. Randomized trial of high-dose adjuvant chemotherapy with autologous hematopoietic stem-cell support versus standard-dose chemotherapy in breast cancer patients with 10 or more positive lymph nodes: overall survival after 6 years of follow-up. Ann Oncol 2008; 19:1082-9.
3. Nitz UA, Mohrmann S, Fischer J, et al. Comparison of rapidly cycled tandem high-dose chemotherapy plus peripheral-blood stem-cell support versus dose-dense conventional chemotherapy for adjuvant treatment of high-risk breast cancer: results of a multicentre phase III trial. Lancet 2005; 366:1935-44.
4. Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003; 21:1431-9.
5. Stadtmauer EA, Schneider CJ, Silberstein LE. Peripheral blood progenitor cell generation and harvesting. Semin Oncol 1995; 22: 291-300.
6. Gómez-Espuch J, Moraleda JM, Ortuño F, et al. Mobilization of hematopoietic progenitor cells with paclitaxel (taxol) as a single chemotherapeutic agent, associated with rhG-CSF. Bone Marrow Transplant 2000; 25:231-5.
7. Weaver CH, Schwartzberg LS, Birch R, et al. Collection of peripheral blood stem cells following administration of paclitaxel, cyclophosphamide, and filgrastim in patients with breast and ovarian cancer. Biol Blood Marrow Transplant 1997; 3:83-90.
8. Burris HA 3rd. Single-agent docetaxel (Taxotere) in randomized phase III trials. Semin Oncol 1999; 26:1-6.
9. Burstein HJ, Manola J, Younger J, et al. Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol 2000; 18:1212-9.
10. O'Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracyclinepretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 2002; 20:2812-23.
11. Gorin NC. Collection, manipulation and freezing of haemopoietic stem cells. Clin Haematol 1986; 15:19-48.
12. Ikeda K, Kozuka T, Harada M. Factors for PBPC collection efficiency and collection predictors. Transfus Apher Sci 2004; 31:245-59.
13. DeLuca E, Sheridan WP, Watson D, et al. Prior chemotherapy does not prevent effective mobilisation by G-CSF of peripheral blood progenitor cells. Br J Cancer 1992; 66:893-9.
14. Teshima T, Harada M, Takamatsu Y, et al. Cytotoxic drug and cytotoxic drug/G-CSF mobilization of peripheral blood stem cells and their use for autografting. Bone Marrow Transplant 1992; 10:215-20.
15. Imamura R, Miyamoto T, Yoshimoto G, et al. Mobilization of human lymphoid progenitors after treatment with granulocyte colonystimulating factor. J Immunol 2005; 175:2647-54.
16. Dührsen U, Villeval JL, Boyd J, et al. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood 1988; 72:2074-81.
17. To LB, Haylock DN, Simmons PJ, et al. The biology and clinical uses of blood stem cells. Blood 1997; 89:2233-58.
18. Burtness BA, Psyrri A, Rose M,et al. A phase I study of paclitaxel for mobilization of peripheral blood progenitor cells. Bone Marrow Transplant 1999; 23:311-5.
19. Baur M, van Oosterom AT, Diéras V, et al. A phase II trial of docetaxel (Taxotere) as second-line chemotherapy in patients with metastatic breast cancer. J Cancer Res Clin Oncol, 2008, 134:125-35.
20. Prince HM, Toner GC, Seymour JF, et al. Docetaxel effectively mobilizes peripheral blood CD34+ cells. Bone Marrow Tansplant 2000; 26: 483-7.
21. Laport GG, Fleming GF, Waggoner S, et al. A phase II trial of docetaxel for peripheral blood stem cell mobilization for patients with breast cancer and ovarian cancer. Bone Marrow Transplant 2001; 27: 677-81.
22. Fleming DR, Goldsmith J, Goldsmith GH, et al. Mobilization of peripheral blood stem cells in high-risk breast cancer patients using G-CSF after standard dose docetaxel. J Hematother Stem Cell Res 2000; 9:855-60.
10.1007/s11670-011-0049-8
2010−08−17;Accepted2010−11−23
This work was supported by a grant from the Beijing Capital Development Foundation for Medical Sciences (No. 2007-2053).
*Corresponding author.
E-mail: renjun9688@yahoo.com
© Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2011
杂志排行
Chinese Journal of Cancer Research的其它文章
- Cancer Incidence And Mortality in China, 2006
- Time Trends of Cancer Incidence in Urban Beijing, 1998-2007
- Health Economic Assessment for Screening of Gastric Cancer in A High Risk Population in Northeastern China
- Prevalence of HPV Infection And Cervical Intraepithelial Neoplasia And Attitudes towards HPV Vaccination among Chinese Women Aged 18-25 in Jiangsu Province
- Plasma Vitamin D Levels And Vitamin D Receptor Polymorphisms Are Associated with Survival of Non-small Cell Lung Cancer
- Current Status of Diagnosis And Treatment of Primary Breast Cancer in Beijing, 2008
