Health Economic Assessment for Screening of Gastric Cancer in A High Risk Population in Northeastern China
2011-07-10LingZhouPengGuanLipingSunQinchengHeYuanYuanBaosenZhou
Ling Zhou, Peng Guan, Li-ping Sun, Qin-cheng He, Yuan Yuan, Bao-sen Zhou*
1Department of Epidemiology, School of Public Health,2Cancer Institute, First Affiliated Hospital, China Medical University, Shenyang 110001, China
Health Economic Assessment for Screening of Gastric Cancer in A High Risk Population in Northeastern China
Ling Zhou1, Peng Guan1, Li-ping Sun2, Qin-cheng He1, Yuan Yuan2, Bao-sen Zhou1*
1Department of Epidemiology, School of Public Health,2Cancer Institute, First Affiliated Hospital, China Medical University, Shenyang 110001, China
Objective:To assess economic cost-effects for the screening programs of gastric cancer in a high risk population in northeastern China.
Methods:The data were collected from November 2001 to December 2003. The multi-stage sampling to define the screening group and the control group was applied in this study. Two stage screening programs were used in the study. An epidemiological survey and serum PG test were carried out in the first stage. The endoscopy and pathological examination were performed in the second stage screening. Effectiveness was assessed by the increased quality adjusted life-year (QALY) because of reduced gastric cancer deaths in screening.
Results:A total of 27,970 participants (n=7,128 screening group, n=20,842 control group) were enrolled in the survey. Twenty nine gastric cancer cases were detected in the screening group with 20 cases in the early stage and 9 cases in the advanced stage, respectively. Eighty six gastric cancer cases were detected in the control group, all of whom were in the advanced stage and had died before the study finished. The screening and treatment of 29 cases cost $152,227 and $5,249 per each case, respectively. The costs were $459 to gain per QALY.
Conclusion:The screening program of gastric cancer used in our study is an economic and society-beneficial measure to detect gastric cancer in high risk area. The methods fit China's present economic development level.
Gastric cancer; Screening; Economics
INTRODUCTION
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related death worldwide, especially in Central Europe, South America, and Asia[1-3]. In China, the incidence of gastric cancer is among the highest of the world, with an estimated 160,000 deaths each year[4,5]. Gastric cancer detected at a symptomatic stage often has poor survival and high recurrence. Early gastric cancer is typically small and asymptomatic, and the low mortality from gastric cancer is mainly due to early detection. When diagnosed at an early stage, 5-year survival rates for gastric cancer exceed 90%, while for an advanced gastric cancer, 5-year survival rates are below 50%[6-8]. Therefore, early detection and treatment is an important approach to achieve good prognosis and reduce death from gastric cancer[9].
Despite the high incidence rate of gastric cancer in China, there is no nationwide screening program to date. Early detection of gastric cancer only relies on the opportunistic screening[9]. A series of comprehensive 1111111preventive methods have been applied since 1997 in China, including primary and secondary prevention, especially in high-risk populations of gastric cancer.
Zhuanghe County is located in of Liaoning Province with a total population of 900,000, and in the seaside of the Huang Sea. The incidence and mortality of gastric cancer in this county are higher than the average level of China[10]. From 1996 to 2000, the gastric cancer death counted 8.25% of all causes death, and 38.94% of all cancer death in Zhuanghe County[11]. The investigations of China Medical University have worked in Zhuanghe for more than 20 years and established a comprehensive gastric cancer prevention network[12].
Serum pepsinogen (PG) has been found to be a marker of gastric mucosal status, and associated with gastric cancer[13]. Measurement of serum PG is a popular non-invasive serological screening test for identifying individuals with extensive gastric atrophy that warrants more intensive surveillance for high-risk of gastric cancer[14]. Endoscopy and pathological examinations have been increasingly used for gastric cancer diagnosis as a golden standard because of a high detection rate.
The two-stage screening program has good sensitivity and specificity in the detection of gastric cancer. Therefore, the relative costs, benefits and long term outcome are important factors to consider when evaluating the publichealth relevance of this intervention. This study aims to evaluate the economic cost-effects of the screening program for gastric cancer in a high-risk population in the Northeast China.
MATERIALS AND METHODS
Definition of Screening Population
The multi-stage sampling to define the screening group and the control group was applied in this study. Firstly, villages were stratified into two parts based on the landform, seaside or hill. Secondly, then each part was divided into two groups, the screening group and the control group, using the method of complete random cluster sampling.
Among the 28 sampled villages, 15 villages were assigned to the screening group and 13 villages were assigned to the control group. There were no difference between two groups in the geographical environment, the humanity environment and other basic characteristics.
Screening
The design of the screening is presented in Figure 1.
The target population was those who had the family history of gastric cancer, and /or were over 35 years old and had the history of gastric illness, or had evident gastric illness symptoms. A two-stage screening program was conducted. An epidemiological survey and serum PG test were carried out in the first stage. If the serum PG I/II ratio was less than 9 and the participant was younger than 50 years old, or the ratio was less than 7.5 and subject was over 50 years old, or PG I was less than 70 μg/L in male, or PG I was less than 60 μg/L in female, the participant would receive the second stage screening with endoscopy and pathological examination.
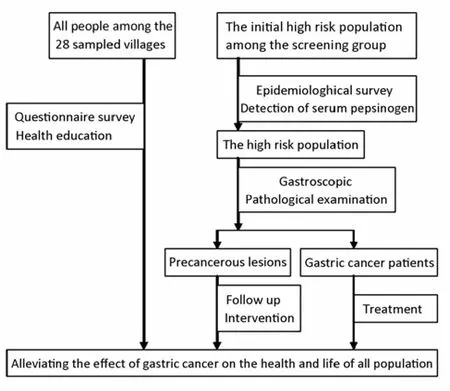
Figure 1.The screening trial sketch
Calculation of Costs
The direct costs for PG tests, endoscopy/pathology and labor in the screening were estimated by the program. The data for the other expenditure for gastric cancer treatment were obtained from hospital finance department, patients and relatives. The treatment costs included out-patient cost, drug cost, surgery cost and hospitalization cost. Because the screening program had certain time span and the medical cost had largely fluctuated, costs and benefits were discounted to the price level in 2001. The discount rate was defined by national retail price general index and resident consumption price general index. A 5% discount rate was adopted in our study.
Effectiveness can be seen from the increased quality adjusted life-year (QALY) because of reduced gastric cancer deaths in screening. We used the World Health Organization Quality of Life (WHOQOL)- BREF to measure the survival quality of the gastric cancer patients, and then to estimate the effectiveness.
Data Collection and Analysis
The data were collected from November 2001 to December 2003. The first stage screening took place in the appointed clinic in each village, while the second stage screening was carried out in the Zhuanghe Central Hospital and Zhuanghe Hospital of Traditional Chinese Medicine. The health workers who provided screening had been trained. Cost data were obtained from hospital finance departments, patients and their relatives. Annual income was acquired from the local government finance department of screening villages. Quantitative data were input and analyzed using SPSS 16.0.
Ethical Issues
This study was approved by the Ethics Committee of China Medical University and implemented under the full knowledge and consent by participants, who were assured of the security of the information. Written, informed consent was received from all study participants before the screening.
RESULTS
A total of 27 970 participants (n=7 128 screening group, n= 20 842 control group) were included in the survey. Of them, 50.86% were males while 49.14% were females in the screening group, and 50.54% were males while 49.46% were females in the control group (Table 1).
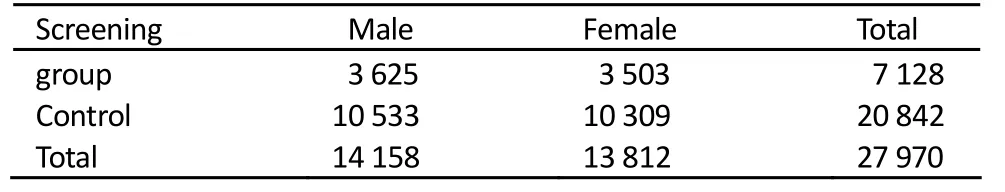
Table 1.Composition of the samples in high risk area of Zhuanghe, China, November 2001 to December 2003
Screening Result
Twenty nine gastric cancer cases were detected in the screening group. Twenty cases were in the early stage, and 9 cases were in the advanced stage. Eighty six gastric cancer cases were founded in the control group, who were all in the advanced stage and had died before the study finished.
Cost-effective Analysis
Costs of the Screening Group
The costs of first stage screening included survey costs and detection costs of serum PG. The second stage costs included the cost of endoscopy, pathological examination and treatment. The cost for two-stage screening for per person and per gastric cancer case was $23.1 and $4,041.1, respectively (Table 2). The total screening and treatment of 29 cases cost $152,227 and $5,249 for each case, respectively (Table 3).
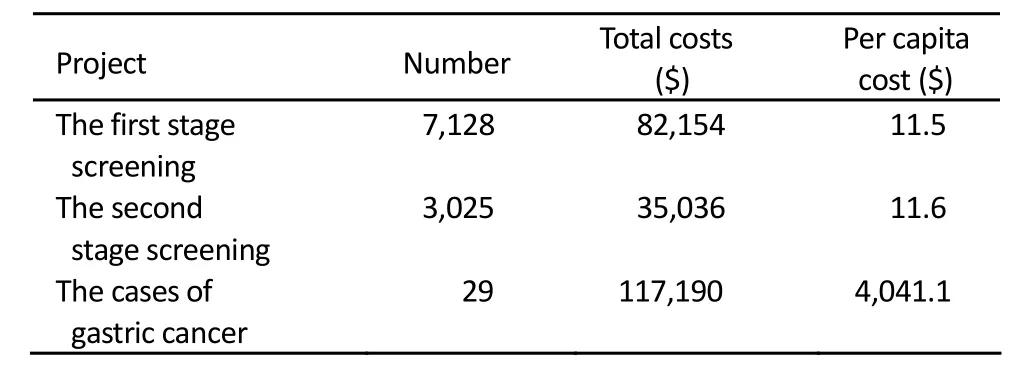
Table 2.Screening costs of gastric cancer in high risk area of Zhuanghe, China, 2001-2003
Costs of the Control Group
There were 86 gastric cancer cases among 20,842 participants. The total costs of diagnosis and treatment were $363,653. The costs of each case were $4,228 (including out-patient costs, diagnosis costs, radiotherapy and chemotherapy costs, surgery and hospitalization costs).
If the 29 cases in the screening group were not detected in screening, they needed $122,627 for diagnosis and treatment according to the costs of diagnosis and treatment in the control group. Screening program had an extra cost of $29,600 ($152,227−$122,627=$29,600) compared to no screening, which means $1,020 for each ($29,600/29= $1,020) to reduce a gastric cancer death.

Table 3.Screening and treatment costs of gastric cancer in high risk area of Zhuanghe, China, 2001-2003
Cost-utility Analysis
Two hundred cases were interviewed (50% were males while 50% were females). The score of each project had been transformed to the standard HRQOL score by forward and standardized processing. The average score was obtained by sex. The computation of the standard HRQOL score used hundred-mark system. The effectiveness value (u) =the standard HRQOL score/100. The range of u was 0-1. u of male was 0.68 while female was 0.66.
The costs were $459 to gain per QALY ($152,227/331.44 =$459). Further description of Information sources is presented in Table 4.

Table 4.Increased QALY due to reduced death of gastric cancer in high risk area of Zhuanghe, China, 2001-2003
DISCUSSION
Although mass screening programs exist in Asian countries with high prevalence of gastric cancer, there is paucity of data for the cost-effectiveness analysis[9]. Comprehensive estimate of the effectiveness should be done before spreading widely in the general population. Health planners in developing countries have far more needs than resources to address them, and even the most effective interventions must be economically feasible to implement[15]. The evaluation of gastric cancer screening programs should include not only accuracy, validity and medicine practice but also the value of health economics. China is the world's most populous developing country and has high incidence of gastric cancer. Although the screenings are relatively inexpensive for each person, population-wide screenings could be enormously costly in China.
To our knowledge, few epidemiology and economic study of gastric cancer screening studies have been conducted in northeastern China. The present study analyzed the costs and effectiveness associated with screening and treatment for the people with high risk.
Serum PG program is a popular non-invasive serological screening test for gastritis and predicting marker for gastric cancer. In a pooled analysis of Japanese studies that assessed about 300,000 subjects, the sensitivity of serum pepsinogen testing for gastric cancer was 77% and the specificity was 73%[16]. The PG I/PG II ratio is an acceptable biomarker for atrophic chronic gastritis, H. pylori-related corpus-predominant or multifocal and predictive of an increased risk of gastric cancer atrophy[17,18]. Those studies suggested that the serum PG test is suitable for the first stagein screening of gastric cancer.
In this study, we found that detecting and treatment for each case needed $5,249 and increasing of per QALY needed $459. In fact, we just calculated the direct cost including screening cost and treatment cost. The indirect, such as the income loss caused by delay work because of screening and treatment, was not considered, which may make our result below the true costs.
The screening program of gastric cancer used in our study is an economic and society-beneficial measure to detect gastric cancer in high risk area. The program fits the present economic development level in China.
Acknowledgements
Thanks to the doctors who work at the 28 sampled villages’clinics in Zhuanghe County..
REFERENCES
1. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354-62.
2. Parkin DM. International variation. Oncogene 2004; 23:6329-40.
3. Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. Lyon: IARC Press; 2004.
4. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Gastric Cancer 2007.
5. Chen XM, Chen GY, Wang ZR, et al. Detection of micrometastasis of gastric carcinoma in peripheral blood circulation. World J Gastroenterol 2004; 10: 804-808.
6. Kikuchi S, Katada N, Sakuramoto S, et al. Survival after surgical treatment of early gastric cancer: surgical techniques and long-term survival. Langenbecks Arch Surg 2004; 389:69-74.
7. Onodera H, Tokunaga A, Yoshiyuki T, et al. Surgical out-come of 483 patients with gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer. Hepatogastroenterology 2004; 51:82-5.
8. Kim JP, Nam SJ, Yang HK, et al. Clinical analysis of early gastric cancer. Korean J Gastroenterol 1991; 23:428-35.
9. Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 2008; 9:279-87.
10. Guo HQ, Peng G, Zhou BS, et al. The Initial Epidemiological Evaluation to Comprehensive Prevention to Gastric Carcinoma. Chin J Health Stat (in Chinese) 2001; 18:69-73.
11. Guo HQ, Guan P, Shi HL, et al. Prospective cohort study of comprehensive prevention to gastric cancer. World J Gastroenterol 2003; 9:432-6.
12. Yuan Y. Comprehensive Prevention and Treatment for High Risk Population in High Risk Area with Gastric Cancer in Zhuanghe Region, Liaoning Province. Bull Chin Cancer (in Chinese) 2005; 14:307-11.
13. Miki K, Ichinose M, Kawamura N, et al. The significance of low serum pepsinogen levels to detect stomach cancer associated with extensive chronic gastritis in Japanese subjects. Jpn J Cancer Res 1989; 80:111-4.
14. Yoshida S, Kozu T, Gotoda T, et al. Detection and treatment of early cancer in high-risk populations. Best Pract Res Clin Gastroenterol 2006; 20:745-65.
15. Ramsey S. Gut check: can cost-effectiveness analysis help eliminate gastric cancer in Asia? Cancer Epidemiol Biomarkers Prev 2007; 16:873-4.
16. Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, et al. Validity of serum pepsinogen I/II ratio for the diagnosis of gastric epithelial dysplasia and intestinal metaplasia during the follow-up of patients at risk for intestinal-type gastric adenocarcinoma. Neoplasia 2004; 6:449-56.
17. Broutet N, Plebani M, Sakarovitch C, et al. Eurohepygast Study Group. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer 2003; 88:1239-47.
18. Kikuchi S, Kurosawa M, Sakiyama T, et al. Long-term effect of Helicobacter pylori infection on serum pepsinogens. Jpn J Cancer Res 2000; 91:471-6.
10.1007/s11670-011-0021-7
2010−07−29;Accepted2010−11−26
This study was supported by National Key Technologies R&D Program of China during the 10th Five-year Plan Period (No. 2004BA703B04-02).
*Corresponding author.
E-mail: bszhou@mail.cmu.edu.cn
© Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2011
杂志排行
Chinese Journal of Cancer Research的其它文章
- Cancer Incidence And Mortality in China, 2006
- Time Trends of Cancer Incidence in Urban Beijing, 1998-2007
- Prevalence of HPV Infection And Cervical Intraepithelial Neoplasia And Attitudes towards HPV Vaccination among Chinese Women Aged 18-25 in Jiangsu Province
- Plasma Vitamin D Levels And Vitamin D Receptor Polymorphisms Are Associated with Survival of Non-small Cell Lung Cancer
- Current Status of Diagnosis And Treatment of Primary Breast Cancer in Beijing, 2008
- Factors Affecting Mesenchymal Stromal Cells Yield from Bone Marrow Aspiration
