Protective effects of glutamine preconditioning on ischemia-reperfusion injury in rats
2011-07-07WanXingZhangLiFangZhouLeiZhangLeiBaoChunChengWangHuiYanMengandWenYin
Wan-Xing Zhang, Li-Fang Zhou, Lei Zhang, Lei Bao, Chun-Cheng Wang, Hui-Yan Meng and Wen Yin
Shijiazhuang, China
Protective effects of glutamine preconditioning on ischemia-reperfusion injury in rats
Wan-Xing Zhang, Li-Fang Zhou, Lei Zhang, Lei Bao, Chun-Cheng Wang, Hui-Yan Meng and Wen Yin
Shijiazhuang, China
BACKGROUND:Hepatic ischemia-reperfusion injury is a common phenomenon in hepatic surgical procedures and can result in further severe damage. This study aimed to investigate the protective effects of glutamine preconditioning on hepatic ischemia-reperfusion injury in rats and its dose-dependency.
METHODS:Thirty-two healthy male Wistar rats were randomly divided into four groups (n=8 per group). One group received 0.9% NaCl (control) and the other three received glutamine (Gln groups) 4 hours before ischemia. The Gln groups were named GL, GM, and GH according to the glutamine dose. The liver was subjected to 1 hour of ischemia and 2 hours of reperfusion. Two hours later, the levels of alanine aminotransferase (ALT), intracellular free calcium (Ca2+), and activity of Na+/K+adenosine triphosphatase (ATPase) and superoxide dismutase (SOD) were assessed, and liver tissue sections were examined under a microscope.
RESULTS:The Gln and control groups differed in the concentration of intracellular free calcium (P<0.05), and the activity of Na+/K+ATPase and SOD in the Gln groups was higher than in the control group (P<0.05). The ALT level was lower in the GM and GH groups than in the control group (P<0.05). The levels of Na+/K+ATPase and SOD rose gradually with increasing glutamine dose (P<0.05), and the concentration of Ca2+declined gradually with increasing glutamine dose (P<0.05). The degree of hepatocyte injury was milder in the Gln groups than in the control group.
CONCLUSIONS:Glutamine preconditioning protected effectively against hepatic ischemia-reperfusion injury. These protective effects were related to the dose of glutamine and due to the reduction of intracellular calcium overload and the improvements in the activity of Na+/K+ATPase and SOD.
(Hepatobiliary Pancreat Dis Int 2011; 10: 78-82)
ischemia-reperfusion injury; liver; glutamine; Na+/K+ATPase; calcium overload
Introduction
Ischemia and hypoxia lead to cell damage, and when the blood supply is restored the damage is not reduced but cell death accelerates. This phenomenon is known as ischemia-reperfusion (IR) injury. Hepatic IR injury is a common pathophysiological process after hepatic surgery. The reduction of IR injury has been a research focus in hepatic surgery for many years, especially in transplantation surgery.[1]Glutamine is the most abundant amino acid in the body, the regulator of protein synthesis and degradation, and it plays an important role in regulating acid-base balance, promoting immune function, and improving adaptation to stress;[2,3]meanwhile it is the precursor of glutathione (GSH) which is the most important antioxidant in the body.[4]The body can make enough glutamine for its regular needs, but in extreme stress (e.g., after injuries, infections, burns, trauma, or surgical procedures), the body may need more glutamine than it can make.[4,5]Studies have shown that glutamine helps to protect organs against IR injury, but the specific mechanism is not clear. This study was undertaken to explore the protective effect of glutamine and the undelying mechanism, and to determine whether the protective effect is dose-dependent in an animal model of hepatic IR injury.
Methods
Animals and grouping
Thirty-two healthy male Wistar rats (weighing 250-300 g) were obtained from the Experimental Animal Center of Hebei Medical University (Shijiazhuang, China). The rats were kept in standard conditions and fed water and rodent chow ad libitum. The animals were randomlydivided into four groups: one control and three glutamine preconditioning groups (Gln, n=8 each). In accordance with the different doses of glutamine they were designated the GL, GM and GH groups.
Experimental protocols
Before the experiment, the rats were fasted for 12 hours but had free access to water. Animals were anesthetized with 10% chloral hydrate (5 ml/kg) by intraperitoneal injection. To induce hepatic ischemia, a laparotomy was performed, and the blood supply to the liver was interrupted with a bulldog clamp for 1 hour. Reflow was initiated by removal of the clamp. Samples were examined 2 hours after the reperfusion. In the control group, 5 ml 0.9% sodium chloride (China Shijiazhuang Pharmaceuticals Corporation, Ltd., Shijiazhuang, China) was injected into the dorsal vein of the penis and 4 hours later the liver was subjected to 1 hour of ischemia and 2 hours of reperfusion. In the Gln groups, 3% glutamine solution (made up from 20% L-alanyl-L-glutamine, Fresenius, Germany, China Huarui Pharmaceutical Corp, Ltd., Beijing, China) was infused into the dorsal penis vein via a microinjection pump (0.5 ml/min), at 0.15 g/kg in the low-dose group (GL), 0.45 g/kg in the mediumdose group (GM), or 0.75 g/kg in the high-dose group (GH). This was followed 4 hours later by ischemia and reperfusion, after which liver samples and 5 ml blood samples from the heart were examined.
Determination of alanine transaminase (ALT) levels in serum
Blood samples taken from the heart after 2 hours of reperfusion were centrifuged at 2500 rpm for 15 minutes to determine the plasma ALT levels with a spectrophotometer (type-756, Shanghai Precision Instrument Factory, Shanghai, China).
Preparation of liver tissue and examination of paraffin sections
From the right lobe of the liver, 100 mg of tissue was harvested. One milliliter of 0.9% NaCl was added to the sample, which was homogenized to give a 10% homogenate of the liver tissue. Then this was centrifuged for 10 minutes at 3000 rpm and 4 ℃, and the supernatant preserved for measurements. Liver tissue was fixed in 4% formaldehyde, cut into sections, stained with hematoxylin-eosin, and then viewed under a light microscope.
Assessment of Ca2+concentration and Na+/K+ATPase activity
To measure the activity of Na+/K+ATPase and the concentration of Ca2+, the instructions with the kit were followed and the following formulae were used.
Concentration of Ca2+(mmol/g prot)=(absorbance experimental tube-absorbance blank tube)/(absorbance standard tube-absorbance blank tube)×absorbance control tube/protein content in tissue (wavelength 610 nm).
Activity of Na+/K+ATPase (μmol Pi/mg protein/ hour)=(absorbance experimental tube-absorbance control tube)/absorbance standard tube×concentration in standard tube×dilution factor of sample×6/protein content in tissue (wavelength 660 nm).
Evaluation of superoxide dismutase (SOD) activity
The SOD activity was measured with an assay kit according to the manufacturer's instructions.
SOD activity (NU/mg protein)=(absorbance control tube-absorbance experimental tube)/absorbance in control tube÷50%×total volume of reaction solution/ sample volume (ml)÷protein content in tissue (mg prot/ ml). Protein content in tissue=(OD (optical density) experimental tube-OD blank tube)/(OD standard tube-OD blank tube)×concentration in standard tube (0.563 g/L).
Statistical analysis
All data were expressed as mean±SD. Values were analyzed using the statistical package SPSS for Windows version 11.5 (SPSS Inc., Chicago, IL, USA). Statistical analyses were performed using one-way analysis of variance (ANOVA) when comparing four groups, and with the LSD post-test for every two-group comparison. APvalue less than 0.05 was considered statistically significant.
Results
ALT levels
The level of ALT was lower in the Gln groups than in the control group (P<0.05) (Table). The difference between the control and GL groups was not significant. Differences were found in the GM and GL groups compared with the control group (P<0.05).
Ca2+concentration, Na+/K+ATPase and SOD activity
The concentration of Ca2+was lower in the Gln groups than in the control group (P<0.05) (Table). The concentration of Ca2+decreased with increasing glutamine, and the difference between these groups was significant.
Pretreatment with glutamine resulted in a highactivity of Na+/K+ATPase in hepatocytes compared to the control group (P<0.05). With the increase of glutamine dose the activity of Na+/K+ATPase increased, and the differences between the Gln groups were significant.
The activity of SOD in the Gln groups was higher than that in the control group (P<0.05) (Table). Among these groups, the activity of SOD increased as the glutamine dose increased (P<0.05).
Observation under a light microscope
In the control group, the central veins and hepatic sinusoids were clearly congested. The sinusoids were narrow. There was granular and vacuolar degenerationto different degrees, and punctiform necrosis was seen occasionally. There was neutrophilic granulocyte aggregation and infiltration, especially in the hepatic central vein areas. Milder congestion was seen in the Gln groups; the structure of the hepatic lobule was relatively normal, cell degeneration was not evident, and neutrophilic granulocyte infiltration was attenuated (Fig.).
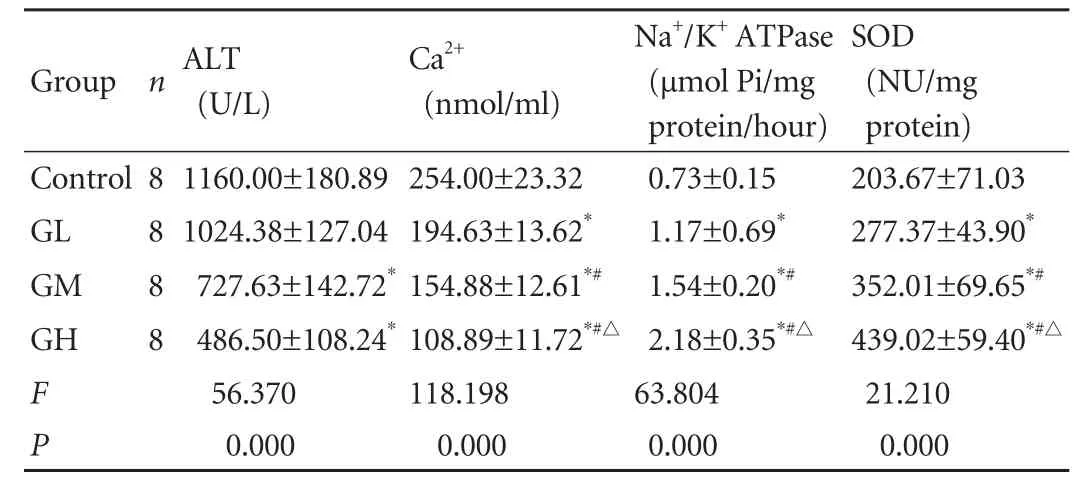
Table. Levels of ALT, Ca2+, Na+/K+ATPase and SOD

Fig. A: In the control group, hepatocytes were disarranged with under-stained cytoplasm. Arrows show hepatocyte vacuolation (HE staining, original magnification ×400); B: In the GL group, the hepatic sinus was dilated and congested. Arrows show inflammatory cell infiltration (HE staining, original magnification ×400); C: In the GM group, congestion and inflammation were attenuated. Arrow shows swollen hepatocyte (HE staining, original magnification ×400); D: In the GH group, hepatocytes showed orderly distribution and had milder hyperemia (HE staining, original magnification ×400).
Discussion
Hepatic IR injury associated with major liver resection and transplantation can lead to a series of metabolic, structural and functional injuries, and therefore may cause serious postoperative complications, such as liver failure and even multiple organ dysfunction syndrome, that increase mortality.[6]The pathogenesis of IR-induced hepatic injury is complicated and still not clear. There are three possible mechanisms: the massive production of deleterious reactive oxygen species (ROS), the overload of intracellular calcium and the activation of neutrophils, which may result in the release of inflammatory cytokines.[7,8]
Glutamine is one of the most abundant amino acids in plasma and plays an important role in nutrient metabolism.[9]It is a conditionally essential nutrient during serious injury or illness, since endogenous glutamine production may be insufficient to meet the increased requirements during catabolic stress.[5]Studies[2,3]have shown that the glutamine content in tissues is closely related to the prognosis and outcome of various diseases, and glutamine supplementation can reduce hospital stay and improve survival rate. Recently, glutamine has been demonstrated to protect against IR injury of the gut, heart, liver and skeletal muscle[10]and the mechanism is still incompletely understood and may be partly related to the preservation of GSH content.[11,12]The GSH system is one of the major mechanisms of reducing oxidative stress in cells.[13]As the precursor of GSH synthesis, glutamine contributes to antioxidant defense.[14]It has been shown that glutamine supplementation helps maintain muscle and small bowel GSH levels and prevent the decrease in hepatic GSH content associated with total parenteral nutrition. Moreover, glutamine possesses immunoregulatory functions.[15]These properties have been put forward to explain the positive impact of glutamine supplementation on outcome in critically ill patients.
The results of our study indicated that after 1 hour of ischemia and 2 hours of reperfusion, liver function and morphology were impaired and glutamine pretreatment protected against this damage.
As a scavenger of ROS,[16]the activity of SOD in the glutamine pretreatment groups was significantly higher than that in the control group, and the difference was related to the glutamine dosage. In the ischemic liver, large amounts of ROS have to be neutralized upon reperfusion, and the antioxidant capacity of the IR-injured tissue is rapidly exhausted, since the SOD cannot keep up with its oxidation.[17,18]Our study demonstrated that glutamine pretreatment effectively increased the activity of SOD, which can reduce the destruction of the ROS, Moreover, supplementation with glutamine may prevent the development of an uncontrolled inflammatory state by improving the local antioxidant defenses. This may also be a part of its protective potential against IR injury.[19,20]
Meanwhile, ROS increases the permeability of the cellular and mitochondrial membranes, which results in decreased ATP production and intracellular calcium overload.[21-23]Intracellular calcium overload activates calcium-dependent neutral proteinase to promote the conversion of xanthine dehydrogenase to xanthine oxidase,[24]which leads to an increase in ROS production and the impairment of hepatocytes. This process forms a vicious cycle between ROS and intracellular calcium, so they magnify eachother's biological effects.[25]In our experiment, the levels of intracellular calcium in the glutamine pretreatment groups were significantly lower than in the control group. This indicated that glutamine stabilized the membrane directly or indirectly and then attenuated the intracellular calcium overload, thereby cutting off the vicious cycle between ROS and Ca2+. In addition, we found that the Na+/K+ATPase activity in liver was improved by glutamine administration. In the hypoxia-ischemia procedure, the intracellular pH decreases (acidosis) and when the flow is restored it forms a concentration gradient of hydrogen ions which can result in augmented Na+/H+exchange and thereby increased Na+/Ca2+exchange.[26]When the Na+/K+ATPase activity is enhanced, the inflow of calcium is reduced and the intracellular calcium overload is relatively attenuated. Consequently, the structure and function of hepatocytes were protected by glutamine pretreatment.
Furthermore, we found that the protective effect was dose-dependent i.e., the protection was enhanced with increased glutamine dose. However, Noh et al[27]found that glutamine does not protect against IR injury in rats. In their research, glutamine was administered intraperitoneally instead of intravenously 24 and 6 hours before ischemia, and the total dose was 1.5 g/kg higher than ours. Besides, another study demonstrated an increased degree of IR injury when glutamine was injected after ischemia.[28]We suppose that the protective effects of glutamine may depend on the dose, experimental design, timing, and mode of administration, which may account for the different results.
Our research provided indirect evidence that glutamine preconditioning is involved in the anti-oxidation process; however the mechanism of its protective effect against IR injury is still incomplete. Further investigation is required in this field as the number of clinically useful methods to attenuate hepatic IR injury is still limited and this is essential to improve clinical outcome.
In conclusion, we found that glutamine effectively protected against hepatic IR injury. The protective effects were related to the dose of glutamine and attributed to the reduction of intracellular calcium overload and the improvement of SOD and Na+/K+ATPase activity.
Funding:None.
Ethical approval:Not needed.
Contributors:ZWX proposed the study. ZLF wrote the first draft. BL analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZWX is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Schuster H, Blanc MC, Bonnefont-Rousselot D, Nakib S, Le Tourneau A, Fürst P, et al. Protective effects of glutamine dipeptide and alpha-tocopherol against ischemia-reperfusion injury in the isolated rat liver. Clin Nutr 2009;28:331-337.
2 Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 2002;30:2022-2029.
3 Melis GC, ter Wengel N, Boelens PG, van Leeuwen PA. Glutamine: recent developments in research on the clinical significance of glutamine. Curr Opin Clin Nutr Metab Care 2004;7:59-70.
4 Wischmeyer PE, Jayakar D, Williams U, Singleton KD, Riehm J, Bacha EA, et al. Single dose of glutamine enhances myocardial tissue metabolism, glutathione content, and improves myocardial function after ischemia-reperfusion injury. JPEN J Parenter Enteral Nutr 2003;27:396-403.
5 Li Y, Chen Y, Zhang J, Zhu JF, Liu ZJ, Liang SY, et al. Protective effect of glutamine-enriched early enteral nutrition on intestinal mucosal barrier injury after liver transplantation in rats. Am J Surg 2010;199:35-42.
6 Zhang WX, Yin W, Zhang L, Wang LH, Bao L, Tuo HF, et al. Preconditioning and postconditioning reduce hepatic ischemia-reperfusion injury in rats. Hepatobiliary Pancreat Dis Int 2009;8:586-590.
7 Pediaditakis P, Kim JS, He L, Zhang X, Graves LM, Lemasters JJ. Inhibition of the mitochondrial permeability transition by protein kinase A in rat liver mitochondria and hepatocytes. Biochem J 2010;431:411-421.
8 Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 2000;32:169-173.
9 Young VR, Ajami AM. Glutamine: the emperor or his clothes? J Nutr 2001;131:2449S-2459S; discussion 2486S-2487S.
10 Jia CJ, Dai CL, Zhang X, Cui K, Xu F, Xu YQ. Alanylglutamine dipeptide inhibits hepatic ischemia-reperfusion injury in rats. World J Gastroenterol 2006;12:1373-1378.
11 Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, et al. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol 2004;10:864-870.
12 Armeni T, Ghiselli R, Balercia G, GoffiL, Jassem W, Saba V, et al. Glutathione and ultrastructural changes in inflow occlusion of rat liver. J Surg Res 2000;88:207-214.
13 Ziegler TR, Evans ME, Fernández-Estívariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr 2003;23:229-261.
14 Roth E, Oehler R, Manhart N, Exner R, Wessner B, Strasser E, et al. Regulative potential of glutamine--relation to glutathione metabolism. Nutrition 2002;18:217-221.
15 Manhart N, Vierlinger K, Spittler A, Bergmeister H, Sautner T, Roth E. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer's patches in endotoxemic mice. Ann Surg 2001;234:92-97.
16 Strauss E. Possible new anti-inflammatory agent. Science 1999;286:209-210.
17 Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact 1991;79:115-136.
18 Fink MP. Cytopathic hypoxia. Is oxygen use impaired in sepsis as a result of an acquired intrinsic derangement in cellular respiration? Crit Care Clin 2002;18:165-175.
19 Carroll PV, Jackson NC, Russell-Jones DL, Treacher DF, Sönksen PH, Umpleby AM. Combined growth hormone/insulin-like growth factoriin addition to glutamine-supplemented TPN results in net protein anabolism in critical illness. Am J Physiol Endocrinol Metab 2004;286:E151-157.
20 Lin MT, Kung SP, Yeh SL, Lin C, Lin TH, Chen KH, et al. The effect of glutamine-supplemented total parenteral nutrition on nitrogen economy depends on severity of diseases in surgical patients. Clin Nutr 2002;21:213-218.
21 Yan S, Jin LM, Liu YX, Zhou L, Xie HY, Zheng SS. Outcomes and mechanisms of ischemic preconditioning in liver transplantation. Hepatobiliary Pancreat Dis Int 2010;9:346-354.
22 Singleton KD, Serkova N, Beckey VE, Wischmeyer PE. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med 2005;33:1206-1213.
23 Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007;12:835-840.
24 Schäfer C, Walther S, Schäfer M, Dieterich L, Kasseckert S, Abdallah Y, et al. Inhibition of contractile activation reduces reoxygenation-induced endothelial gap formation. Cardiovasc Res 2003;58:149-155.
25 Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 2009;1787:1395-1401.
26 De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med 2005;33:1125-1135.
27 Noh J, Behrends M, Choi S, Bedolli MA, Yardi J, Hirose R, et al. Glutamine does not protect against hepatic warm ischemia/reperfusion injury in rats. J Gastrointest Surg 2006; 10:234-239.
28 Fukatsu K, Ueno C, Hashiguchi Y, Hara E, Kinoshita M, Mochizuki H, et al. Glutamine infusion during ischemia is detrimental in a murine gut ischemia/reperfusion model. JPEN J Parenter Enteral Nutr 2003;27:187-192.
July 27, 2010
Accepted after revision October 29, 2010
Author Affiliations: Department of Hepatobiliary Surgery (Zhang WX, Zhou LF, Bao L, Wang CC, Meng HY and Yin W), and Department of Pharmacy (Zhang L), Hebei Provincial General Hospital, Shijiazhuang 050051, China
Wan-Xing Zhang, MD, Department of Hepatobiliary Surgery, Hebei Provincial General Hospital, Shijiazhuang 050051, China (Tel: 86-311-85988739; Email: zhangwx12@hotmail.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Primary hepatic carcinosarcoma
- Aberrant methylation frequency of TNFRSF10C promoter in pancreatic cancer cell lines
- Effects of suppressing glucose transporter-1 by an antisense oligodeoxynucleotide on the growth of human hepatocellular carcinoma cells
- Peroxisome proliferator-activated receptor gamma inhibits hepatic fibrosis in rats
- Hepatocyte differentiation of human fibroblasts from cirrhotic liver in vitro and in vivo
