电化学转盘法处理切削液废水
2011-05-18钟登杰
钟登杰
(重庆理工大学 化学化工学院,重庆400054)
在金属加工过程中经常会用到切削液,其主要作用是降低摩擦、润滑表面、冷却、带走刨花等。切削液主要含有油、水、表面活性剂和各种添加剂(防泡剂、杀菌剂和防锈剂)。切削液在使用过程中由于热降解和加工过程中所产生的微粒增多而失去润滑和冷却作用,所以需要替换,从而会产生大量的废液。这些废液如果不经处理直接排放,其所含的重金属和热降解所产生的有害物质将对土壤和水体造成污染。处理切削液废水的传统方法主要有蒸发[1]、冷冻[2]、膜过滤[3-6]和吸附等,但这些方法只是将有害物质从废水中分离出来,有害物质并没有被降解成无害物质。另外,切削液废水具有毒性,所以很难用生物法去处理[7-10]。水热氧化法能有效地处理切削液废水[11-12],但该法所需条件苛刻,难以满足。
电化学方法是一种“绿色高级氧化”废水处理技术,具有经济、高效、所需空间小等优点。电化学方法已被成功应用于各种废水处理中,如染料废水[13-15]、壬基酚废水[16]、含酚废水[17]、含氰废水[18-19]、制革废水[19]和橄榄油废水处理[20-21]。
为了提高电化学反应的效率、降低副反应的发生,近年来研究者们发现了新的电极材料[22-25]和反应器[26]。对于电化学反应而言,传质速率和溶解氧浓度都是重要的影响因素,会限制整个反应速率。本文设计了一种新型电化学反应装置,并用于处理切削液废水。阳极和阴极交替均匀地分布在一个圆盘上,用导线分别连接。通过转动圆盘,电极表面静止液膜的厚度减少,这在很大程度上会提高传质速率。同时,转盘有一半暴露在空气中,另一半在溶液中,这样随着转盘的转动,可以加速氧气从空气中溶解到主体溶液中,加速氧化[27]。在阴阳极之间分布活性炭颗粒。处理废水时,活性炭颗粒在电场作用下产生极化,生成很多微小电池。这样就可以加大反应器的有效电极面积,缩短有机物的迁移距离。
1 材料和方法
从某机械厂所取的切削液废水的主要成分为:COD 14 356 mg/L,含油量4 326 mg/L,pH值为7.75。COD采用重铬酸钾氧化法测定,含油量采用420 oil IR红外分析仪测定,pH值采用PHS-3B酸度计测定。
电化学反应装置如图1所示,主要包括1个弧形电解槽、电化学转盘和直流电源。电化学转盘是一个直径为18 cm,厚度为3.6 mm的有机玻璃盘。阴极为6 cm长、1.2 cm宽、2.1 cm厚的铁片。阳极为6 cm长、1.2 cm宽、2.7 cm厚的石墨片。颗粒活性炭(平均粒度为1.0 mm)分布在阴阳极之间,阳极和阴极通过导线分别与电源的正负极相连。通过调速器来控制转盘的转速。图2是一个传统的电化学反应器,电极材料和面积均与电化学转盘法相同,采用磁力搅拌。
每次实验所用水样为400 mL。颗粒活性炭在使用之前先用切削液废水浸泡,达到饱和。电解电压控制在10 V。

2 结果与讨论
2.1 转速的影响
在电压为10 V的条件下,将转盘的转速分别控制在0,30,60和90 r/min。从图3可以看出,随着转速的提高,切削液废水中油的去除率得到提高。这是由于电化学转盘的转动能加快主体溶液和电极表面之间的传质。当转速超过60 r/min后,油的去除率相对稳定,提高电化学转盘的转速不再能大幅度提高废水中油的去除率。

图3 转盘转速对废水中油去除的影响
2.2 电化学转盘法和传统电解法去除油含量的比较
从图4可以看出,在去除切削液废水中的油量方面,电化学转盘法明显优于传统电解法。
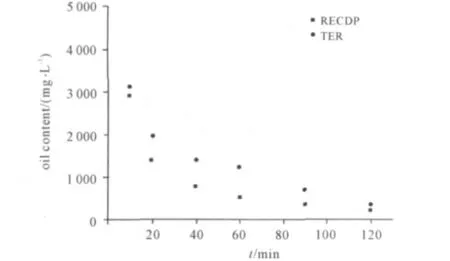
图4 电化学转盘法与传统电解法在油含量去除效果方面的比较
2.3 COD去除率
从图5可以看出,切削液废水COD去除率随反应时间的延长而上升。在反应的前90 min内,COD去除率上升很快。反应90 min后,COD去除率上升不明显。经过90 min电解,切削液废水中的COD去除率能达到83.7%,但切削液废水剩余的COD还有约2 000 mg/L,所以,电化学转盘法可以作为切削液废水的预处理方法。

图5 电化学转盘法去除废水中的COD
2.4 降解机理初步研究
电化学方法总是有自由基参与反应[14,29-30]。自由基反应是有机物氧化的关键[29]。电化学反应过程中所产生的自由基一旦生成,就立即无选择性地与反应介质中的有机物发生反应。反应的初步机理为:有机物分子吸附到电极表面→电化学反应过程中产生的自由基将有机物分子氧化→有机物分子降解成有机小分子、最后降解成无害的无机小分子(如CO2和H2O)。
3 结论
1)电化学转盘法是一种新电化学方法,阳极和阴极交替分布,转盘的转动可以加强传质、加快空气中的氧气溶解于主体溶液,有利于有机物的降解。
2)电化学转盘法能有效地去除切削液废水中的油量和COD。当转速为60 r/min,电压为10 V,pH值为7.75时,电解120 min,油和COD去除效率能分别达到94.9%和83.7%。与传统的电解法相比,电化学转盘法在去除切削液废水的含油量方面具有优势。
3)由于切削液废水的初始COD太高,COD去除率达到83.7%,其剩余的COD还有2 000 mg/L,所以电化学转盘法用于切削液废水的预处理。
[1]Hadj-Ziane Z A,Moulay S.Microemulsion breakdown using the pervaporation technique:application to cutting oil models[J].Desalination,2004,170:91-97.
[2]Lorain O,Thiebaud P,Badorc E.Potential of freezing in wastewater treatment:soluble pollutant application[J].Water Research,2001,35(2):541-547.
[3]Hu XG,Bekassy-Molnar,Erika Vatai Gyula.Ultrafiltration of oily emulsion for metal cutting fluid:role of feed temperature[J].Environment Protection Engineering,2005,31(3/4):109-118.
[4]Lin S W,Heriberto E G.Development of energy-saving spinning membrane system and negatively charged ultrafiltration membranes for recovering oil from waste machine cutting fluid[J].Desalination,2005,174(2):109-123.
[5]Madaeni,Sayed Siavash,Yeganeh.Microfiltration of emulsified oil wastewater[J].Journal of Porous Materials,2003,10(2):131-138.
[6]Nidal Hilal,Gerald Busca,Federico Talens-Alesson,et al.Treatment of waste coolants by coagulation and membrane filtration[J].Chemical Engineering and Processing,2004,43:811-821.
[7]Taylor G T.Evaluation of the potential of biodegradation for the disposal of cutting fluid from the machining of uranium[J].International Biodeterioration&Biodegradation,2001,47(4):215-224.
[8]Van der Gast C J,Whiteley A S,Thompson I P.Temporal dynamics and degradation activity of a bacterial inoculum for treating waste metal-working fluid[J].Environmental microbiology,2004,6(3):254-263.
[9]Van der Gast C J,Knowles C J,Starkey M,et al.Selection of microbial consortia for treating metal-working fluids[J].Journal of Industrial Microbiology&Biotechnology,2002,29(1):20-27.
[10]Karimov R R,Shulaev M V,Emel'yanov V M,et al.Study of biosorption treatment of spent cutting fluids under anaerobic conditions using various adsorbents[J].Khimicheskaya Promyshlennost(St.Petersburg,Russian Federation),2004,81(9):485-488.
[11]Juan R P,López J,Nebot E,et al.Elimination of cutting oil wastes by promoted hydrothermal oxidation[J].Journal of Hazardous Materials,2001,B88:95-106.
[12]Juan R P,Sanchez-Onet,Jezabel,et al.Hydrothermal oxidation of oily wastes:An alternative to conventional treatment methods[J].Engineering in Life Sciences,2003,3(2):85-89.
[13]Wang A,Qu J,Liu H,et al.Degradation of azo dye Acid Red 14 in aqueous solution by electrokinetic and electrooxidation process[J].Chemosphere,2004(55):1189-1196.
[14]Shen Z,Wang W,Jia J P,et al.Degradation of dye solution by an activated carbon fiber electrode electrolysis system[J].Journal of Hazardous Materials,2001,84(1):107-116.
[15]Roessler A,Jin X.State of the art technologies and new electrochemical methods for the reduction of vat dyes[J].Dyes and Pigments,2003,59:223-235.
[16]Kuramitza H,Jun S,Toshiaki H.Electrochemical removal of p-nonylphenol from dilute solutions using a carbon fiber anode[J].Water Research,2002,36:3323-3329.
[17]Korbahti B,Tanyolac A.Continuous electrochemical treatment of phenolic wastewater in a tubular reactor[J].Water Research,2003,37(7):1505-1514.
[18]Lanza M,Bertazzoli R.Cyanide oxidation from waste-water in a flow electrochemical reactor[J].Industrial&Engineering Chemistry Research,2002,41(1):22-26.
[19]Rao N,Somasekhar K,Kaul S.Electrochemical oxidation of tannery wastewater[J].Journal of Chemical Technology and Biotechnology,2001,76(11):1124-1131.
[20]Israilides C J,Vlyssides A G,Mourafeti V N,et al.Olive oil wastewater treatment with the use of an electrolysis system[J].Bioresource Technology,1997,61(2):163-170.
[21]Gotsi M,Kalogerakis N,Psillakis E,et al.Electrochemical oxidation of olive oil mill wastewaters[J].Water Research,2005,39(17):4177-4187.
[22]Subbiah A,Chellammal S,Basha C M.Electrochemical treatment of xylenol orange dye wastewater at RuO2/Ti anode[J].Bulletin of Electrochemistry,2003,19(3):127-132.
[23]Polcaro A,Mascia M,Palmas S,et al.Electrochemical degradation of diuron and dichloroaniline at BDD electrode[J].Electrochimica Acta,2004,49:649-656.
[24]Zanta C,Michaud P,Comninellis C,et al.Electrochemical oxidation of p-chlorophenol on SnO2-Sb2O5based anodes for wastewater treatment[J].Journal of Applied Electrochemistry,2003,33(12):1211-1215.
[25]Zollinger D,Griesbach U,Putter H,et al.Electrochemical cleavage of 1,2-diphenylethanes at borondoped diamond electrodes[J].Electrochemistry Communications,2004,6(6):605-608.
[26]Xiong Y,He C,An T C,et al.Removal of formic acid from wastewater using three-phase three-dimensional electrode reactor[J].Water Air and Soil Pollution,2003,144(1):67-79.
[27]Zhong D J,Yang J,Jia J P,et al.Degradation of dye wastewater containing reactive brilliant blue X-BR using a rotating electrochemical disc process[C]//Progress in Natural Science.[S.l.]:special issue,2005:149-154.
[28]Zhou K,Zhou D.Promoting the electrolysis efficiency of a bipolar electrolyser by adding a coated activated carbon[J].China Journal of Environmental Science,1994,15(2):38-40.
[29]Yang J,Jia J P,Liao J,et al.Removal of Fulvic Acid from Water Electro-chemically Using Active Carbon Fiber Electrode[J].Water Research,2004,38(20):4353-4360.
[30]Jia J P,Yang J,Liao J,et al.Treatment of dying wastewater with ACF electrodes[J].Water Research,1999,33(3):881-884.
