Synthesis of Methyl Isopropyl Ketone and Diethyl Ketone over Ni-Na/ZrO2-MnO2-ZnO Catalyst*
2011-03-22JIYongjun纪永军andYANGJianguo杨建国
JI Yongjun (纪永军)** and YANG Jianguo (杨建国)
Shanghai Key Laboratory of Green Chemistry and Chemical Process, Department of Chemistry, East China Normal University, Shanghai 200062, China
1 INTRODUCTION
Methyl isopropyl ketone (MIPK) and diethyl ketone (DEK) are important organic chemical raw materials and solvents, which are widely used in producing synthetic dyes, pharmaceutical products, organic solvents,etc[1-4]. Methyl isopropyl ketone can be used to synthesize dye intermediates, extract precious metal tantalum and niobium and so on. Diethyl ketone can be used to synthesize organic materials, and it is also widely used in the pharmaceutical and pesticide industry, as well as many other fields.
There are several methods to synthesize MIPK and DEK [4-13], but in common industrial applications, there are two main methods: the condensation method based on carboxylic acid or carboxylic acid salt and the aldol condensation method based on aldehydes and ketones. These two methods have many disadvantages. The former one requires high temperature, and while producing ketones, some byproducts containing carbon dioxide and water are released.Therefore, the energy consumption is high. The latter method usually requires three complicated processes,the aldol condensation, the dehydration and the hydrogenation, and the equipment cost is also very high.In recent years, the one-step synthesis of MIPK and DEK by methanol (MeOH) and methyl ethyl ketone(MEK) has attracted wide interests [14-18]. This process can produce the two organic materials MIPK and DEK simultaneously, and meanwhile, it is also advantageous in terms of raw material cost. Therefore, it has a great potential for industrial applications. Luoet al. [17]reported that the catalyst of Pd-Na/ZrO2-MnO2-ZnO showed good catalytic performance on this reaction,but using Pd as the active material was not feasible in industrial production in view of the noble metal cost.
In this study, we developed the catalyst of Ni-Na/ZrO2-MnO2-ZnO, which possessed multiple functions, including dehydrogenation, aldol condensation, dehydration and hydrogenation. Ni was used as the active material, which reduced greatly the cost of raw materials, and the conversion and selectivity were comparable to that using Pd as the active material.Therefore, this catalyst would have good prospects for industrial application.
2 EXPERIMENTAL
2.1 Catalyst preparation
ZrOCl2·8H2O, 50% of Mn(NO3)2solution and Zn(NO3)2·6H2O were dissolved into the distilled water according to a certain molar ratio (Zr∶Mn∶Zn=2∶2∶1) to get a solution with Zr of 0.4 mol·L-1, Mn of 0.4 mol·L-1and Zn of 0.2 mol·L-1, and then, 0.3 L of the resulted solution was transferred into a round bottom flask. NaOH aqueous solution of 0.3 mol·L-1was employed as the precipitation agent, and it was added dropwise until the solution pH reached 9. The resulting solution was firstly stirred at 82 °C for 4 h,then filtered, and the cake was washed with distilled water till neutral. After drying at 120 °C for 16 h and further calcined at 450 °C for 4 h in air, 31 g of Zr-Mn-Zn mixed oxides were obtained [17]. Subsequently, 0.055 L of 10% NaOH solution was used to impregnate the mixed oxides for 6 h (the incipient wetness technique), where the amount of Na added was 0.24% of the mass of the mixed oxides. After drying at 120 °C for 16 h and calcined at 400 °C for 3 h in air, the above-mentioned oxides were then impregnated with the 10% Ni(NO3)2solution followed by a similar procedure. Finally, the catalyst was obtained.
2.2 Experiment process and methods
The reactions were carried out in a fixed bed stainless steel microreactor. Typically, 2 ml of catalyst was packed between two layers of quartz wool. The activity measurements were carried out at several temperatures from 310 °C to 430 °C with an interval of 30 °C. The catalyst was firstly reduced in the reactor with hydrogen (100 ml·min-1) at 330-490 °C for 2 h prior to each measurement, and then the temperature was adjusted to the reaction temperature. The reactants were fed in by a microdosing pump in different liquid hourly space velocity (LHSV). The products were collected in an ice-bath cold trap, and analyzed quantitatively by Shimadzu GC-14B Gas Chromatograph (DB-WAX capillary: 30 m long, 0.25 mm i.d.)equipped with a flame ionization detector (FID). The conversion and selectivity were calculated by applying the area normalization method without the corrected factor. The following temperature program was employed: detector temperature at 250 °C, injection temperature at 250 °C, streaming, isothermal at 40 °C for 2 min, then heating to 250 °C with a rate of 10°C·min-1, isothermal at 250 °C for 25 min.
The Scanning Electron Microscopy (SEM) image was taken on a Hitachi S-4800 microscope. The X-ray Powder Diffraction (XRD) was analyzed in a Bruker D8 Advance Diffractometer with CuKαradiation operated at 35 kV and 25 mA at a scanning rate of 5 (°)·min-1.The N2adsorption was carried out at -196 °C in an BELSORP-max system (Micromeritics) with the BET model. The sample was preheated at 200 °C for 10 h before the nitrogen absorption.
3 RESULTS AND DISCUSSION
3.1 Catalysts characterization

Figure 1 SEM image of fresh catalyst
The result of SEM characterization was shown in Fig. 1. It can be observed that the particle size was about 50 nm, and the particle distribution was uniform.The result of XRD characterization was shown in Fig. 2. It was clearly showed that the diffraction angle of 30 °, 35 °, 50 ° and 60 ° symboled by * were the characteristic diffraction peaks of zirconia, while the angle around 45 ° and 76 ° symboled by + were the characteristic diffraction peaks of metal nickel. The shape of the peaks indicated that they were in very high crystallinity.

Figure 2 XRD patterns of catalysts at different Ni loading(by mass)
3.2 Impacts of catalyst preparation conditions on catalytic properties
3.2.1Impact of Ni loading

Entry Ni loading/% (by mass)Selectivity/%Conversion of MEK/% MIPK DEK

Table 1 Catalytic activities at different Ni loading①
The catalysts containing different Ni loading were tested to show its impact on the catalytic activity and selectivity (Table 1). When Ni loading was low(between 0.25% and 0.5%), the active sites of the hydrogenation/dehydrogenation were much less than the acidic active sites, and the conversion was also low.This was due to that this reaction required the matching of the two types of active sites. With the Ni loading being increased from 5% to 25%, the conversion was improved, and the byproducts were decreased significantly. This was because that the active sites of the hydrogenation/dehydrogenation matched better with the acidic active sites, and they also inhibited the side reaction triggered by the excess acidic active sites.When the Ni loading was increased to the level higher than 25%, the change of conversion and selectivity became insignificant. It is showed that the catalytic performance was determined by the matching of the two types of active sites in the bifunctional catalyst.The optimal Ni loading was 25%, which was consistent with the results of XRD.
3.2.2Impact of calcination temperature
The impact of calcination temperature on the catalyst performance was shown in Table 2. It can be seen that with the increase of the calcination temperature, the conversion of MEK was improved, and the peak value was observed at 400 °C. The overall selectivity of MIPK and DEK had almost no change. This was because that if the calcination temperature was too high, the specific surface area would decrease.Therefore, the most suitable calcination temperature was 400 °C.

Table 2 Specific surface area of catalysts and catalytic activities of various calcination temperature①
3.2.3Impact of reduction temperature
The impact of reduction temperature on the catalyst performance was shown in Table 3, indicating that the increased reduction temperature improved the conversion of MEK, and the peak value was observed at 410 °C. Meanwhile, the overall selectivity of MIPK and DEK decreased slightly. This was because that the surface area reached the largest value when the reduction temperature was at 410 °C. If the temperature continued to rise, the increase of the nickel particle size would decrease the surface area. Therefore, the optimal reduction temperature was 410 °C.
3.3 Impact of reaction conditions on catalytic properties
3.3.1Impact of reaction temperature on catalytic performance
Reaction was carried out at different temperatures under the same raw material molar ratio and space ve-locity. Both the catalyst activity and the product composition were found to change with changing reaction temperature as shown in Table 4. It is evident that the conversion of MEK was also improved with increasing temperature from 310 °C to 400 °C. However, the overall selectivity of MIPK and DEK showed a general trend of decrease with the maximum at 310 °C,and the selectivity of DEK decreased at higher temperature 400 °C. Therefore, to achieve the best yield,400 °C was the appropriate temperature.

Table 3 Specific surface area of catalysts and catalytic activities of various reduction temperature①

Table 4 Specific surface area of catalysts and catalytic activities of various reaction temperature①
3.3.2Impact of LHSV on catalytic performance
The impact of LHSV on the catalytic performance was shown in Table 5. When LHSV was at 0.5 h-1, the conversion of MEK could reach 41.7%, which was comparable to 38.1% achieved by using Pd.Meanwhile, the selectivity of MIPK and DEK could reach 83.3%, comparable to 82.2% achieved by using Pd [17]. When LHSV was increased, the conversion of MEK decreased continuously for shorter contact time with the catalyst, but the overall selectivity was observed at lower LHSV.
3.3.3Impact of raw materials ratio on catalytic performance
The impact of raw materials ratio on the catalytic performance in Table 6 showed that with the increaseof the molar ratio of MeOH and MEK, the conversion of MEK was improved, while the total selectivity of MIPK and DEK dropped. 1/1 of (MeOH)/(MEK)seemed to be an appropriate molar ratio. In addition,with the increase of water, both the conversion of MEK and the selectivity of MIPK were increased at the beginning, and then started to drop after reaching a certain threshold value. Adding water into the materials could inhibit the coking of the catalyst, but the excess water would reduce the efficiency of the raw materials,leading to the increase of energy consumption. Therefore, the optimal molar ratio of (MeOH)/(MEK)/(H2O)was 1/1/1.
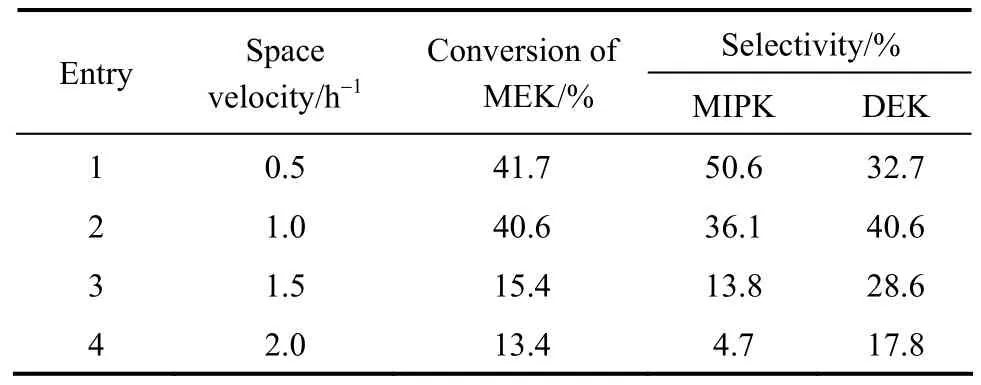
Table 5 Catalytic activities of various liquid hourly space velocity①

Table 6 Catalytic activities of various raw materials ratio①
3.4 Stability of catalyst
The stability of the catalyst was tested at 400 °C and atmospheric pressure for about 315 h time on stream (TOS), as shown in Fig. 3. It can be observed from this figure that the conversion of MEK remained more or less constant (>38%) for the period studied(<300 h). However, after 300 h, the conversion started to drop rapidly, and the catalyst became inactive in a short time. These results were also tested for reproducibility and found to be consistent.
4 CONCLUSIONS

Figure 3 Relation between activity of catalyst and time on stream(Ni loading 25%, calcination temperature 400 °C,reduction temperature 410 °C, reaction temperature 400 °C, atmospheric pressure, LHSV of raw material 0.5 h-1, the molar ratio of(MeOH)/(MEK)/(H2O) being 1/1/1)
When the Ni loading was 25%, the calcination temperature was 400 °C and the reduction temperature was 410 °C, the Ni-Na/ZrO2-MnO2-ZnO catalyst had good catalytic performance on the one-step synthesis reaction of MIPK and DEK from MEK and MeOH.The suitable reaction conditions were 400 °C for reaction; atmospheric pressure for reaction; LHSV of raw material of 0.5 h-1; the molar ratio of (MeOH)/(MEK)/(H2O) being 1/1/1. Under such conditions, the conversion of MEK could achieve 41.7%, and the overall selectivity of MIPK and DEK could achieve 83.3%, which was comparable to the conversion of 38.1% and the selectivity of 82.2% achieved by using Pd as the active material. The good stability made this catalyst have good prospects for industrial application.
1 Liang, C., “Production and development of methyl isopropyl ketone”,Chemical Technology and Development, (4), 33-34 (2002).(in Chinese)
2 Liang, C., “Market and development of methyl isopropyl ketone”,Fine and Specialty Chemicals, (24), 11-12 (2000). (in Chinese)
3 Gui, Y.C., Wang, H., Guo, W. L., “Study of synthesis of methyl isopropyl ketone”,Chemistry and Bonding, (2), 76-77 (1999). (in Chinese)
4 Zhu, R.H., “Study of synthetic technology of methyl isopropyl ketone”,Jiangsu Chemical Industry, (6), 23-25 (1999). (in Chinese)
5 Chen, A., “Synthesis of methyl isopropyl ketone”,Chemical Industry and Scientific Technology, 2, 25-34 (1994). (in Chinese)
6 Frankenthanl, W.H., Friedelsheim, L.H., “Preparation of ketones”, U.S. Pat., 4866210 (1990).
7 Ludwigshafen, C.S., Mutterstadt, K.E., “Preparation of ketones”, U.S. Pat., 4950763 (1976).
8 Olah, G.A., Mathew, T., Marinez, E.R., Esteves, P.M., Etzkorn, M.,Rasul, G., Prakash, G.K.S., “Acid-catalyzed isomerization of pivalaldehyde to methyl isopropyl ketoneviaa reactive protosolvated carboxonium ion intermediate”,J.Am.Chem.Soc., 123,11556-11560 (2001).
9 Joseph, P., Monroeville, B., Jeffreys, S., “Preparation of methyl isopropeny1 ketone”, U. S. Pat., 5072051 (1991).
10 Olah, G.A., Tse-Lok, H., Prakash, G.K.S., “Synthetic methods and reaction: A genera1 ketone synthesis by the Friedel-Crafts acylation of alkylsilanes”,J.Am.Chem.Soc., 10, 677-678 (1977).
11 Kamimura, Y., Sato, S., Takahashi, R., Sodesawa, T., Akashi, T.,“Synthesis of 3-pentanone from 1-propanol over CeO2-Fe2O3catalysts”,Appl.Cata1.A, 252, 399-410 (2003).
12 Sakagami, H., Ohta, N., Endo, S., Harada, T., Takahashi, N., Matsuda, T., “Location of active sites for 3-pentanone formation during ethene hydroformylation on Rh/active-carbon catalysts”,J.Catal.,171, 449-456 (1997).
13 Claridge, J.B., Green, M.L.H., Tsang, S.C., “Conversion of propanol to 3-pentanone using lanthanide oxides”,J.Chem.Soc.Faraday.Trans., 89, 1089-1094 (1993).
14 Luksza, M., Gaustrasse, D., “Process for the manufacture of methyl isopropyl ketone and diethyl ketone”, EP. Pat., 0224218A1 (1987).(in German)
15 Tian, L., Jiang, W.F., Luo, H.Y., Ding, Y.J., “Synthesis of methyl isopropyl ketone and diethyl ketone over ZrO2/MnOx/ZnO catalyst”,Petrochemical Technology, 33, 1060-1062 (2004). (in Chinese)
16 Tian, L., Jiang, W.F., Luo, H.Y., “Preparation and characterization of Na-Pd/ZrO2-MnOx-ZnO catalyst for synthesis of methyl isopropyl ketone and diethyl ketone”,Fine Chemicals, (5), 365-368 (2005).(in Chinese)
17 Luo, H.Y., Ding, Y.J., Tian, L., He, D.P., “A catalyst for synthesis of methyl isopropyl ketone and diethyl ketone and production method and application”, CN. Pat., 1733360A (2006). (in Chinese)
18 Liu, D., Cao, W.H., Shen, L., “Study of catalyst for synthesis of methyl isopropyl ketone and diethyl ketone”,Journal of ZheJiang University of Technology, 35, 194-197 (2007). (in Chinese)
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Adsorptive Recovery of Uranium from Nuclear Fuel Industrial Wastewater by Titanium Loaded Collagen Fiber*
- Salting-out Extraction of 2,3-Butanediol from Jerusalem artichoke-based Fermentation Broth*
- Investigation of Mg2+/Li+ Separation by Nanofiltration*
- Vapor-Liquid Equilibrium of Ethylene + Mesitylene System and Process Simulation for Ethylene Recovery*
- Purification and Characterization of a Nonylphenol (NP)-degrading Enzyme from Bacillus cereus. Frankland*
- Sponge Effect on Coal Mine Methane Separation Based on Clathrate Hydrate Method*
