Vapor-Liquid Equilibrium of Ethylene + Mesitylene System and Process Simulation for Ethylene Recovery*
2011-03-22GUOJing郭敬WUXianghong武向红JINGShuhong荆树宏ZHANGQian张谦andZHENGDanxing郑丹星
GUO Jing (郭敬), WU Xianghong (武向红), JING Shuhong (荆树宏), ZHANG Qian (张谦)and ZHENG Danxing (郑丹星)*
College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
1 INTRODUCTION
The refinery off-gas is an important resource in petrochemical industry. It is mainly from the secondary processing of crude oil and contains 14%-22%ethylene. Foreign refineries use the refinery off-gas as the raw material for production of ethylene, which has been industrialized in 1980s, but the refinery off-gas is often used as fuel in China, wasting the resource and causing environmental pollution.
For economic and environmental concerns, effective recovery of ethylene from the gas mixture has been the subject of study for chemical engineers.
Several new separation technologies for ethylene have been developed. Imperial Chemical Industries(I.C.I) [1] developed CuNO3and ethanolamine absorbent for chemical absorption of ethylene. Mitariten [2]used pressure swing absorption (PSA) to recover ethylene from fluid catalytic cracking (FCC) off-gas, in which the adsorbent is zeolite molecular sieve. Sikavitsaset al. [3] used magnetic stabilized bed (MSB)in olefin separation process. Eriksenet al. [4] used membrane separation method with Ag+hollow fiber membranes in an ethylene separation process. In China,PSA and middle low temperature absorption are usually used to recover ethylene from refinery off-gas.For physical absorption, the key is to find a suitable ethylene absorbent. Some physical absorbents such as alkanes, cycloalkanes, and aromatic hydrocarbons [5]can be used.
With different raw materials, processing programs,catalysts and process conditions, the refinery off-gas is different. A typical refinery off-gas composition is shown in Table 1 [6].
Vapor-liquid equilibrium (VLE) data is the basis of absorption and separation process. In order to simulate the process for recovering ethylene from therefinery off-gas with Aspen Plus, the model equations and parameters and the VLE data of the gas components and absorbent mesitylene are required, but relevant literature data are scarce. For those systems lacking of experimental data, it is better to measure the VLE data. In this study, physical absorption is used and mesitylene is selected as a novel absorbent for ethylene [7, 8]. Table 1 shows that except for ethylene,the content of methane and ethane is higher than that of other compositions, so methane and ethane can be recycled as raw material for reproduction. In the previous work [9], the binary interaction parameters were calculated and it was found that the solubility of methane is worse than that of ethylene and the solubility of ethane is close to that of ethylene in mesitylene. Thus three steps are to be used in this study. First,high pressure binary VLE data for ethylene + mesitylene system are measured. Secondly, parameters in equations of state are regressed. Finally, for the middle low temperature absorption process, ethylene recovery process with mesitylene is simulated.
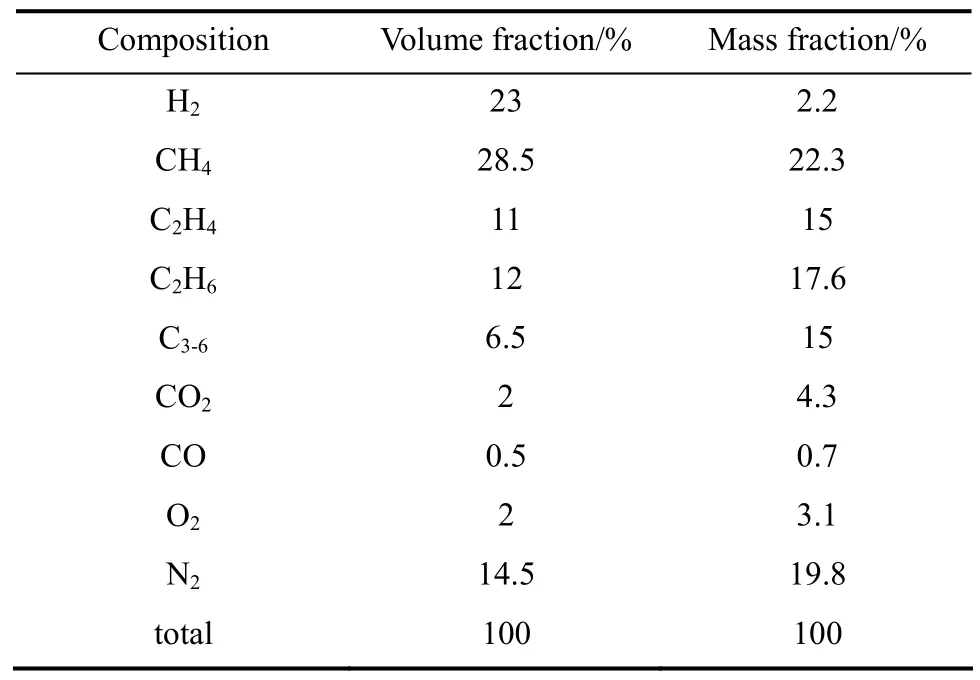
Table 1 Refinery off-gas composition
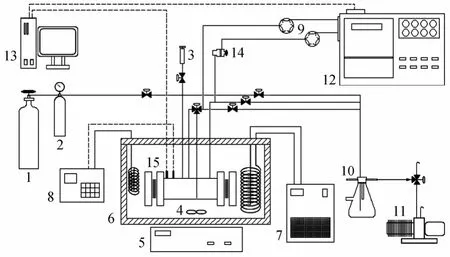
Figure 1 Schematic diagram of the experimental apparatus1—gas cylinder; 2—gas storage tank; 3—liquid injector; 4—stirrer; 5—magnetism mixer; 6—constant temperature water bath; 7—refrigerator; 8—temperature controller; 9—six-way valves; 10—cushion tank; 11—vacuum pump; 12—gas chromatography; 13—computer; 14—relief valve; 15—equilibrium cell
2 EXPERIMENTAL
2.1 Chemicals
In the experiment, the purity of ethylene was not less than 99.99%. Ethylene was purchased from Zhaoge Gas Co. Mesitylene was a special-grade reagent from Beijing Shengda Chemical Co, with 98% purity and a little impurities of water and acetone. All materials were used without further purification.
2.2 Apparatus
The study was carried on a device developed previously [10], which was based on the static-analytical method with liquid and vapor phase sampling. The apparatus was modified as follows. A six-way valve(Valco Co.) was used to control the injection volume and the experimental repeatability was greatly improved. The volume of stainless steel equilibrium cell was enlarged from 120 ml to 160 ml to reduce the impact of balance when taking the sample. The thermal regulator (Model LC-6, Julabo Co.) was used, so that the temperature stability of the bath was improved from ±0.10 K to ±0.03 K, and the cooling unit (Eyela Co.) was used to improve low temperature performance of the system. The phase equilibrium apparatus in this study is shown in Fig. 1.
The composition of mixture was analyzed by a gas chromatography (GC), produced by Shimadzu Co.(Model GC-9A) with a Thermal Conductivity Detector (TCD). The operation conditions of GC were: oven temperature 130 °C, injection temperature 170 °C,detector temperature 170 °C, detector current 170 mA,hydrogen with a flow rate of 50 ml·min-1as carrier gas [11-17].
2.3 VLE data
At five selected temperatures, 253.15 K, 273.15 K,293.15 K, 313.15 K and 333.15 K, binary vapor-liquid equilibrium data for ethylene + mesitylene system were measured under the pressure from 0 to 3 MPa.The VLE data are shown in Table 2.
The pressure-composition data from ethylene +mesitylene vapor-liquid equilibrium experiments are shown in Figs. 2 and 3. Ethylene concentration in liquid phase reduces as temperature increases and increases with pressure, while ethylene concentration in vapor phase increases with pressure. The solubility of ethylene is large in mesitylene. At pressure 2.1 MPa and temperature 253.15 K, the concentration of ethylene in the liquid phase is up to 0.91. It shows that mesitylene has a potential as a novel ethylene absorbent.
2.4 Correlations
Peng-Robinson (PR) equation is commonly used in chemical engineering. The phase equilibrium is correlated with the PR equation of state with mixing rules [18-21]. The explicit form for this equation is expressed as

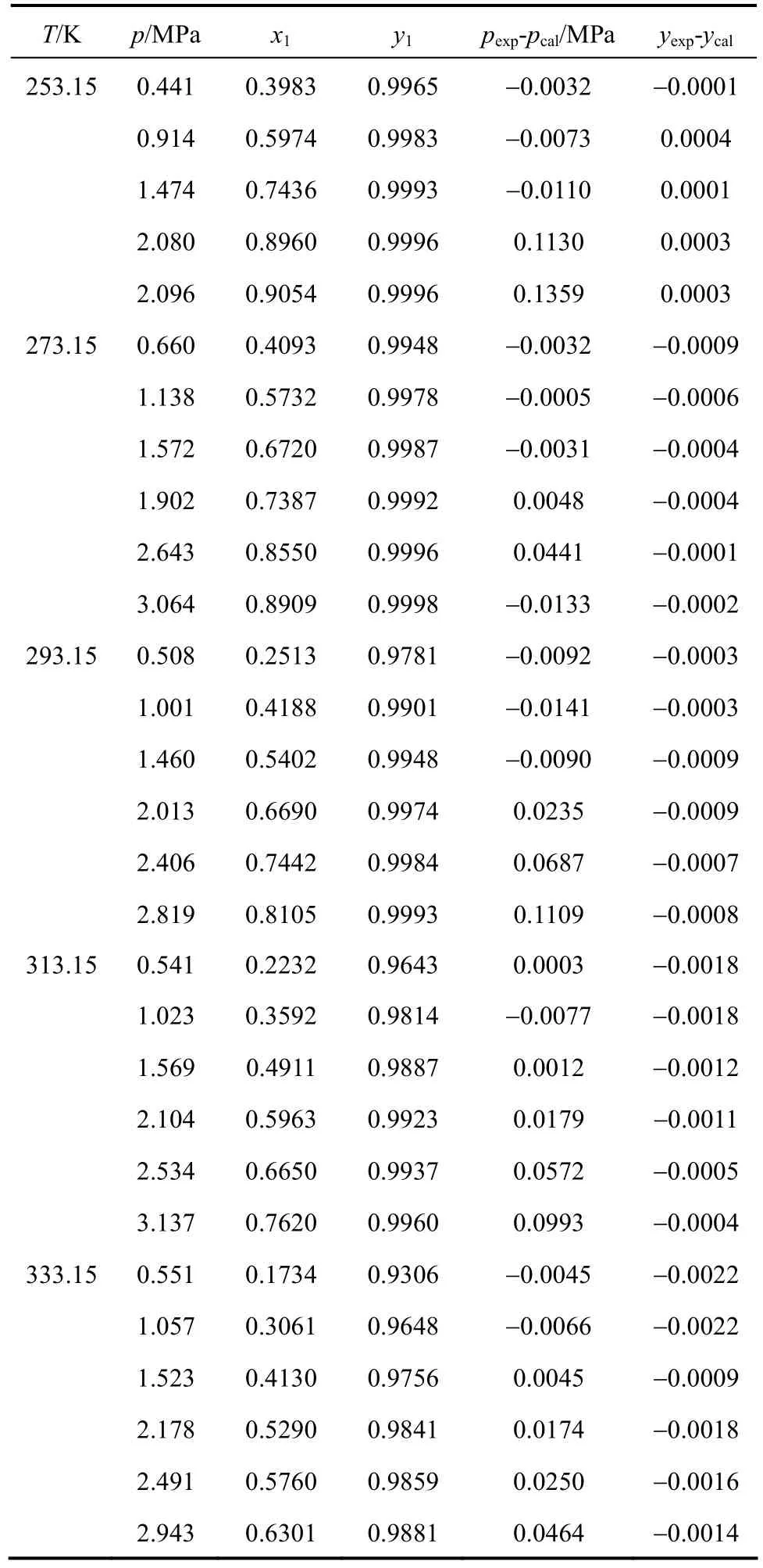
Table 2 VLE data for C2H4(1) + C9H12(2) system from T=253.15 K to 333.15 K
wherep,R,T,vandωdenote the pressure, gas constant, temperature, molar volume, and acentric factor,respectively,a,bandαare parameters, subscriptscandrdenote the critical properties and relative properties, respectively.
The following mixing rules are used with binary interaction parameterkij


Figure 2 Liquid phase diagram of C2H4(1) + C9H12(2) system T/K: + 253.15; △ 273.15; ● 293.15; ■ 313.15; ▲ 333.15

Figure3 Vapor phase diagram of C2H4(1) + C9H12(2) system T/K: + 253.15; △ 273.15; ● 293.15; ■ 313.15; ▲ 333.15

Figure 4 Vapor phase composition deviations from the experimental dataT/K: + 253.15; △ 273.15; ● 293.15; ■ 313.15; ▲ 333.15
wherekij=kji,kii=0,yis the mole fraction, subscriptsiandjdenote the components.
In the range of our experimental temperature and pressure, the gas and liquid phases in equilibrium are non-ideal. In this paper, the VLE data for ethylene +mesitylene binary system is calculated by the PR equation with its mixing rule. The binary interaction parameterkijof the equation is -0.1872.
The vapor phase deviations of the calculated results by PR equation of state from the experimental data are shown in Fig. 4. The system pressure deviations of the calculated results from the experimental data are shown in Fig. 5. The calculation data accord with the experimental data well, so the PR equation of state can be used to express the VLE data of ethylene +mesitylene system. All values in Figs. 4 and 5 are listed in Table 2.

Figure 5 Pressure deviations from the experimental data T/K: + 253.15; △ 273.15; ● 293.15; ■ 313.15; ▲ 333.15
3 PROCESS SIMULATION FOR ETHYLENE RECOVERY
According to the middle low temperature absorption process patent [22], the main equipment parameters refer to ethylene recovery process of liquefied petroleum gas, establishing mesitylene absorption of ethylene with the Aspen Plus, the process is calculated with the PR equation, and the equation parameters are obtained by the experimental data regression. The binary interactive parameters of methane + mesitylene and ethane + mesitylene are obtained from literature [9],and the binary interactive parameters of other components of refinery off-gas and mesitylene are considered as zero. Aspen Plus provides parameters for all refinery off-gas components in vapor phase. We use the absorption tower, desorption tower, crude separation column and four flash equipment in the mesitylene absorption for ethylene. RadFrac model is used to simulate the equipment in Aspen plus.
Figure 6 is the schematic diagram of ethylene recovery. Refinery off-gas, which contains ethylene,first enters the absorption tower, in which absorbent mesitylene absorbs ethylene from the refinery off-gas.When the gas passes four flasher equipment and the desorption tower, the bottom fluid of desorption tower contains almost 100% mesitylene, which can be recycled as ethylene absorbent through a pump, and the top fluid of desorption tower contains ethylene and ethane, which are separated in a crude separation column at low temperature. At last, we obtain ethylene and ethane from the top and bottom separately.
With the sensitivity analysis tools of Aspen Plus to determine the operating conditions for absorption tower, desorption tower and crude separation column,we obtain these values as shown in Table 3. The operating pressure in the towers is lower, and it needs lesstheoretical trays. The operating pressures in the four flash equipment are 1.2 MPa, 0.7 MPa, 0.4 MPa, and 0.1 MPa. Besides, the liquid gas ratio (mass) of absorption tower is 4, and the reflux ratio (mole) of desorption tower and crude separation column is 2. While the inlet flow rate of refinery off-gas is 1000 kg⋅h-1, the mass flow rate of ethylene in refinery off-gas is 208.25 kg·h-1. The composition of inlet refinery off-gas, outlet ethylene product and outlet ethane product in the process simulation are shown in Table 4 in terms of patent CN 101063048A, in which C3-C5are assumed as alkane in terms of their carbon atom number and composition of C3-C5.

Table 3 Operating conditions of main equipment
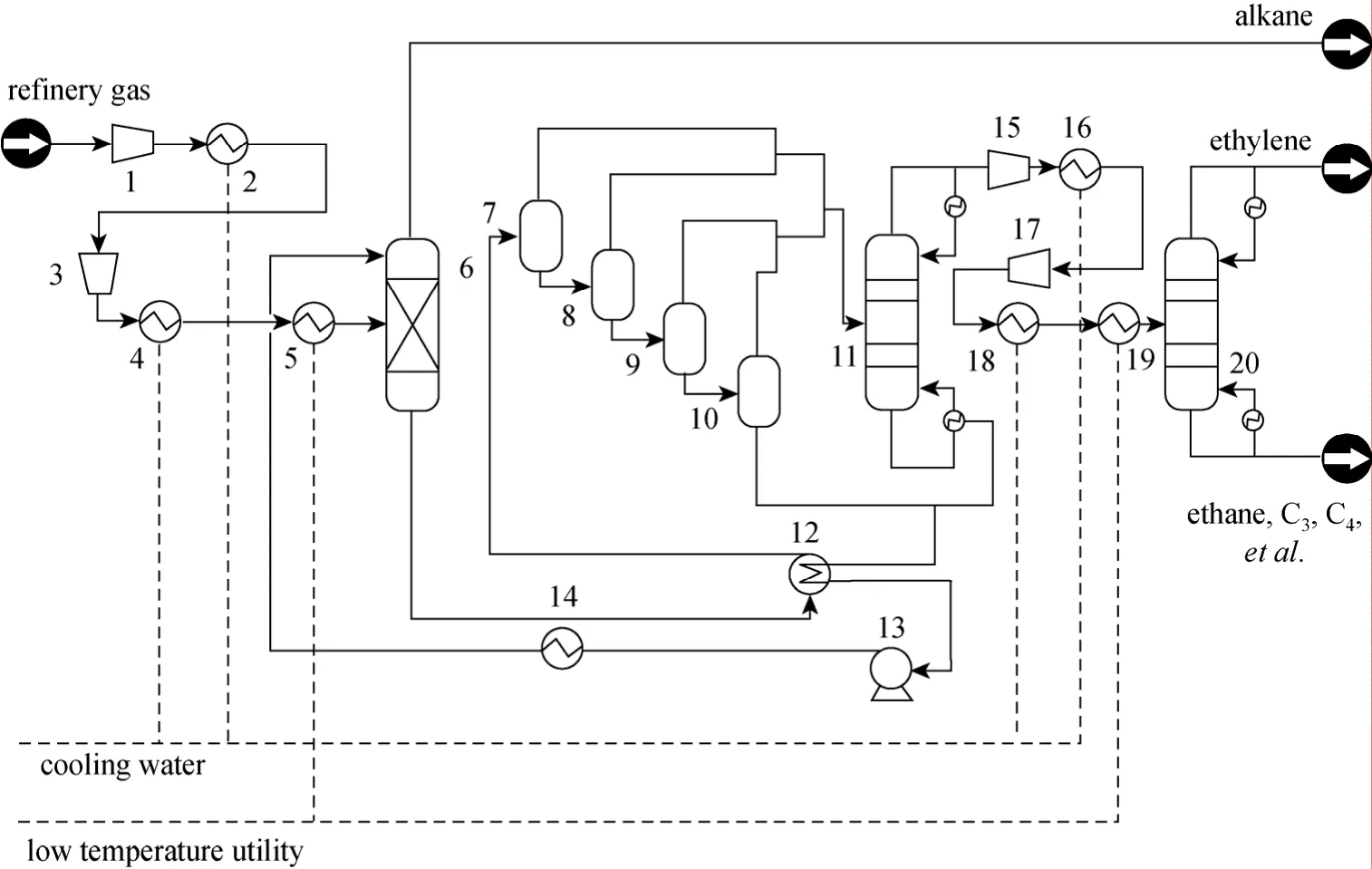
Figure 6 Schematic diagram of ethylene recovery1—compressor; 2—heat exchanger; 3—compressor; 4—heat exchanger; 5—heat exchanger; 6—absorption tower; 7-10—flash equipment; 11—desorption tower; 12—heat exchanger; 13—pump; 14—heat exchanger; 15—compressor; 16—heat exchanger; 17—compressor; 18—heat exchanger; 19—heat exchanger; 20—crude separation column

Table 4 Composition of inlet refinery off-gas, outlet ethylene product and outlet ethane product

Table 5 Effect of ethylene absorption process (mesitylene process)
After passing through the mesitylene process, the mass flow rate of ethylene in the product is 207.87 kg⋅h-1. The effect of ethylene absorption is shown as Table 5. From Tables 3 and 5, we can see that the mesitylene process at lower pressure gives large ethylene recovery ratio, high purity of ethylene in product and low absorbent loss rate, which can be recycled as ethylene absorbent for the absorption tower.
In this process, the flash equipment does not need energy supply. Solute and solvent can flash or absorb in the device at the balanced temperature and pressure.Besides, the operating pressure of equipment is all less than 2 MPa. Therefore, electric energy consumption is less. The utility energy consumption in the mesitylene process can be calculated with Aspen Plus. According to the process simulation results, the total electric load is 71.24 kW, total cooling water load is 144.82 kW,total cold facility load is 285.27 kW, and total steam load is 438.98 kW. When the total energy consumption is converted into standard coal unit, for the mesitylene process to recover 1 t ethylene from refinery off-gas, the process will consume 557.07 kg coal equivalent, which meets the criteria [23, 24] of ethylene product consumption limitation (<935 kg-coal equivalent per ton-ethylene).
4 CONCLUSIONS
VLE data of ethylene + mesitylene binary system are measured. PR equation is used to regress the experimental data and well describes the system.
With Aspen plus and the regressed equation parameters, the recovery process of ethylene with mesitylene from refinery off-gas is simulated. With the operating conditions assessed by sensitivity analysis tool, the process using mesitylene as ethylene absorbent presents following advantages: (1) less theoretical trays and low operating pressure of towers, with the desorption tower operated under atmospheric condition; (2) less absorbent loss, 0.02%; (3) low energy consumption, with 557.07 kg coal equivalent per ton recovered ethylene; (4) high ethylene recovery ratio,up to 99.8%.
1 Douglas, J.S., Eldridge, R.B., “Olefin/paraffin separations by reactive absorption: a review”,Ind.Eng.Chem.Res., 37 (7), 2571-2581(1998).
2 Mitariten, J.M., Peekskill, N.Y., “PSA process for recovery of ethylene”,US Pat., 5245099, (1993).
3 Sikavitsas, V.I., Yang, R.T., Burns, M.A., Langenmayr, E.J., “Magnetically stabilized fluidized bed for gas separations: Olefin-paraffin separations by π-complexation”, Ind. Eng. Chem. Res., 34 (8),2873-2880 (1995).
4 Eriksen, O.I., Dahl, I.M., Vik, I.B., Posey, M.L., “Facilitated transport membranes: separation of ethene from ethane with silver ionexchanged nafion hollow fibers”, Polymer Membranes for Gas and Vapor Separation., 733 (9), 115-126 (1999).
5 Mehra, Y.R., Lam, W.K., Mullins, D.W., “Absorption method of recovery ethylene and hydrogen”, CN Pat., 1075707, (1993). (in Chinese)
6 Fang, Y.D., Xie, C.L., “The research of ethylene recycle and utilization in catalytic exhaust gas”, Petrochem. Techn., 32, 102-104(2003).
7 Zheng, D.X., Ji, P.J., Qi, J.P., “Maximum excess Gibbs function of working pairs and absorption cycle performance”, Int. J. Refrig., 24(8), 834-840 (2001).
8 Zhu, C.F., Wu, X.H., He, W., Jing, S.H., “Measurement and correlation of vapor-liquid equilibria for the system carbon dioxidediisopropyl ether”, Fluid Phase Equilibria, 264, 259-263 (2007).
9 Deng, W., Zheng, D.X., Wu, X.H., Guo, L., “Research on CH4+m-C9H12binary vapor-liquid equilibrium system and CH4+ C2H4+m-C9H12ternary vapor-liquid equilibrium system”, J. of Beijing Univ.of Chem. Techn., 37, 5-7 (2010). (in Chinese)
10 He, W., Zheng, D.X., Wu, X.H., “The isothermal measurement of ethylene + toluene VLE data”, Petrochem. Techn., 36 (4), 362-365(2007). (in Chinese)
11 Lee, L., Ou, H., Hsu, H., “The experiments and correlation of the solubility of ethylene in toluene solvent”, Fluid Phase Equilibria,231 (2), 221-230 (2005).
12 Shi, Y.F., Ma, H.L., Gao, Y., Yuan, W.K., “High-pressure vapor-liquid equilibrium for ethylene + benzene”, J. Chem. Eng. Data, 44 (1),30-31 (1999).
13 Srinivasa, S., Gao, W., Gasem, K.A., Robert, L.R., “Solubility of methane in toluene at temperatures from 313 to 423 K at pressures to 8.9 MPa”, J. Chem. Eng. Data, 43 (4), 623-625 (1998).
14 Ng, H.J., Huang, S.S., Robinson, D.B., “Equilibrium phase properties of selected m-xylene binary systems. m-xylene-methane and m-xylene carbon dioxide”, J. Chem. Eng. Data, 27 (2), 119-122(1982).
15 Williams, B., Prodany, N.W., “Vapor-liquid equilibriums in methane-hydrocarbon systems”, J. Chem. Eng. Data, 16 (1), 1-6 (1971).
16 Chang, H.L., Kobayashi, R., “Vapor-liquid equilibrium of the methane-toluene system at low temperatures and high pressures”, J.Chem. Eng. Data, 12 (4), 517-520 (1967).
17 Ma, Q.L., Chen, G.J., Ma, C.F., “Study of vapor-hydrate two-phase equilibria”, Fluid Phase Equilibria, 265, 84-93 (2008).
18 Stanley, M.W., Phase Equilibria in Chemical Engineering, Butterworth Publishers, London, 333-386 (1985).
19 Tada, Y., Tamakoshi, A., Kato, Y., Nagatsu, Y., “Generalized Peng-Robinson equation of state with pair potential parameters for liquid n-alkenes”, Fluid Phase Equilibria, 262, 236-243 (2007).
20 Perry, R.H., Green, D.W., Perry’s Chemical Engineering Handbook,McGraw-Hill, New York, 226-273 (2001).
21 Smith, J.M., van, Ness, H., Abbott, M.M., Introduction to Chemical Engineering Thermodynamics, McGraw-Hill, Boston, 329-354(2001).
22 Cheng, J.M., Mao, W.X., Li, D.F., Dai, W., Liao, L.H., Wang, J.,“Using middle low temperature absorption method to separate refinery exhaust gas”, CN Pat., 101063048A, (2007). (in Chinese)
23 Shen, Y.L., Liu, A.Y., Li, W.D., Cao, Q.G., Jiang, Y., Li, W.F., Ding,S., Lu, S.G., Wang, X.S., Li, D., Chen, W.J., Wang, L., Wang, W.,Mu, W.L., “The limitation of ethylene product consumption”,DB37/751-2007, 2007-11-01 (2007). (in Chinese)
24 Wang, J.D., Hu, H.F., “The limitation of ethylene product consumption and the calculation method”, DB33/808-2010, 2010-09-19(2010). (in Chinese)
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Adsorptive Recovery of Uranium from Nuclear Fuel Industrial Wastewater by Titanium Loaded Collagen Fiber*
- Salting-out Extraction of 2,3-Butanediol from Jerusalem artichoke-based Fermentation Broth*
- Investigation of Mg2+/Li+ Separation by Nanofiltration*
- Purification and Characterization of a Nonylphenol (NP)-degrading Enzyme from Bacillus cereus. Frankland*
- Sponge Effect on Coal Mine Methane Separation Based on Clathrate Hydrate Method*
- Modeling of Surface Tension and Viscosity for Non-electrolyte Systems by Means of the Equation of State for Square-wellChain Fluids with Variable Interaction Range*
