Solvent Recovery from Soybean Oil/Hexane Miscella by PDMS Composite Membrane*
2011-03-22CAIWeibin蔡卫滨SUNYanzhi孙艳芝PIAOXianglan朴香兰LIJiding李继定andZHUShenlin朱慎林
CAI Weibin (蔡卫滨), SUN Yanzhi (孙艳芝), PIAO Xianglan (朴香兰), LI Jiding (李继定)** and ZHU Shenlin (朱慎林)
State Key Laboratory of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
1 INTRODUCTION
Vegetable oil is mostly processed by solvent extraction using hexane as solvent at present. The solvent content of the miscella (mixture of extracted oil and solvent) in the extractor exit is always 70% to 75% (by mass). The miscella is then evaporated to separate the oil and hexane. Since the process involves solvent phase change, the evaporation process requires considerable amount of energy (about 530kJ per kg of hexane) [1]. In addition, the explosive vapor in the vegetable oil plant also brings a safety problem. Since most of the membrane separation process does not involve phase change, if evaporation is partially replaced by membrane separation, great energy can be saved.
According to Cheryan [2], a process using membranes when compared to conventional distillation may promote substantial cost reduction for vegetable oil processing due to the pre-concentration of the miscella and the opportunity to use more compact evaporators. In addition, this new process can have environmental and safety advantages, since risks of explosion, for instance, are minimized [3].
By analyzing the difference between the triacylglycerols (oil) and hexane, it is found that solvent recovery from miscella by membrane is feasible. The first difference is their molecular weight. Triacylglycerols has a molecular weight about 900 g·mol-1and hexane, 86 g·mol-1. Thus their molecular volume differs markedly. Moreover, they have different diffusivities. This make the membrane separation become possible. An ideal membrane for solvent recovery should combine specific properties such as high rejection of oil and a suitable permeate fluxes that are adequate for industrial scale, as well as mechanical, thermal and chemical resistances compatible with the process [1].
Several papers reported recovery of hexane from miscella by membrane separation. In their research UF(untrafiltration), NF (nanofiltration) and RO (reverse osmosis) membrane are tested [4-8], but the results are not satisfactory. The separation performance (permeate fluxes and rejection) were rather limited,i.e., either the permeate fluxes is low, or the rejection is too low. One reason is that the membranes used in these works are mostly hydrophilic, which are mainly used in water treatment, but hexane is hydrophobic and the hydrophobicity is very strong. At the same time, it was found that membrane swelling is a severe problem.Only a few membranes could permeate hexane without being destroyed, while most membranes were destroyed very quickly. These issues greatly limited research work in this area, and till now, hexane recovery by membrane has been investigated only at bench scale.
PDMS (polydimethylsiloxane) is one of the hydrophobic membrane materials. It is found that PDMS membrane is stable in organic solvent, and the separation performance is satisfactory [9-12]. For this reason,PDMS was selected as membrane material in this study. Cross-linked PDMS/PVDF composite membranes were prepared, in which asymmetric microporous PVDF membrane prepared with phase inversion was acted as the microporous supporting layer in the flat-plate composite membrane. It is found that,when filled with zeolite, separation performance can be improved [13-17]. Thus, zeolite filled PDMS/PVDF composite membranes were also prepared. The separation performances of the prepared membranes were then used to recover hexane from soybean oil/hexane miscella.
2 EXPERIMENTAL
2.1 Materials
PVDF of 1.76 g·cm-3in density, 170,000 in molecular weight was purchased from the Solvay Company, Belgium. PDMS prepolymer with a viscosity of 20 Pa·s was received from Beijing Chemical Reagents Corporation. Hexane,n-heptane, dibutyltin dilaurate(DBTL) and tetraethylorthosilicate (TEOS) were obtained from Beijing Jingyi Chemical Reagents Corporation, Beijing, China. Zeolite is provided by The Catalyst Plant of Nankai University. Miscella was prepared from 25% (by mass) refined commercial soybean oil (Yihai Kerry Group) and 75% (by mass) hexane.PVDF was used for the preparation of porous membrane supporter. Triethyl phosphate (TEP, reagent grade,Beijing Chemical Corporation) was used as the solvent for PVDF UF membrane formation. All reagents were used as received unless otherwise mentioned.
2.2 Preparation of PDMS/PVDF composite membranes
The PVDF support layer was prepared by dissolving PVDF in TEP solvent to form a 15% (by mass)solution. The solution was then cast on non-woven fiber and then immersed into water to induce polymer precipitation. The residual solvents was fully exchanged with ethanol and evaporated at room temperature. The thickness of PVDF layer was controlled in the range of 45-50 μm.
PDMS, crosslinking-agent ethyl orthosilicate and catalyst dibutyltin dilaurate were dissolved inton-heptane at room temperature. Zeolite of ZSM-5 particles was added into the solution under stirring. The resulting suspension was then sonicated for 30 min to promote the dispersion of ZSM-5 particles. After degassed under vacuum, the solution was cast onto the PVDF membrane. The membrane was first vulcanized under room temperature to evaporate the solvent, and then placed in an oven at different temperature for 5h to complete crosslinking.
Zeolite used was ZSM-5 particles with Si/A1 ratio of 360, the size is about 4-6 μm.
2.3 Membranes characterization
2.3.1SEM
The morphologies of PVDF membrane and PDMS/PVDF composite membrane were obtained by scanning electron microscope (SEM, JEOL JSM-7401F,Japan). Membranes were coated with Au/Pd in vacuum for three minutes.
2.3.2FTIR-ATR analysis
The change of chemical structures of PDMS before and after cross-linking was confirmed using Fourier transform infrared (FTIR) spectrometer (Nicolet,IR560) in the ranger of 4000-400 cm-1.
2.4 Membrane conditioning
According to Kesting [18], before each test, conditioning of polymeric membranes should be performed. Membrane conditioning consists of its immersion in a work fluid and filtration under appropriate pressure in order to remove conservants and humectants from the surface and their pores. After conditioning it is possible to increase solvent permeation and to improve membrane global performance.
According to vad der Bruggenet al. [19], immersing polymeric membranes in hexane may cause agglomeration of hydrophobic and hydrophilic sites presented in the active layer. As a consequence, hydrophilic membranes may have their hydrophilicity reduced. Thus, before each test, membranes were immersed in oil/hexane miscella for 24 h and then filtered at room temperature, 2.4 MPa for 1 h, and 1.0 MPa for 1 h.
2.5 Separation experiments
Separation experiment apparatus used in this study can be seen in Fig. 1. The membrane was positioned in the stainless steel permeation cell with an effective area of 21.0 cm2. The feed solution continuously circulated from the feed tank to the upstream side of the membrane in the cell at a desired pressure,and the permeate was collected after a steady state was obtained. The compositions of the feed solution and the permeate were analyzed by gas chromatography.
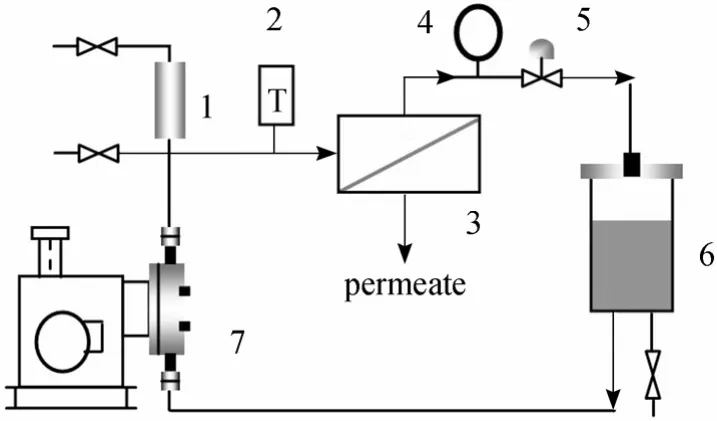
Figure 1 Schematic flow diagram of membrane evaluation equipment1—bumper; 2—electric thermometer; 3—membrane unit; 4—pressure gauge; 5—counterbalance valve; 6—feed tank; 7—plunger pump
Separation performances of the membranes can be evaluated on the basis of total flux and retention coefficient. The permeate fluxJwas determined by measuring the mass of permeate collected and divided by time and the membrane’s effect surface area as show in Eq. (1):

where Δmis the mass of permeate during the operation time Δtat steady state, andAis the effective membrane area.
The retention coefficient for the oil was defined as:
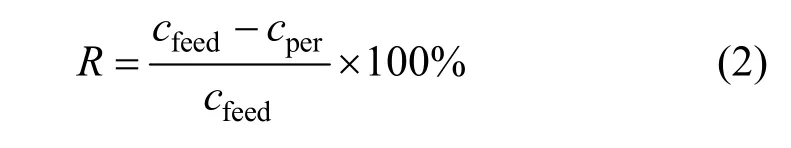
wherecfeedandcperare the oil concentration in the feed and permeate, respectively.
3 RESULTS AND DISCUSSION
3.1 SEM photographs of membrane
SEM permits imaging cross section and surface membrane morphology. The morphologies of the composite membrane and PVDF membrane prepared in this study were presented in Fig. 2.
As can be seen in Fig. 2 (a), the surface morphology of the PVDF UF membrane was rough,which was good for it to combine with PDMS layer.Fig. 2 (b) is the surface image of PDMS/PVDF composite membrane. The PDMS layer, functioning as the basis of selectivity, had a nonporous and tight structure. The surface was dense and there is no any pinhole or crack, which was important for the practical application. With the filling of zeolite, as can be seen in Fig. 2 (c), the surface became rough. This was for the presence of zeolite in the surface, but the surface was still featureless.
The cross-section morphology of the composite membrane was shown in Figs. 2 (d) and (e). As demonstrated in the SEM photograph, there was a clear boundary between the top layer and the PVDF support layer, and the top layer combines with the support one tightly.
3.2 FTIR results
ATR (attenuated total reflection) Fourier transform infrared spectroscopy is commonly used to characterize chemical structure of the surface [20]. The ATR technique enables the identification of specific molecules and groups located within 100 nm from the surface layer. To obtain detailed information about the structural changes of PDMS/PVDF membranes resulted from crosslinking, FTIR spectra of the surface of PDMS/PVDF membranes and PDMS prepolymer were recorded in Fig. 3 using the ATR technique.
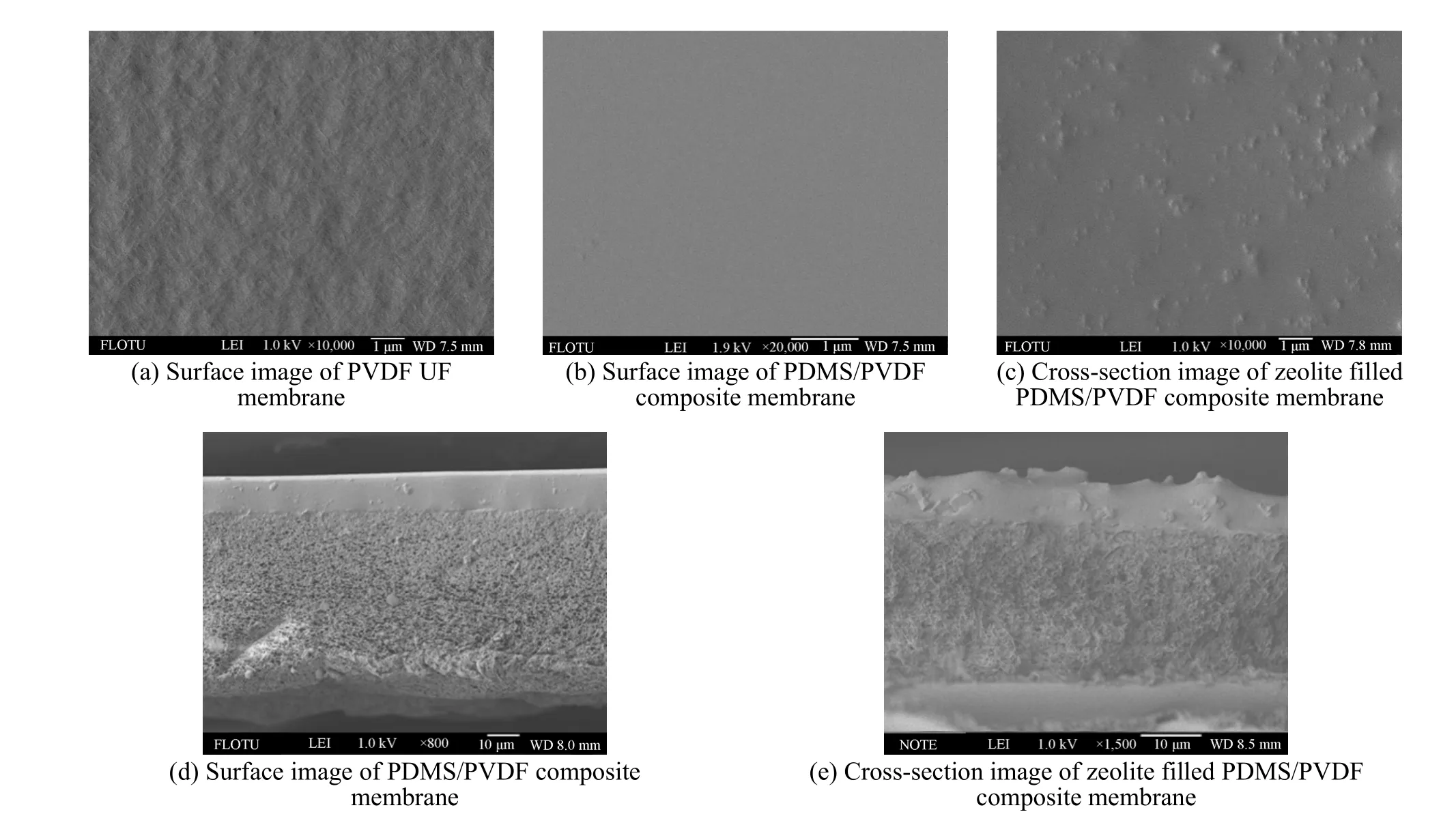
Figure 2 The surface and cross-section SEM image

Figure 3 FTIR spectrums of uncrosslinked and crosslinked PDMS
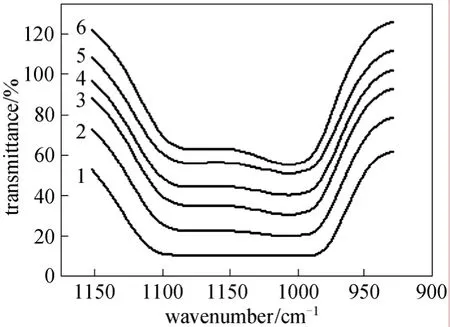
Figure 4 FTIR-ATR spectrums of magnified area of SiOH in 1150-1000 cm-1 area 1—PDMS prepolymer;crosslinking temperature/°C: 2—30; 3—60; 4—80; 5—110;6—140
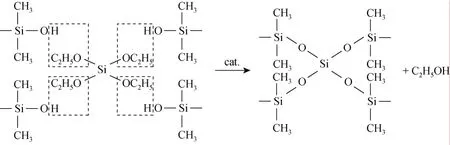
The PDMS used in this study is hydroxyl-terminated one. The degree of the crosslinking reaction can be learned from the absorbance intensity of SiOH. The characteristic absorption bands of SiOH groups appeared at 3000-2890 cm-1and 1150-1000 cm-1.Comparing with the uncrosslinked PDMS (Fig. 3), the SiOH absorbance signals of the crosslinked membrane obviously weakened. And it could also be found that with the increase in crosslinking temperature, the absorption peak weakened (Fig. 4). These changes were the evidences of crosslinking reaction of hydroxyl-terminated PDMS with TEOS by DBTL catalysis, and with the increase in crosslinking temperature, the crosslinking degree increase, too. The crosslinking reaction is shown as the following formula [21]:
3.3 Separation performance of the PDMS/PVDF composite membrane
3.3.1Effect of crosslinking temperature and pressure
Impacts of crosslinking temperature on permeate flux and oil retention was depicted in Figs. 5 and 6,respectively. It could be found that with the increase of membrane crosslinking temperature, the permeate flux decreased, while the retention of oil increased at first till reached a maximum value at 110 °C and then began to decline. This could be explained that when the crosslinking temperature is low, the crosslinking degree is low too and the PDMS layer is relatively‘loose’, leading to large permeate flux and low oil retention. With the increase of crosslinking temperature, crosslinking degree increase, which resulted in the rise of density and caused smaller available free volume of polymer matrix for diffusion. Thus, flux dropped and oil retention increased. But if crosslinking temperature is too high (140 °C), the membrane prepared became curl and defect may occur, decreasing the oil retention.
Although membrane prepared under low crosslinking temperature had larger permeate flux, the oil retention was low, which made it not so competitive in practical use. Considering both flux and oil retention,crosslinking temperature in the region of 80-110 °C is preferable for the PDMS/PVDF composite membrane.
From Figs. 5 and 6 the influence of feed pressure on separation performance could be found. The permeate flux increased quickly with a nearly linear increase of feed pressure. This can be explained by the pore-based model. In this model the permeate flux,J,can be calculated as follows [22]:
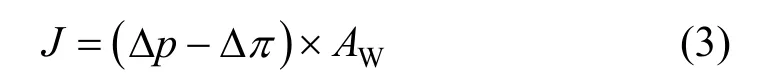
where Δpand Δπrepresent pressure and osmotic pressure differential between feed and permeate, respectively, andAWis the permeability coefficient.
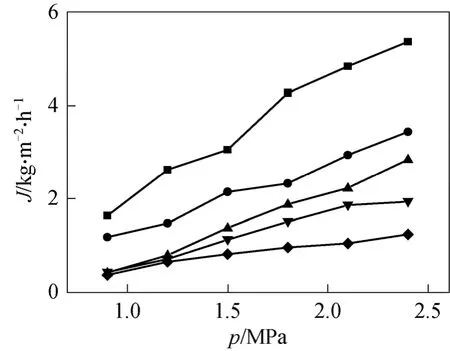
Figure 5 Effect of crosslinking temperature and experiment pressure on membrane permeate flux (membrane thickness: 45 μm)crosslinking temperature/°C: ■ 30; ● 60; ▲ 80; ▼ 110; ◆ 140
The pressure of permeate is zero and Δπdose not change much when oil content in permeate is low, and thus the relation of permeate fluxJand feed pressure is nearly linear.
From Fig. 6 it showed that with the increase of feed pressure oil retention increased at first and then decreased. The maximum feed pressure appeared at a range of about 1.5-1.8 MPa. For application in industry, feed pressure should be adjusted to optimize flux and oil retention.
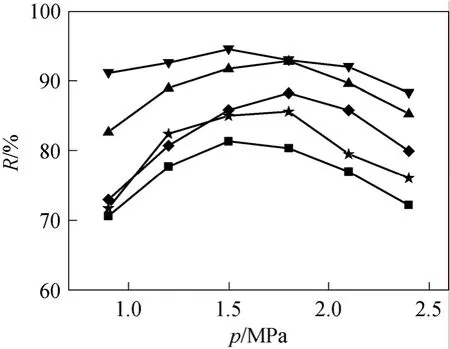
Figure 6 Effect of crosslinking temperature and experiment pressure on oil retentioncrosslinking temperature/°C: ■ 30; ★ 60; ▲ 80; ▼ 110; ◆ 140
3.3.2Effect of PDMS layer thickness
To research the influence of PDMS layer thickness on separation performance, various PDMS composite membranes with different thickness (12, 30 and 45 μm) were prepared. The separation performance can be seen from Fig. 7.
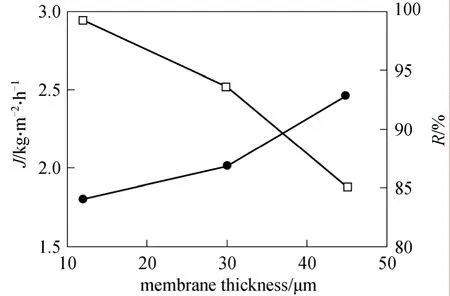
Figure 7 Effect of membrane thickness on separation performance (pressure: 1.8 MPa)□ flux; ● oil retention
As the thickness of membrane increased, the flux showed a remarkable decrease, which was due to the increase in mass transport resistance, while the oil retention exhibited a slightly increase from 84% to 93%. It is generally recognized that membrane selectivity is determined by two factors: solubility and diffusivity. For a selected system, when membrane thickness increases, the selectivity for the component which diffusion quickly can be improved. In this work,the separation layer of the composite membrane is hydrophobic PDMS, which makes it dissolve hexane preferentially. On the other hand, hexane is much smaller than soybean molecular, which makes hexane diffuse more quickly than soybean oil. Thus, oil retention increases with the increase of membrane thickness. The result confirmed the conclusion. The oil retention does not increase much, showing that diffusivity is not the major factor in this process.
3.3.3Separation performance of the Zeolite filled PDMS/PVDF composite membrane
In order to see whether there was an enhancement in separation performance, Zeolite filled PDMS/PVDF composite membrane was prepared and tested (Fig. 8).In this study, ZSM-5 particles with Si/A1 ratio of 360 was selected, and the ZSM-5 content is 10% (by mass)of PDMS.
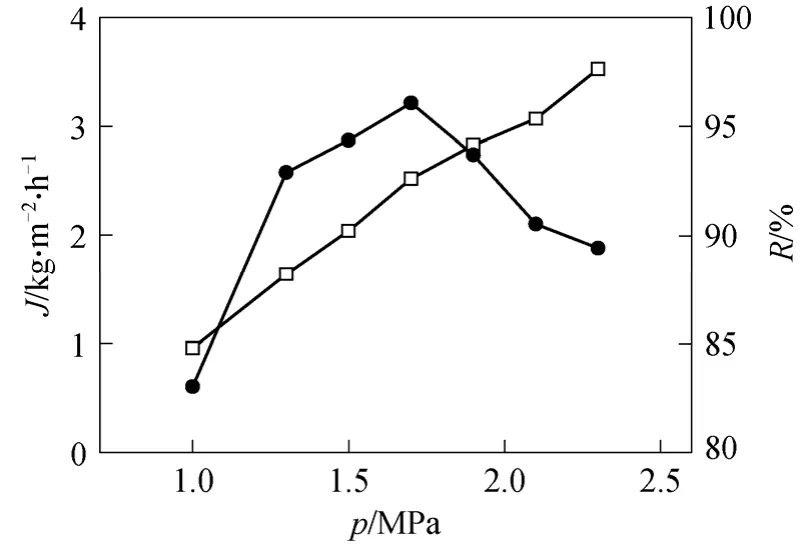
Figure 8 Separation performance of zeolite filled composite membrane [crosslinking temperature: 80 °C, Zeolite content: 10% (by mass) of PDMS]□ flux; ● oil retention
Comparing with the PDMS composite membrane crosslinked under 80 °C in Figs. 5 and 6, both permeate flux and oil retention increase remarkably. A maximum oil retention of 96.1% is observed at feed pressure 1.7 MPa with corresponding oil content of 0.97% and permeate flux of 2.52 kg·m-2·h-1. The permeate can be reused to extraction directly.
The enhancement of separation performance for zeolite filled composite membrane can be explained in two aspects. First, ZSM-5 is highly hydrophobic,which makes it more affinitive to hexane than soybean oil. On the other hand, ZSM-5 has many inner pores with size about 0.51 nm×0.55 nm, which is similar to the size of hexane but much smaller than oil molecular.Thus hexane can pass the hole freely, while soybean oil is too large to go through.
4 CONCLUSIONS
Crosslinked PDMS/PVDF and zeolite PDMS/PVDF composite membrane were prepared, characterized by FTIR, SEM, and employed in hexane recovery from hexane/soybean oil miscella. Experimental results indicated that permeate flux decreased with the increase of crosslinking temperature, while oil retention increase at the beginning and then decreased. Temperature range of 80-110 °C is preferable for crosslinking. The increase of PDMS layer thickness resulted in a sharp drop in permeate flux and a slightly increase in oil retention. The permeate flux increased with the increase of feed pressure, while oil retention had a maximum value. Zeolite filled PDMS composite membrane can increase separation performance remarkably. Under the feed pressure of 1.7 MPa, permeate flux is 2.52 kg·m-2·h-1and oil retention is 96.1%. The result demonstrated that the PDMS/PVDF composite membranes are effective for recovering hexane from hexane/oil miscella.
1 Koseoglu, S.S., Engelgau, D.E., “Membrane applications and research in the edible oil industry”,J.Amer.Oil Chem.Soc., 67, 239-249 (1990).
2 Cheryan, M., “Membrane technology in the vegetable oil industry”,Membr.Technol., 207, 5-7 (2005).
3 Stafie, N., Stamatialis, D.F., Wessling, M., “Insight into the transportation of hexane-solute systems through tailor-made composite membranes”,J.Membr.Sci., 228, 103-116 (2004).
4 Koseoglu, S.S., Lawhon, J.T., Lusas, E.W., “Membrane processing of crude vegetable oil: Pilot plant scale removal of solvent from oil miscellas”,JAOCS., 67, 315-322 (1990).
5 Ribeiro, A.P.B., de Moura, J.M.L.N., Goncalves, L.A.G., Petrus,J.C.C., Viotto, L.A., “Solvent recovery from soybean oil/hexane miscella by polymeric membranes”,J.Membr.Sci., 282, 328-336(2006).
6 Liu, H.X., Zhu, J.H., Zhao, J.T., Li, D., “Membrane applications in edible oil degumming and solvent recovery”,Cereals and Oils Processing, 9, 78-81 (2006).
7 Wu, C. S., Lee, E., “Ultrafiltration of soybean oil/hexane extracted by porous ceramic membranes”,Journal of Membrane Science, 154,251-259 (1999).
8 Koike, S., Subramanian, R., Nabetani, H., Nakajima, M., “Separation of oil constituents in organic solvents using polymeric membranes”,JAOCS, 79 (9), 937-942 (2002).
9 Chen, J., Li, J. D., Lin, Y. Z., Chen, C. X., “Pervaporation performance of polydimethylsiloxane membranes for separation of bezene/cyclohexane mixtures”,J.App.Poly.Sci., 112, 2425-2433 (2009).
10 Zhao, C. W., Li, J. D., Qi, R. B., Chen, J., Luan, Z. K., “Pervaporation separation ofn-heptane/sulfer species mixtures with polydimethylsiloxane membranes”,Sep.Purif.Technol., 63, 220-225(2008).
11 Zhang, X.Y., Chen, C.X., Chen, Z., Hao, J., Li, J.D., “Preparation of silicone polymer membrane for separation of solvents from lube oil”,Membr.Sci.and Technol., 25, 19-23 (2003).
12 Okamoto, K.I., Butsuen, A., Tsuru, S., “Pervaporation of water-ethanol mixtures through polydimethylsiloxane block-copolymer membrane”,Polymer J., 19, 747-756 (1987).
13 Zhan, X., Li, J.D., Chen, J., “Pervaporation of ethanol/water mixtures with high flux by zeolite-filled PDMS/PVDF nanofiltration membranes”,Chin.J.Polym.Sci., 27 (6), 771-780 (2009).
14 Jia, M.D., Peinemann, K.V., Behling, R.D., “Preparation and characterization of thin-film zeolite-PDMS composite membranes”,J Mem Sci., 73, 119-128 (1992).
15 Vankelecom, I.F.J., De Beukelaer, S., Uytterhoeven, J.B., “Sorption and pervaporation of aroma compounds using zeolite filled PDMS membranes”,J PhysChem B., 101, 5186-5190 (1997).
16 Moermans, B., Beuckelaer, W.D., Vankelecom, I.F.J., “Incorporation of nano-sized zeolites in membranes”,Chem.Commun., 24,2467-2468 (2000).
17 Bowen, T.C., Meier, R.G., Vane, L.M., “Stability of MFI zeolite-flled PDMS membranes during pervaporative ethanol recovery from aqueous mixtures containing acetic acid”,J.Membr.Sci., 298,117-125 (2007).
18 Kesting, R.E. “Synthetic Polymeric Membranes: A Structural Perspective”, Wiley-Interscience Publication, New York, 1985.
19 Van der, Bruggen, B., Geens, J., Vandecasteele, C., “Fluxes and rejections for nanofiltration with solvent stable polymeric membranes in water, ethanol andn-hexane”,Chem.Eng.Sci., 57, 2511-2518(2002).
20 Hillborg, H., Gedde, U.W., “Hydrophobicity changes in silicone rubbers”,IEEETrans.Dielectr.Electr.Insul., 6, 5-12 (1999).
21 Chen, C.Y., Wang, J., Chen, Z., “Surface restructuring behavior of various types of poly(dimethylsiloxane) in water detected by SFG”,Langmuir, 20, 10186-10193 (2004).
22 Mulder, M., Basic Principles of Membrane Technology, 2nd ed.,Kluwer Academic Publishers, Dorcrecht (1996).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Adsorptive Recovery of Uranium from Nuclear Fuel Industrial Wastewater by Titanium Loaded Collagen Fiber*
- Salting-out Extraction of 2,3-Butanediol from Jerusalem artichoke-based Fermentation Broth*
- Investigation of Mg2+/Li+ Separation by Nanofiltration*
- Vapor-Liquid Equilibrium of Ethylene + Mesitylene System and Process Simulation for Ethylene Recovery*
- Purification and Characterization of a Nonylphenol (NP)-degrading Enzyme from Bacillus cereus. Frankland*
- Sponge Effect on Coal Mine Methane Separation Based on Clathrate Hydrate Method*
