氯化铯催化氢氧化钾促进二烷基二硒醚的合成*
2011-03-06喻爱和赵文杰李宁波许新华
喻爱和,赵文杰,李宁波,许新华
(湖南大学化学化工学院,湖南长沙 410082)
氯化铯催化氢氧化钾促进二烷基二硒醚的合成*
喻爱和,赵文杰,李宁波,许新华†
(湖南大学化学化工学院,湖南长沙 410082)
针对氢氧化钠作用下水合肼还原硒形成Na2Se2与磺酸酯反应制备二烷基二硒醚需要在高温(100℃)下才能有效进行,且仅适用于制备不含官能团的二烷基二硒醚的问题.由于铯离子体积大,与阴离子之间的静电作用弱,使与之键合的阴离子表现出强的碱性和亲核性,利用铯碱作缩合剂,能显著降低反应的活化能,使许多反应在温和条件下就能有效进行.根据离子交换原理,采用氯化铯和TBAI作催化剂,以无水DMF作溶剂,4A分子筛作吸水剂,氢氧化钾促进肼还原硒,随后与含官能团的卤代烃或磺酸酯反应,高收率地形成对应的二烷基二硒醚.本方法不仅可以合成含官能团的二硒醚,而且具有反应条件温和、产率高等优点.
氯化铯;催化;二烷基二硒醚;肼;硒
有机硒化合物具有重要的生物活性,有机硒基团在有机合成转化中起着重要的作用,对有机硒化合物的研究日益得到生物、化学及材料等领域学者的重视.二硒醚是有机硒化学中重要的中间体.由于硒原子最外层具有孤对电子,同时又有空的4d轨道,所以二硒醚既是亲核试剂又是亲电试剂.尽管制备二硒醚的方法有多种[1-4].但是常用的方法是格氏试剂法,即硒插入C—Mg键,随后进行水解、氧化[5].这一方法主要应用于制备二烷基二硒醚,由于涉及到格氏试剂的制备,所以要求在十分严格的无水、无氧下操作,且对于带有官能团如羰基、硝基的底物不适用.最近文献报道了用(Et4N)2WSe4与卤代烃反应[6]及在氢氧化钠作用下水合肼还原硒形成Na2Se2与磺酸酯反应[7]制备二烷基二硒醚的方法.尽管前一方法在室温就可有效进行,可以制备含官能团的二烷基二硒醚,但是要使用重金属试剂;后一方法需要在高温(100℃)下才能有效进行,仅适用于制备不含官能团的二烷基二硒醚.所以,开发新的简便有效方法制备含官能团的二烷基二硒醚具有一定应用价值.
近几年,关于氢氧化铯在有机合成中的应用有许多报道[8-14].由于铯离子体积大,与阴离子之间的静电作用弱,使与之键合的阴离子表现出强的碱性和亲核性.利用铯碱作缩合剂,能显著降低反应的活化能,使许多反应在温和条件下就能有效进行.但是与KOH相比,CsOH价格昂贵,采用等当量的CsOH,对于工业化生产,成本很高.根据离子交换原理,本文设计用催化剂CsCl催化KOH促进水合肼还原硒制备含官能团的二烷基二硒醚.
1 实验部分
1.1 主要仪器和试剂
1H NMR(以CDCl3作溶剂,TMS为内标)用BRUKER AC-P400型仪测定;质谱由VG Auto Spec-300仪测定;Yanaco CHN CORDER MT-3型自动元素分析仪,硅胶为青岛海洋化工厂产品;溶剂DMF用无水硫酸钙干燥,减压蒸馏;氢氧化铯、硒粉从Aldrich公司购买;其他试剂均为分析纯.
1.2 实验步骤
向50 m L单口瓶中依次加入KOH(1.5 mmol),2.0 m L无水DMF,CsCl(0.1 mmol),79 mg硒粉(1 mmol),0.17 m L水合肼,TBAI(0.1 mmol),4A分子筛(100 mg),氮气保护下室温搅拌15 min.,然后向反应体系中加入卤代烃或对甲苯磺酸酯(1.0 mmol),在室温下搅拌,TLC跟踪反应,当原料反应完毕,向体系中加入10 m L水,用乙酸乙酯萃取(3×10 m L),合并有机相,水洗(2×10 m L),无水硫酸钠干燥,过滤,旋转去掉溶剂,柱层析纯化,用石油醚/乙酸乙酯(V石油醚∶V乙酸乙酯=2∶1)作洗脱剂,得产物.

2 结果与讨论
实验表明,在10%摩尔CsCl与相转移催化剂存在下,氢氧化钾促进肼还原硒,形成亲核的硒负离子与卤代烃或磺酸酯在室温就能有效反应形成二烷基二硒醚,其结果见表1.由表1可知,不论是活泼的亲电试剂如磺酸酯、α-溴代乙酸乙酯还是活性小的亲电试剂如氯乙醇、β-溴代酯及1-溴乙酰基葡萄糖、烷基溴都能获得高产率的二烷基二硒醚.本方法不仅能够在室温下合成二烷基二硒醚,还可合成含官能团如酯基及羟基的二烷基二硒醚,对于二(替加氟乙基)及二(四乙酰基半乳糖)二硒醚的制备,也具有高的收率.氯化铯催化氢氧化钾促进肼还原硒合成二烷基二硒醚过程如图1所示.
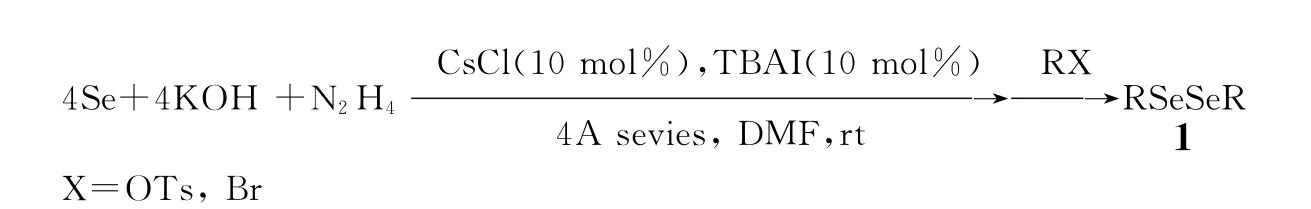
图1 氯化铯催化氢氧化钾促进肼还原硒合成二烷基二硒醚Fig.1 Synthesis of diaklyldiselenides by hydrazine reduction of selenium in the presence of potassium hydroxide catalyzed by chloride catalyzed
反应机理如图2所示.
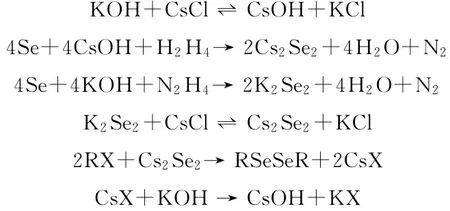
图2 氯化铯催化机理Fig.2 The catalytic mechanism of cesium chloride
由图2可知,通过离子交换产生CsOH,在CsOH或KOH促进下肼还原硒形成亲核的硒负离子.CsOH参与的反应形成Cs2Se2,KOH参与的反应形成K2Se2,后者通过与CsCl离子交换转化为Cs2Se2.Cs2Se2与RX进行亲核取代形成产物二硒醚,同时产生CsX,通过离子交换后,又形成CsOH.所以采用催化剂的CsCl就可实现上述反应,相转移催化剂TBAI可以加速反应进行,4A分子筛可以吸收反应产生的水,推动反应向正反应方向移动.
我们考察了在没有CsCl存在室温反应下,1a的收率仅为63%,加热到100℃,收率可以达到90%.
3 结 论
本文采用氯化铯做催化剂,价格便宜的氢氧化钾为促进剂促进肼还原硒合成二烷基二硒醚,此方法具有反应条件温和,收率高的优点,为官能化二烷基二硒醚的合成提供了一条有效途径.
[1] KRIEF A,DELMOTTE C,DUMONT W.Chemoselective reduction of organoselenocyanates to diselenides and selenolates[J].Tetrahedron,1997,53(36):12147-12149.
[2] KRIEF A,DUMONT W.Synthesis of primary-alkyl selenols and selenides from primary-alkyl thiols involving diphenyl sulfonium salts[J].Chem Commun,2005:2167-2169.
[3] KRIEF A,DUMONT W,DELMOTTE C.Reaction of organic selenocyanates with hydroxides:the one-pot synthesis of dialky diselenides from alky bromides[J].Angew Chem Int Ed,2000,39(9):1669-1671.
[4] SLAMA P,BERNARD C.Chemoselective synthesis of func-tionalized diselenides[J].Tetrahedron Lett,1995,36(32):5711-5713.
[5] NOGUEIRA C W,ZENI G,ROCHA J B T.Organoselenium and organotellurium compounds:toxicology and pharmacology[J].Chem Rev,2004,104:6255-6290.
[6] SARAVANAN V,PORHIEL V.Tetraethylammonium tetraselenotungstate:a new and efficient selenium transfer reagent for the chemoselective synthesis of functionalised diselenides[J].Tetrahedron Lett,2003,44(11):2257-2259.
[7] SCIANOWSKI J.Convenient route to dialkyl diselenides from alkyl tosylates.Synthesis of di(cis-myrtanyl)diselenide[J].Tetrahedron Lett,2005,46(19):3331-3335.
[8] TZALIS D,KNOCHEL P.Cesium hydroxide:a superior base for the catalytic alkynylation of aldehydes and ketones and catalytic alkenylation of nitriles[J].Angew Chem Int Ed,1999,38:1463-1468.
[9] ZOU K B,LIU W Q,QIU R H,etal.Stereoselective synthesis of(Z)-1,2-diarylthio-1-alkene via the reaction of diaryl disulfides with terminal alkynes catalyzed by cesium hydroxide[J].Synthetic Commun,2009,39(14):2464-2471.
[10]ZOU K B,QIU R H,FANG D W,etal.Cesium hydroxide-catalyzed reaction of terminal alkynes with diarylditellurides to synthesize alkynyl tellurides[J].Synthetic Commun,2008,38(13):2237-2241.
[11]夏湘,邹康兵,李若信,等.氢氧化铯催化二硫醚、二碲醚与端炔反应研究[J].化学学报,2008,66(14):1749-1752.
XIA Xiang,ZOU Kang-bing,LI Ruo-xin,etal.Study of cesium hydroxide-catalyzed reactions of diaryl disulfides and ditellurides with terminal acetylenes[J].Acta Chin Sinica,2008,66(14):1749-1752.(In Chinese)
[12]夏湘,李剑平,邹康兵,等.氢氧化铯促进下硒、端炔及二芳基碘盐反应合成炔基芳基硒醚[J].有机化学,2009,29(10):112-115.
XIA Xiang,LI Jian-ping,ZOU Kang-bing,etal.Cesium hydroxide promoted reaction of element selenium with terminal alkynes and diaryliodonium salts to synthesize acetylenic arylselenides[J].Chin J Org Chem,2009,29(10):112-115.(In Chinese)
[13]TZALIS D,KORADIN C,KNOCHEL P.Cesium hydroxide catalyzed addition of alcohols and amine derivatives to alkynes and styren[J].Tetrahedron Lett,1999,40:6193-6195.
[14]XU Zhen-yuan,ZHANG Yong-min.An atom-economical and environmentally benign preparation of unsymmetrical bis-allyl ethers via dimerization of Baylis-hillman adducts catalyzed by cesium hydroxide monohydrate[J].Synlett,2005:2999-3001.
The Synthesis of Dialkyl Diselenides Promoted by Potassium Hydroxid Catalyzed by Cesium Chloride
YU Ai-he,ZHAO Wen-jie,LI Ning-bo,XU Xin-hua†
(College of Chemistry and Chemical Engineering,Hunan Univ,Changsha,Hunan 410082,China)
The preparation of dialkyl diselenides by the reaction of tosylates with disodium diselenide derived from hydrazine reduction of selenium promoted by sodium hydroxide needs high temperature(100℃),and this method is only suitable for preparation of the diselenides without functional group.Cesium ion(Cs+)is bulky,and the electrostatic interaction between cesium ion(Cs+)and anion is very weak.So the anion attached to Cs+has strong alkality and nucleophilicity.The reaction proceeds more readily under mild reactive conditions using alkali of cesium as a catalyst because of the remarkable decline in activation energy.However,CsOH is very expensive compared to KOH.In this paper,according to the principle of ion exchange,using cesium chloride(CsCl)and TBAI as catalysts and dry DMF as solvent,hydrazine reduced selenium promoted by potassium hydroxide,followed by treatment with functional alkyl halide or tosylates at room temperature to afford corresponding diaryl diselenides in high yields.The method not only prepares diselenides containing functional group but has the advantages of mild conditions and high yields.
cesium chlorides;catalysis;dialkyl diselenide;hydrazine;selenium
O626.4
A
1674-2974(2011)06-0060-04*
2010-11-20
湖南省自然科学基金资助项目(2009NK3162)
喻爱和(1965-),男,湖南平江人,湖南大学访问学者,湖南机电职业技术学院副教授
†通讯联系人,E-mail:xhx1581@yahoo.com.cn
