The influence of autophagy on mouse inflammatory responses caused by Salmonella enterica serovar Typhimurium with spv genes
2011-01-24LIYuanYuanWUShuYanCHUYuanYuanLIAOLILIQiongHUANGRui
LI Yuan-Yuan, WU Shu-Yan, CHU Yuan-Yuan, LIAO LI, LI Qiong, HUANG Rui
Department of Medical Microbiology, Medical School of Soochow University, Suzhou 215123, China
Salmonellaentericaserovar Typhimurium (S.typhimurium) is a Gram-negative bacterium that causes gastroenteritis in humans and a typhoid-like systemic infection in mice. Infection results in food intoxication of millions of people each year. The disease caused byS.typhimuriumrequires functional expression of virulence genes. Kuritaetal. have identified a highly conserved 8-kb region responsible for the bacterial virulence phenotype, which has been designated asSalmonellaplasmid virulence gene (spv), and presents on the plasmid of pathogeneticSalmonellaspp.[1]. Thespvgenes appear to promote rapid growth and survival ofSalmonellawithin host cells, characteristics that are important for maintaining systemic infection in experimental animals[2].
Autophagy is a cellular process that mediates
the degradation of long-lived proteins and unwanted organelles in the cytosol by delivering them to the lysosomes[3]. Autophagy allows for the targeting and elimination of intracellular microbes as an important part of the innate immune response and also enhances the delivery of pathogen-related peptides to the lysosomes and affects the adaptive immunity[4]. Due to the conservation of autophagy, pathogens evolve mechanisms to escape from the host defense system, and they can even utilize autophagy as an advantage for their own survival[5]. Jiaetal. found that inCaenorhabditiselegansandDictyosteliumdiscoideum, genetic inactivation of the autophagy pathway increases intracellularS.typhimuriumreplication, decreases animal lifespan, and results in apoptotic-independent death[6].
Apoptosis has a great significance in maintaining host stability in normal growth and metabolism, and is also involved in defending the infections of viruses, bacteria, and parasites. Apoptosis, therefore, is regarded as an effective host mechanism to control the progression of the infection; however, some pathogens have developed strategies to overcome host killing and can use apoptotic cells as a tool for spreading[7]. The relationship between autophagy and apoptosis is very complicated[8]. Autophagy can serve as the origin of apoptosis and inhibition of autophagy may delay the occurrence of apoptosis. Otherwise, autophagy may antagonize apoptosis and suppression of autophagy will enhance cell sensitivity to apoptotic signals[9,10].
In the present study, the standardS.typhimuriumvirulence strain SR-11, which carriesspvgenes was employed;S.typhimuriumstrain BRD509 withoutspvwas used as the negative control. To evaluate the effects ofspvgenes on autophagy, apoptosis and inflammatory responses caused by the host bacteriainvivo, pathological changes of livers and spleens fromS.typhimurium-infected mice, the expression of autophagy and apoptosis proteins, the levels of inflammatory cytokines such as interleukin 12 (IL-12) and interferon γ (IFN-γ), and the viable bacterial quantities at various time points post-infection were examined.
1 Materials and methods
1.1 Mice
Female C57BL/6 mice, aged 8-12 weeks, were purchased from the Experimental Animal Centre, Chinese Academy of Sciences. All mice were maintained under specific-pathogen-free conditions. Animal experiments complied with the standards set out in the Guidelines for Care and Use of Laboratory Animals of Soochow University, China.
1.2 Bacterial strains and culture conditions
S.typhimuriumstrain SR-11 is a virulent wild type strain carryingspvgenes. BRD509 is an attenuated virulentS.typhimuriumstrain withoutspvgenes. Both strains were grown to mid-logarithmic phase at 37 ℃ in Luria-Bertani broth, and quantified spectrophotometrically by determining the optical density at 600 nm along with viable plate counts. Then they were centrifuged at 2 300gfor 5 min and re-suspended in RPMI 1640 medium without antibiotics.
1.3 Infection of mice
Mice were randomly assigned to 5 groups. Three groups of mice (n=36/group) were injected intraperitoneally (i.p.) with 200 colony-forming units (CFUs) of SR-11, BRD509, and 200 μl of phosphate buffered saline (PBS), respectively. Mice (n=6 per time point) were sacrificed at 6 h, 1 d, 2 d, 5 d, 7 d, and 10 d post-infection. The other two groups of mice (n=24/group) were injected i.p. (consecutively) with autophagy agonist rapamycin (RAPA,0.25 mg/kg·d, 9 d) 1 d after SR-11 or BRD509 infection according to previous study[11]and our preliminary experiment. Mice were then sacrificed at 2 d, 5 d, 7 d, and 10 d. Serum was collected for cytokine analysis.
1.4 Haematoxylin and eosin (HE) stain
Livers and spleens from mice were aseptically removed and washed twice with normal saline. Organs were cut into small samples and fixed for 24 h with formaldehyde solution, and then washed and dehydrated by a series of graded ethanol. Samples were embedded with paraffin after xylene treatment, and stained with HE. The pathological changes of organs were observed with an Olympus microscope (CX21, Japan) and Jieda software (China).
1.5 Immunohistochemical analysis
Livers and spleens from mice were dissected into appropriately sized pieces. Organ sections (7 μm) were cut, mounted, and air-dried. Detection of surface markers was conducted using a standard horseradish peroxidase (HRP) method. Sections were fixed in ice-cold acetone, re-hydrated in PBS, and blocked with 20% normal goat serum in PBS for 30 min. Primary multi-antibodies (mAbs) Caspase-3 (Santa Cruz, USA) or Beclin-1 (Cell Signaling, USA) were added and reacted at 4 ℃ overnight; secondary mAbs were added and reacted at 37 ℃ for 30 min. Staining was visualized by adding diaminobenzidine (DAB) for 10 min at room temperature. Sections were counterstained with hematoxylin. Finally, sections were dehydrated and mounted. Assessment was conducted on an Olympus microscope (CX21, Japan) fitted with Jieda software (China) and ImagePro Plus 5.0.
1.6 Cytokine analysis
Mice sera were collected and stored at -80 ℃. Titers of IL-12 and IFN-γ were tested by double sandwich enzyme-linked immunosorbent assay (ELISA) kits (Biosource, USA) according to manufacturer’s protocol.
1.7 Assessment of bacterial survival in organs
Livers and spleens were aseptically removed and homogenized in a glass tissue homogenizer with 2 ml of normal saline per 0.5 g tissues. A series of diluted tissue homogenates were placed on SS agar plates for the viable plate counts.
1.8 Statistical analysis
Data were expressed as mean±SD, andttest was performed by SPSS13.0 for analyzing the differences. Differences were considered significant if thePvalue was less than 0.05. Each experiment was repeated at least twice and accepted as valid only when trials showed similar results.
2 Results
2.1 Pathological organ changes
To evaluate the pathogenicity ofS.typhimuriumwithspvgenes, mice were injected i.p. with SR-11 and BRD509. Then mouse livers and spleens were assessed by the naked eye and HE stain. At 4 d post-infection, organs of SR-11-infected mice appeared significantly different and point-like lesions were found on the liver and spleen surface. It was observed that the livers showed cell degeneration, inflammatory cell infiltration, and focal necrosis. Spleens demonstrated multinucleated giant cells in diffuse hyperplasia, focal necrosis, splenic sinus expansion, and transparent thrombosis. Organs of BRD509-infected mice showed no significant differences compared with the control mice. After RAPA intervention, swelling of organs from SR-11-infected mice decreased and liver cells showed only slight degeneration, while BRD509-infected mice demonstrated liver cell degeneration and splenic lymphocyte proliferation (Fig.1, Fig.2).
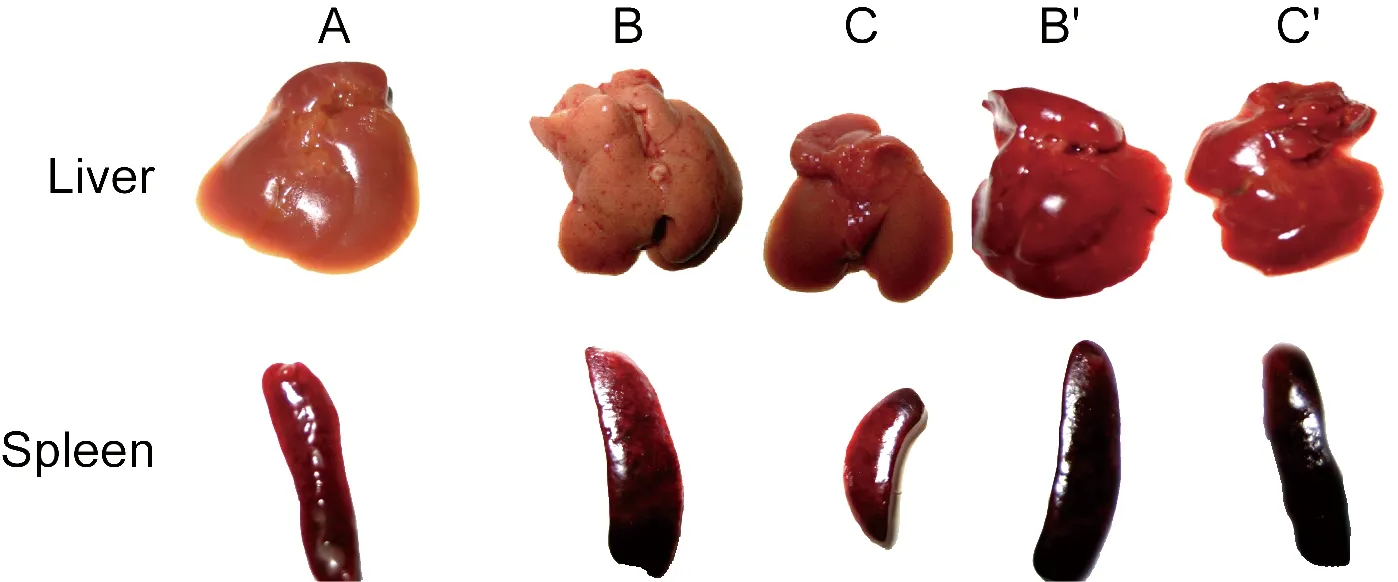
A: Organs of control mice. B: Organs of SR-11-infected mice. C: Organs of BRD509-infected mice. B′: Organs of mice injected with SR-11 and RAPA. C′: Organs of mice injected with BRD509 and RAPA.
Fig.1Theappearanceofmouseliversandspleensat4dpost-infection

A, C: Organs of SR-11-infected mice. B, D: Organs of BRD509-infected mice. E, G: Organs of mice injected with SR-11 and RAPA. F, H: Organs of mice injected with BRD509 and RAPA. I, J: Organs of control mice.Fig.2 The pathological change of mouse livers (40×) and spleens (20×) at 4 d post-infection detected by HE stain
2.2 Beclin-1 and Caspase-3 expression in mouse organs
Beclin-1 is an important autophagy protein and Caspase-3 is a protein associated with apoptosis. To estimate the level ofS.typhimuriumwithspvgenes caused autophagy and apoptosis, Beclin-1 and Caspase-3 expression in mouse livers and spleens were assayed by immunohistochemistry. The number of Beclin-1-positive cells in livers and spleens of BRD509-infected mice were significantly greater than that in the SR-11 group at 6 h, 1 d, and 2 d post-infection. At 4 d post-infection, Beclin-1 expression in both groups started to decrease. After RAPA intervention, Beclin-1 expression in both groups was increased (Fig.3).
At 6 h, 1 d, and 2 d post-infection, few Caspase-3-positive cells were observed in the livers and spleens of mice in both SR-11 and BRD509 groups. At 4 d post-infection, Caspase-3 expression in the SR-11 group was significantly increased while expression in the BRD509 group remained low. After RAPA intervention, Caspase-3 levels in the SR-11 group decreased while levels in the BRD509 group increased (Fig.4).
2.3 Cytokine production in mouse serum
One challenge faced by the immune system during bacterial infection is effectively removing the invasive pathogens while minimizing host injury. IL-12 and IFN-γ titers in mouse serum were detected in this study. It was observed that both strains led to notable production of IL-12 and IFN-γ at 1 d post-infection. At 2 d post-infection, the cytokine levels in the SR-11 group were significantly lower than those observed in the BRD509 group (P﹤0.05) while no obvious change was observed after RAPA intervention. Since 4 d post-infection, IL-12 and IFN-γ secretions in the SR-11 group were significantly higher than that observed in the BRD509 group (P﹤0.05). After RAPA intervention, cytokine titers in the SR-11 group were decreased while those in the BRD509 group were increased (P﹤0.05). At 4 d post-infection, cytokine levels in both groups reached a peak and then began to decline along with the infection time (Tab.1).
2.4 Bacterial survival in mouse organs
To identify the invasive ability of thespv+strain, mice were infected with SR-11 and BRD509 and bacterial survival in livers and spleens was examined at various time points post-infection. Viable bacterial amounts were observed in livers and spleens in both SR-11 and BRD509 groups and continued to rise from 2 d post-infection, increasing exponentially. The quantity of viable bacteria in the SR-11 group was significantly greater than that in the BRD509 group and this difference became obvious along with the infection time (P﹤0.05). Four days after RAPA intervention, bacterial amounts in the SR-11 group decreased significantly while those in the BRD509 group increased (P﹤0.05) (Fig.5).
Tab.1MouseserumcytokinesassayedbyELISA(pg/ml,mean±SD,n=6)

TimeGroupsIL-12IFN-γ6 h1 d2 d4 d7 d10 dSR-112.43×10±2.261.32×10±3.01BRD5092.33×10±2.131.35×10±2.32Control2.41×10±1.831.31×10±2.70SR-117.40×10±5.926.86×10±7.88BRD5098.15×10±5.217.10×10±15.32Control2.33×10±2.241.32×10±2.05SR-111.39×102±1.01×10*1.52×102±2.38×10*BRD5091.89×102±1.04×102.17×102±3.54×10SR-11+RAPA1.43×102±1.16×101.68×102±3.01×10BRD509+RAPA1.91×102±0.99×102.14×102±1.90×10Control2.53×10±2.181.33×10±1.70SR-111.58×103±3.43×102*#1.36×103±1.15×102*#BRD5091.38×103±2.59×1021.29×103±1.07×102SR-11+RAPA1.39×103±2.22×1021.14×103±1.23×102BRD509+RAPA1.66×103±2.71×102*1.48×103±1.13×102*Control2.33×10±3.111.32×10±1.90SR-111.38×103±2.14×102*#6.14×102±6.53×10*#BRD5091.27×103±2.23×1025.78×102±6.71×10SR-11+RAPA1.01×103±2.28×1025.55×102±5.13×10BRD509+RAPA1.53×103±3.41×102*7.43×102±5.27×10*Control2.43×10±2.631.30×10±2.20SR-111.28×103±2.19×102*#5.56×102±4.51×10*#BRD5091.18×103±1.02×1024.46×102±1.76×10SR-11+RAPA0.94×103±1.11×1024.21×102±6.03×10BRD509+RAPA1.37×103±3.49×102*6.43×102±3.21×10*Control2.38×10±2.291.31×10±0.73
Values are mean±SD (six mice per group) for cytokine production.*P﹤0.05vsBRD509 group,#P﹤0.05vsSR-11+RAPA group.
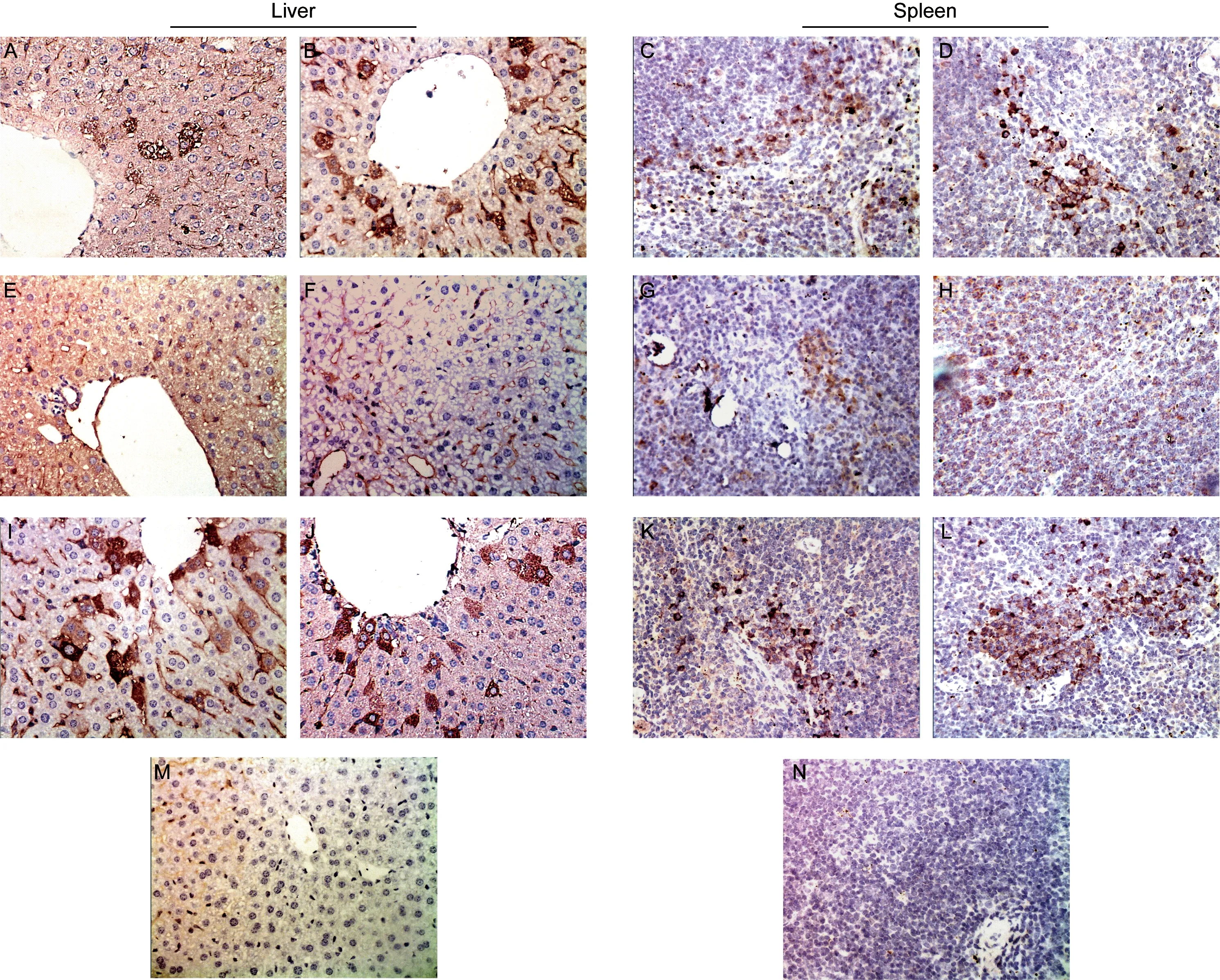
A, C: 1 d post-infection with SR-11. B, D: 1 d post-infection with BRD509. E, G: 4 d post-infection with SR-11. F, H: 4 d post-infection with BRD509. I, K: 2 d post-infection with SR-11 and RAPA. J, L: 2 d post-infection with BRD509 and RAPA. M, N: The normal control group.Fig.3 Beclin-1 expression in mouse livers (40×) and spleens (20×) detected by immunohistochemistry
3 Discussion
The ability ofSalmonellaspp. to resist and evade the antimicrobial arsenal of phagocytes is a prerequisite for virulence[12]. In recent years, eliminating intracellular bacteria by enhancing host defense mechanisms may be used as new methods for treatment of infectious diseases[13]. Our previous studies demonstrated that thespv-containing strain affected macrophage autophagy activity and increased intracellular viable bacterial numberinvitro, so as to induce rapid and massive cell apoptosis[14]. The present study aims to investigate the influence of autophagy on inflammatory response in mice infected byS.typhimuriumcontainingspvgenes.
Although autophagy and apoptosis are two different ways of programmed cell death, they are closely related to each other[15]. Beclin-1, an important autophagy protein, is a sign of autophagy initiation and a positive regulatory factor for autophagy[16]. Caspase-3-dependent phagocyte apoptosis during systemicS.typhimuriuminfection in mice has been reported[17]. Our research demonstrated that at the early stage of bacterial infection, Beclin-1 expression in livers and spleens of SR-11-infected mice was lower than that observed in the BRD509 group, while at the late stage, Caspase-3 expression in the SR-11 group was higher than that in the BRD509 group. After RAPA intervention, Beclin-1 expression in the both groups increased and occurred earlier, while Caspase-3 expression in the SR-11 group decreased and increased in the BRD509 group. These data showed that in the early stage SR-11 inhibited autophagy but induced apoptosis, which may result in a serious inflammatory response. The induction of moderate autophagy by RAPA could protect the host from injury as a result of the infection.
Both IL-12 and IFN-γ play an important role in anti-intracellular bacteria, viruses, parasites, and in tumor immunity. Results revealed that IL-12 and IFN-γ levels in the SR-11 group were significantly lower than those in the BRD509 group at 2 d post-infection; however, there was no obvious change after RAPA intervention. Since 4 d post-infection, both cytokines in the SR-11 group were significantly higher than those in the BRD509 group; after RAPA intervention, levels in the SR-11 group apparently decreased while those in the BRD509 group increased during advanced stages of infection. In addition, we observed that the pathological changes that occurred in the livers and spleens of SR-11-infected mice were more severe than those observed in the BRD509 group. Viable bacterial amounts in the organs were also higher in the SR-11 group compared with those in the BRD509 group. After RAPA intervention organ injury in the SR-11 group was decreased but was aggravated in the BRD509 group. Furthermore, viable bacterial quantity in the SR-11 group decreased while it increased in the BRD509 group. These results suggest that at the early stage of infection,S.typhimuriumwithspvgenes can inhibit the secretion of inflammatory factors to evade the host clearance of bacteria. During advanced stage of infection, SR-11 promoted the production of inflammatory cytokines resulting in severe injury in mice, while moderate autophagy induced by RAPA could protect the host by affecting cytokine production to relieve organ damage. However, the combined effect of the antagonism between RAPA-activated autophagy and SR-11-inhibited autophagy determined the extent of host autophagy. This combined effect was delayedinvivo, so that cytokine production and host organ pathological changes demonstrated significant differences on the 4th day after RAPA intervention, while at the early stage of infection, there was no obvious change in cytokine levels.
Our results revealed an effect ofspvgenes on bacterial virulence and the “double-edged sword” function of autophagy.S.typhimuriumwithspvgenes (SR-11) displayed more virulence thanS.typhimuriumwithoutspvgenes (BRD509), triggering an increase in apoptosis and cell injury by suppressing autophagy. Autophagy inhibition may possibly be a self-protection mechanism by virulent strains, in order to avoid degradation. Under these conditions, autophagy is a protective mechanism to lessen bacterial infection and improve cell survival. While mice infected by a low virulent strain may resist bacterial invasion and remain vital, by inducing moderate autophagy. After interference with RAPA, autophagy in the BRD509 group was over-enhanced, which led to an up-regulation of the apoptosis rate and augmented cell damage. Nevertheless, suppression of autophagy in the SR-11 group was activated appropriately, resulting in a certain protective effect.
In summary,S.typhimuriumwithspvgenes could exacerbate infection by inhibiting autophagy and affecting the production of inflammatory cytokines. RAPA-enhanced autophagy could improve the secretion of cytokines to protect the host from damaging bySalmonellainfection. These results shed new light on the role of cellular autophagy in the prevention and control for some infectious diseases.
Acknowledgements
We are grateful to Prof. Roy Curtiss III, The School of Life Sciences, Arizona State University, USA, for kindly supplyingS.typhimuriumstrain SR-11.
[1] Kurita A, Gotoh H, Eguchi M, Okada N, Matsuura S, Matsui H, Danbara H, Kikuchi Y.Intracellular expression of the Salmonella plasmid virulence protein, SpvB, causes apoptotic cell death in eukaryotic cells [J]. Microb Pathog, 2003, 35(1): 43-48.
[2] Soto SM, Rodríguez I, Rodicio MR, Vila J, Mendoza MC. Detection of virulence determinants in clinical strains of Salmonella enterica serovar Enteritidis and mapping on macro-restriction profiles [J]. J Med Microbiol, 2006, 55(Pt 4): 365-373.
[3] Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms [J]. Nat Rev Microbiol, 2004, 2(4): 301-314.
[4] Yi X, Chinnaswamy J, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity[J]. Immunity, 2007, 27(1): 135-144.
[5] Birmingham CL, Higgins DE, Brumell JH. Avoiding death by autophagy: interactions of Listeria monocytogenes with the macrophage autophagy system [J]. Autophagy, 2008, 4(3): 368-371.
[6] Jia K, Thomas C, Akbar M, Sun Q, Adams-Huet B, Gilpin C, Levine B. Autophagy genes protect against Salmonella typhimurium infection and mediate insulin signaling-regulated pathogen resistance [J]. Proc Natl Acad Sci USA, 2009, 106(34):14564-14569.
[7] Early J, Fischer K, Bermudez LE. Mycobacterium avium uses apoptotic macrophages as tools for spreading [J]. Microb Pathog, 2011, 50(2): 132-139.
[8] Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis [J]. Nat Cell Biol, 2006, 8(10): 1124-1132.
[9] Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy [J]. Cell, 2005, 122(6): 927-939.
[10] Birmingham CL, Brumell JH. Methods to monitor autophagy of Salmonella enterica serovar typhimurium [J]. Methods Enzymol, 2009, 452: 325-343.
[11] Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells [J]. Blood, 2002, 100(3): 1084-1087.
[12] Wu S, Li Y, Xu Y, Li Q, Chu Y, Huang R, Qin Z. A Salmonella enterica serovar typhi plasmid induces rapid and massive apoptosis in infected macrophages [J]. Cell Mol Immunol, 2010, 7(4): 271-278.
[13] Schwegmann A, Brombacher F. Host-directed drug targeting of factors hijacked by pathogens [J]. Sci Signal, 2008, 1(29): re8.
[14] Wu SY, Li Q, Chu YY, Li YY, Huang R, Qin ZH. The influence of autophagy on apoptosis of macrophage induced by Salmonella typhimurium [J]. Microbiol China, 2010, 37(5): 776-782.
[15] Madden DT, Egger L, Bredesen DE. A calpain-like protease inhibits autophagic cell death [J]. Autophagy, 2007, 3(5): 519-522.
[16] Sun Q, Fan W, Zhong Q. Regulation of Beclin 1 in autophagy [J]. Autophagy, 2009, 5(5): 713-716.
[17] Grant AJ, Sheppard M, Deardon R, Brown SP, Foster G, Bryant CE, Maskell DJ, Mastroeni P.Caspase-3-dependent phagocyte death during systemic Salmonella enterica serovar typhimurium infection of mice [J]. Immunology, 2008, 125(1): 28-37.
