Prognostic models for acute liver failure
2010-12-14WeiBoDuXiaoPingPanandLanJuanLi
Wei-Bo Du, Xiao-Ping Pan and Lan-Juan Li
Hangzhou, China
Prognostic models for acute liver failure
Wei-Bo Du, Xiao-Ping Pan and Lan-Juan Li
Hangzhou, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 122-128)
acute liver failure;prognosis;the King's College Hospital criteria;model for end-stage liver disease score;liver transplantation
Introduction
Acute liver failure (ALF), characterized by the sudden onset of hyperbilirubinemia, hepatic encephalopathy and coagulopathy with no underlying liver disease, remains a dramatic and unpredictable disease with high morbidity and mortality.[1,2]Orthotopic liver transplantation is the ultimate procedure of proven bene fi t, which improves the one-year survival rate of patients ranging from 50% to 75%.[2,3]However,liver transplantation is limited by the high medical costs, the long-term application of immunosuppressive agents, and crucially the persistent scarcity of donor livers.Fortunately, with the help of intensive care and arti fi cial liver treatment, a substantial proportion of patients suffered from ALF have the capacity for spontaneous recovery,[4,5]thus avoiding unnecessary liver transplantation and the risk of mortality from its sequential complications.
Early and accurate prognostic assessment of patients with ALF is dif fi cult, but critically important for optimum clinical pathway, especially the appropriate utilization of liver transplantation. Over the last two decades, a number of prognostic models have been proposed to aid in decision-making for patients with ALF to be treated either medically or by liver transplantation. The well accepted multi-variable prognostic models including the King's College Hospital (KCH) criteria[6-11]and the model for end-stage liver disease (MELD) score[8,12-19]have been improved by incorporation with other clinical and biochemical indices.[20-24]Single variable prognostic models such as serum Gc-globulin,[25-27]arterial blood lactate,[21,22]serum phosphate,[23,28,29]arterial blood ammonia,[30-32]serum alpha-fetoprotein,[33-36]or prothrombin time[37]also appear to be promising. They have been applied in many hospitals but should be further assessed by future large-scale perspective studies.
In this paper we review the available prognostic indicators of ALF and their pros and cons.
KCH criteria and modi fi ed KCH criteria KCH criteria
The KCH criteria, initially proposed by O'Grady et al for the selection of patients with end-stage liver disease for liver transplantation in 1989,[7]are etiologically speci fi c prognostic models for both acetaminophen-induced and non-acetaminophen-induced ALF. The criteria are based on many available clinical and biochemical variables,including grade of hepatic encephalopathy, arterial blood pH, prothrombin time, and serum creatinine,which can re fl ect the severity of hepatic coma, acidosis,coagulopathy and renal dysfunction in the course of ALF (Table 1).[7]
In the earliest large cohort of 588 patients with AHF between 1983 and 1985 at the Liver Unit of King's College Hospital, O'Grady et al identi fi ed that the KCH criteria were able to indicate a poor prognosis in both acetaminophen-induced and non-acetaminophen-induced ALF, with the positive predictive value of 84% and 98%,the negative predictive value of 86% and 82%, and the diagnostic accuracy of 85% and 95%, respectively.[7]The criteria were further validated in a cohort of 175 patients between 1986 and 1987 at the same institution.[7]Since then, the KCH criteria have been con fi rmed by a number of studies (Table 2), and have been used in selecting patients with ALF for liver transplantion.[6,8-11,15-17,19]
However, not all studies have shown that the diagnostic accuracy of the KCH criteria is as good as reported by O'Grady et al.[7,8,11]Recent studies have con fi rmed that the diagnostic accuracy of the KCH criteria was 70%-88% in acetaminophen-induced ALF,and 55%-92% in non-acetaminophen-induced ALF,respectively. More importantly, many evidences show that the ful fi llment of the KCH criteria is of high predictive value for poor outcome, and is a strong indication for liver transplantation.[9,10]However, lack of the ful fi llmentcould not predict spontaneous survival.[9,10]Therefore,a signi fi cant proportion (23%-70%) of patients, particularly non-acetaminophen-induced ALF cases died without meeting the KCH criteria.[7,9,10,19,38]Contrarily,about 21% of patients with ALF who ful fi ll the KCH criteria will survive without a liver transplant.[7,9,10,19,38]
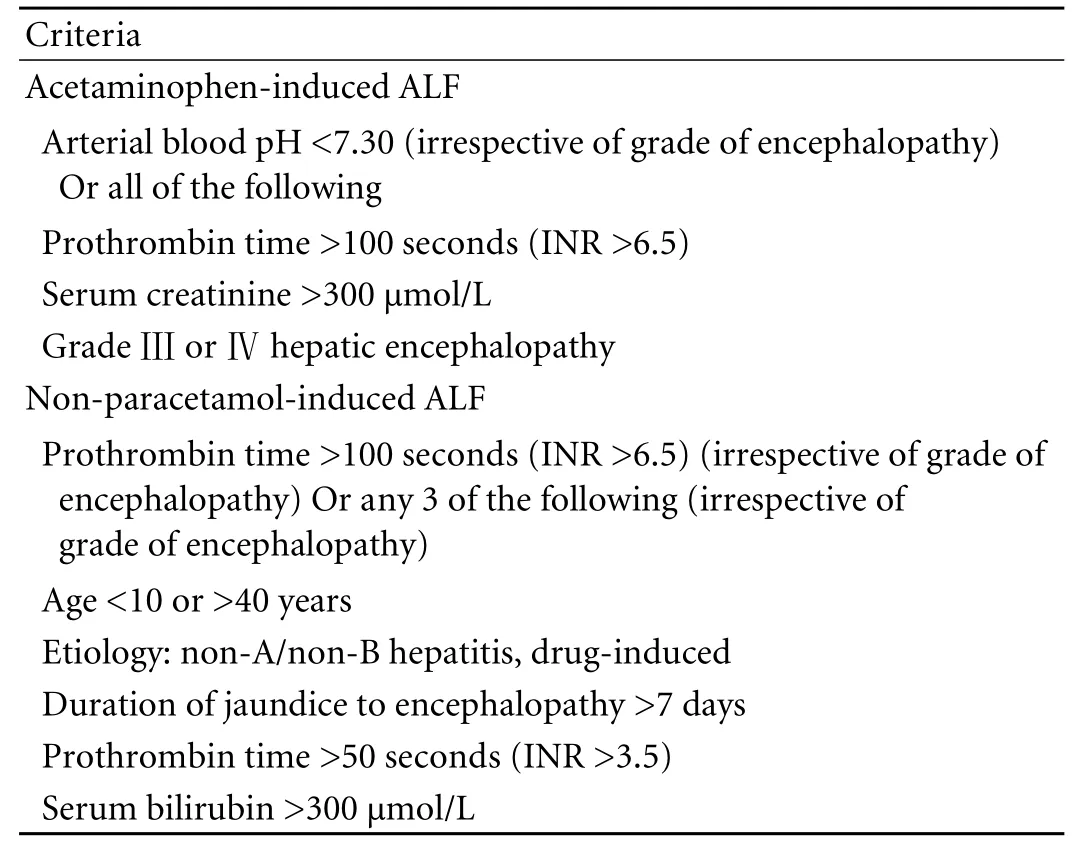
Table 1. The KCH criteria[7]
Modi fi ed KCH criteria
For the purpose of improving the diagnostic accuracy, the KCH criteria have recently been modi fi ed to include other indices such as blood lactate[21,22]and serum phosphate,[23]but the results are far from satisfactory. Bernal et al[21]found that addition of blood lactate concentration to the KCH criteria could improve the speed of identi fi cation and the negative predictive value, but decrease the positive predictive value. Schmidt et al[22]identi fi ed that the blood lactate modi fi cation of the KCH criteria did result in an improved sensitivity.However, the speci fi city was reduced to 50%, and showed no clear advantages over the KCH criteria.[22]In another cohort of 38 patients with ALF, Chung et al[23]found that the addition of serum phosphate to the KCH criteria slightly improved the negative predictive value,but there was no difference in positive predictive value between the combined criteria and the KCH criteria.Thus, those modi fi ed KCH criteria still need further assessment in larger patient populations.
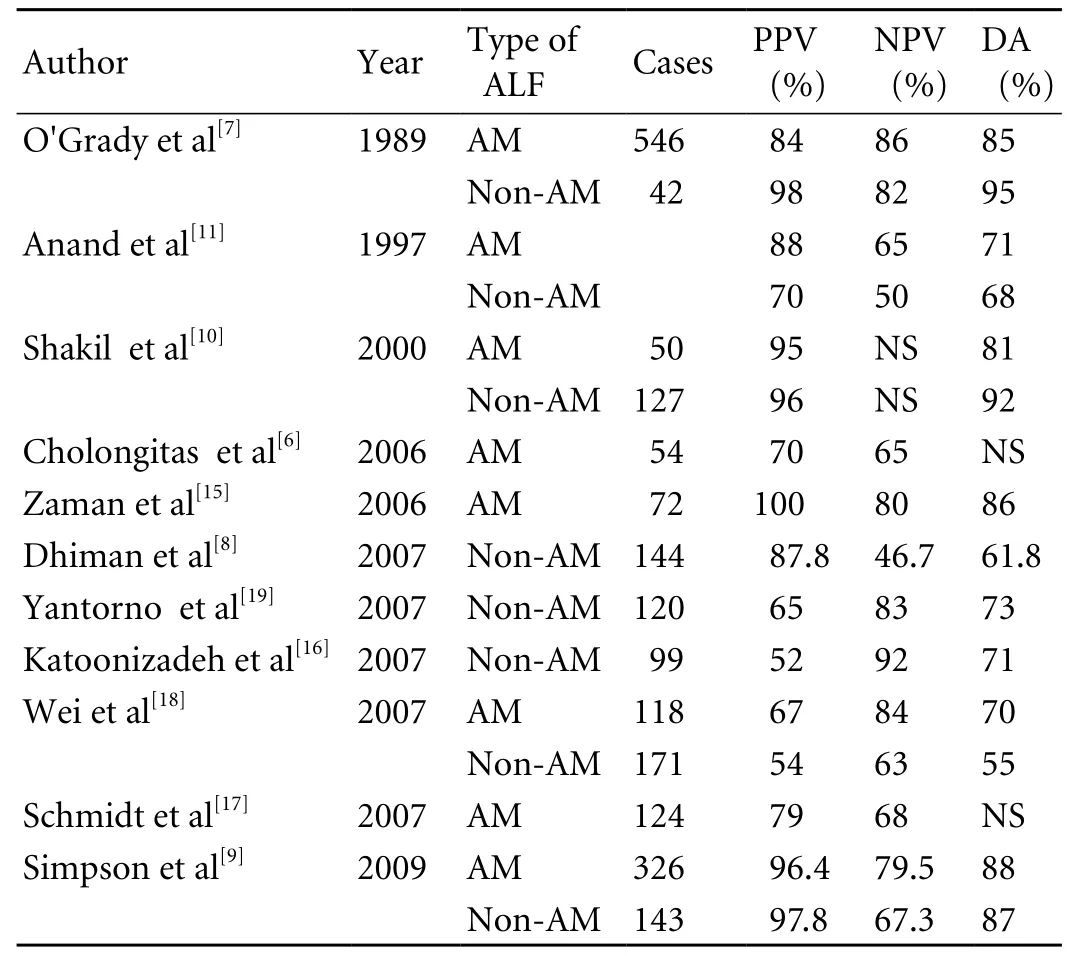
Table 2. Positive prognostic value, negative prognostic value and diagnostic accuracy of the KCH criteria in detecting patients with acetaminophen-induced and non-acetaminophen-induced ALF
PPV: positive prognostic value; NPV: negative prognostic value;DA: diagnostic accuracy; AM: acetaminophen-induced ALF;Non-AM: non-acetaminophen-induced ALF; NS: not stated.
MELD score and modi fi ed MELD score MELD score
The MELD score was initially developed to estimate 3-month mortality risk in patients with hepatic cirrhosis treated with transjugular intrahepatic portosystemic shunt procedure.[12]It is based on a composite of three available objective biochemical variables including serum bilirubin, creatinine and international normalized ratio for prothrombin time [MELD score=9.57×ln creatinine(mg/dl)+3.78×ln bilirubin (mg/dl)+11.2×ln INR+6.43],and currently can be easily calculated with a website calculator (Webpage: http://www.unos.org/resources/meldpeldcalculator.asp). The MELD score has been extensively applied and validated in patients with endstage liver disease of diverse etiology and severity.[39,40]Since 2002, the MELD score has been adopted as the new model to assess disease severity and determine organ allocation by the United Network of Organ Sharing(UNOS).[41]Recently, the MELD score has been used for the assessment of the mortality in patients with ALF(Table 3).[8,13-17,19]Some evidences[18,19]show that the MELD score is superior to the KCH criteria to assess prognosis in patients with ALF. In 279 patients with acetaminophen-induced ALF, Wei et al[18]found that the MELD score more than 30 had a positive predictive value of 21% and a negative predictive value of 94%,whereas the KCH criteria had a positive predictive value of 67% and a negative predictive value of 84%. Similarly,Yantorno et al[19]found that the MELD score was more than 30 in 94% of the patients who died from non liver transplantation and lower than 30 in 91% of the patients who survived with medical therapy. Logistic regression analysis demonstrated that the concordance levels were signi fi cantly higher for the MELD score compared to the KCH criteria.
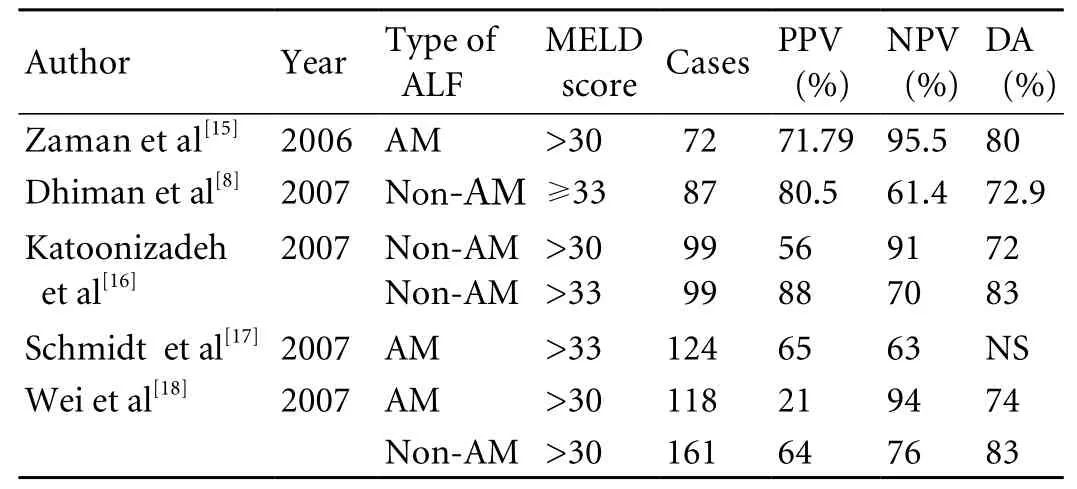
Table 3. Positive prognostic value, negative prognostic value and diagnostic accuracy of the MELD score in detecting patients with acetaminophen-induced and non-acetaminophen-induced ALF
However, different laboratory methods and reagents for determination of bilirubin, creatinine and international normalized ratio may result in a signi fi cant variation of the MELD score.[42-45]Of the 3 indices, international normalized ratio has the greatest variability because of different laboratory methods.[17,44]Xiol et al[45]evaluated the differences in concentrations and scores among 3 laboratories, and con fi rmed that there were unexpected,clinically relevant differences in serum creatinine,serum bilirubin, international normalized ratio, and the MELD score. The most important inter-laboratory variation was secondary to international normalized ratio. The MELD score updated by assigning lower weight to creatinine and international normalized ratio and higher weight to bilirubin may be better in predicting mortality.[46]
Modi fi ed MELD score
Several studies have shown that the predictive value of the MELD score is increased by adding clinical or laboratory variables.[47]Serum sodium is a readily available, reliable, and objective laboratory test, and is recently considered as a useful predictor of mortality in patients with end-stage liver disease, and may improve the accuracy if added to the MELD score.[24]Some reports have suggested that the incorporation of sodium into the MELD, called MELD-Na, can predict a more accurate survival than the MELD alone.[20,45]In 735 patients with end-stage liver disease, the modi fi ed MELD scores of 20, 30 and 40 were associated with 6%, 16% and 37% of risk of death within 6 months,respectively.[20]However, serum sodium determination is varied from laboratory to laboratory, and is not an easily reproducible parameter, thus resulting in signi fi cant differences similar to or even greater than those obtained by the MELD score.[45]
Serum Gc-globulin and actin-free Gc-globulin Serum Gc-globulin
Gc-globulin, also known as vitamin D binding protein,is a highly expressed, multifunctional and polymorphic serum protein that is synthesized in the liver.[48-50]One of its main physiologic functions is to clear actin from the circulation during cell necrosis and tissue injury and protect against disseminated intravascular coagulation induced by polymerized forms of actin.[48-50]Gcglobulin has been suggested as a prognostic marker in ALF.[25-27]It was reported that serum Gc-globulin level was markedly decreased in patients with acetaminophen intoxication compared with normal individuals, and this decrease was correlated with the degrees of hepatic encephalopathy.[51]In patients with ALF, non-survivors had a lower serum Gc-globulin level and a higher ratio of Gc-globulin to monomeric actin complex than survivors, and the predictive properties of Gc-globulin were consistent with the KCH criteria when a cutoff level of 100 mg/L was used.[25,48]Surprisingly, serum Gc-globulin level was associated with the subsequent development of multiple organ failure including cardiovascular failure and intracranial hypertension.[52]The number of failing organs was inversely correlated with serum Gc-globulin level in patients with hepatic encephalopathy grade Ⅲ or Ⅳ.[48]
Serum actin-free Gc-globulin
Serum actin-free Gc-globulin was postulated to be a more sensitive marker than total Gc-globulin.[26]A recent study revealed that in 252 patients with varying etiologies from the US ALF study group registry, serum actin-free Gc-globulin levels were signi fi cantly higher in spontaneous survivors than in patients who died or underwent transplantation. Moreover, actin-free Gcglobulin provided the same prognostic information as the KCH criteria did. A cutoff level of 40 mg/L yielded positive and negative predictive values of 68% and 67% respectively in contrast to 72% and 64% for the KCH criteria.[26]The predictive value of actin-free Gcglobulin was validated in another study involving 61 patients with ALF and 91 patients with liver cirrhosis.[27]Serum actin-free Gc-globulin level was the lowest in the patients with ALF (10% of control values) similar to those with poor prognosis as detected by the acute physiology and chronic health evaluation (APACHE)Ⅱscores. Similarly, serum actin-free Gc-globulin level was markedly decreased in patients with cirrhosis, which was recognized as an independent predictor of mortality in a logistic regression.[27]
Arterial blood lactate
Hyperlactatemia is frequent in patients with ALF, with an increase in lactate production as well as a decrease in lactate clearance secondary to hepatic dysfunction.[22,53,54]More recent studies have demonstrated that arterial blood lactate may be an early prognostic marker of survival in patients with ALF.[21,22,53,55,56]High lactate concentration may indicate the severity of both the sustained hepatic injury and the subsequent multiple organ failure.[21]In a large cohort of patients with acetaminophen-induced ALF, arterial blood lactate concentrations were related to survival. With a threshold value of 3.5 mmol/L early after admission and 3.0 mmol/L after adequate fl uid resuscitation, arterial blood lactate concentrations alone could identify non-surviving patients earlier in their clinical course with a predictive accuracy similar to that of the KCH criteria.[21]These results were con fi rmed in another study involving 101 patients with grade Ⅲ-Ⅳ.hepatic encephalopathy.[22]However, it is ambiguous whether incorporating blood lactate into the KCH criteria is superior to the existing criteria.[21,22]
Arterial blood ammonia
Hyper-ammonia is common in experimental and human ALF, and seems to be closely associated with severe cerebral complications including intracranial hypertension, hepatic coma, seizures and cerebral herniation, etc.[30-32,57,58]In 80 patients with ALF , Bhatia et al[30]found that arterial ammonia concentration higher than 124 μmol/L was associated with severe hepatic encephalopathy and cerebral edema, and could predict the mortality with a sensitivity of 78.6%, a speci fi city of 76.3%, and a diagnostic accuracy of 77.5%. Bernal et al[31]also found that an ammonia concentration higher than 100 μmol/L could predict the onset of severe hepatic encephalopathy with an accuracy of 70% in 165 ALF patients with severe hepatic encephalopathy.Moreover, an additional measurement of ammonia to the MELD score could improve the diagnostic accuracy of hepatic encephalopathy, but a higher blood ammonia concentration (more than 200 μmol/L) failed to identify the risk of intracranial hypertension although its complications are closely related to the devastating consequence of ALF.[31]
Serum phosphate
Hypophosphatemia is a common laboratory abnormality in patients with ALF although its cause is still unclear. Hypophosphatemia is related to hepatic regeneration, and is strongly associated with the severity of liver damage.[23,29,59]Recently, the concentration of serum phosphate has been reported as an important predictive indicator in patients with ALF.[23,29,59]In patients with acetaminophen-induced ALF, Schmidt et al[29]found that phosphate concentration was signifi cantly higher in non-survivors than in survivors,which serves as a highly speci fi c and sensitive predictor of non-survivors in acetaminophen-induced cases. In 38 patients with ALF, serum phosphate level more than 2.5 mg/dl was a predictor of mortality.[23]Interestingly, the phosphate criteria appeared to have a higher sensitivity,accuracy and positive and negative predictive values than the KCH criteria.[23,29]However, addition of serum phosphate level to the KCH criteria did not improve the positive predictive values.[23]
Serum alpha-fetoprotein
Alpha-fetoprotein is a single-strand glycoprotein which belongs to the albumoid gene family, and is highly expressed in the normal fetal liver. Serum alphafetoprotein has been found markedly increased in patients with hepatocellular carcinoma, and modestly increased in patients with chronic hepatitis. It has been used as an effective marker for the diagnosis of hepatocellular carcinoma. Some evidences have con fi rmed that serum alpha-fetoprotein might be of predictive value in ALF.[34-36]In the course of liver injury associated with extensive necrosis, an increased serum alpha-fetoprotein level seems to be a sign of hepatic regeneration in response to liver injury, and is strongly associated with a favorable outcome in ALF.[35,36]In 239 patients with acetaminophen-induced ALF, Schmidt et al[34]found that a threshold alpha-fetoprotein 3.9 μg/L on the day after appearance of peak ALT for identi fi cation of non-survivors had a sensitivity of 100%, a speci fi city of 74%, a positive predictive value of 45%, and a negative predictive value of 100%. However,in a large prospective study from the US ALF study group, Schiodt et al[35]found that changes in alphafetoprotein (alpha-fetoprotein ratio) during ALF seem to be of prognostic value, whereas higher absolute values of serum alpha-fetoprotein do not predict a favorable outcome.
Other prognostic models for ALF
Other prognostic models include APACHEⅡ scores,[27]Clichy's criteria,[19]liver biopsy,[60]computed tomographyderived liver volume,[61-63]serum leukocyte cell-derived chemotaxin-2,[64]biliary carnitine,[65]etc. Some of them are promising, but more investigations are needed to assess their reliability.
Problems and prospects
ALF is a devastating disease with a very high mortality rate.[1,2]It remains a great clinical challenge to identify those patients who would die if liver transplantation is not performed, or who have the potential capacity to survive if treated medically. It is clear that the prognostic models of ALF are far from being satisfactory although some have been widely used. Most of the prognostic models are based on objective biochemical variables,but not all of these variables, for example, serum Gcglobulin,[25-27]are commonly used and easily accessed.Moreover, selection of laboratory methodologies and reagents for determination of the biochemical prognostic variables including serum bilirubin, creatinine, sodium,and international normalized ratio leads to a signi fi cant inter-laboratory variation.[42-45]More importantly, some prognostic models such as the KCH criteria have proven to have a low sensitivity to determine outcome, although their speci fi city is acceptable.[7,9,10,19,38]Therefore, a reliable prognostic model to be developed in the future should re fl ect both the severity of liver cell necrosis and its subsequent fatal complications, as well as the potential capacity of proceeding liver regeneration.Thus the model should not only have a predictive value for poor outcome, but also predict a favorable outcome without a transplant. Furthermore, it would be timedependent and could be monitored dynamically. Hence further studies are required to assess the diagnostic accuracy of any new indicators.
Funding: This work was supported by grants from the National S&T Major Project for Infectious Disease Control of China(2008ZX10002-005), the National High Technology Research and Development Program of China (2006AA02A140), the National Natural Science Foundation of China (30630023), and Zhejiang Health Science Foundation (2009A076).
Ethical approval: Not needed.
Contributors: DWB wrote the main body of the article under the supervision of LLJ. PXP provided advice on medical aspects. LLJ is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Sass DA, Shakil AO. Fulminant hepatic failure. Liver Transpl 2005;11:594-605.
2 Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology 2005;41:1179-1197.
3 Bismuth H, Samuel D, Castaing D, Williams R, Pereira SP.Liver transplantation in Europe for patients with acute liver failure. Semin Liver Dis 1996;16:415-425.
4 Detry O, Arkadopoulos N, Ting P, Kahaku E, Watanabe FD,Rozga J, et al. Clinical use of a bioarti fi cial liver in the treatment of acetaminophen-induced fulminant hepatic failure. Am Surg 1999;65:934-938.
5 Li LJ, Zhang YM, Liu XL, Du WB, Huang JR, Yang Q, et al.Arti fi cial liver support system in China: a review over the last 30 years. Ther Apher Dial 2006;10:160-167.
6 Cholongitas EB, Betrossian A, Leandro G, Shaw S, Patch D,Burroughs AK. King's criteria, APACHE II, and SOFA scores in acute liver failure. Hepatology 2006;43:882.
7 O'Grady JG, Alexander GJ, Hayllar KM, Williams R.Early indicators of prognosis in fulminant hepatic failure.Gastroenterology 1989;97:439-445.
8 Dhiman RK, Jain S, Maheshwari U, Bhalla A, Sharma N,Ahluwalia J, et al. Early indicators of prognosis in fulminant hepatic failure: an assessment of the Model for End-Stage Liver Disease (MELD) and King's College Hospital criteria.Liver Transpl 2007;13:814-821.
9 Simpson KJ, Bates CM, Henderson NC, Wigmore SJ, Garden OJ, Lee A, et al. The utilization of liver transplantation in the management of acute liver failure: comparison between acetaminophen and non-acetaminophen etiologies. Liver Transpl 2009;15:600-609.
10 Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J.Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl 2000;6:163-169.
11 Anand AC, Nightingale P, Neuberger JM. Early indicators of prognosis in fulminant hepatic failure: an assessment of the King's criteria. J Hepatol 1997;26:62-68.
12 Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J,ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts.Hepatology 2000;31:864-871.
13 Du WB, Li LJ, Huang JR, Yang Q, Liu XL, Li J, et al. Effects of arti fi cial liver support system on patients with acute or chronic liver failure. Transplant Proc 2005;37:4359-4364.
14 Yu JW, Wang GQ, Li SC. Prediction of the prognosis in patients with acute-on-chronic hepatitis using the MELD scoring system. J Gastroenterol Hepatol 2006;21:1519-1524.
15 Zaman MB, Hoti E, Qasim A, Maguire D, McCormick PA,Hegarty JE, et al. MELD score as a prognostic model for listing acute liver failure patients for liver transplantation.Transplant Proc 2006;38:2097-2098.
16 Katoonizadeh A, Decaestecker J, Wilmer A, Aerts R, Verslype C, Vansteenbergen W, et al. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int 2007;27:329-334.
17 Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology 2007;45:789-796.
18 Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S,Almer S, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med 2007;262:393-401.
19 Yantorno SE, Kremers WK, Ruf AE, Trentadue JJ, Podestá LG,Villamil FG. MELD is superior to King's college and Clichy's criteria to assess prognosis in fulminant hepatic failure. Liver Transpl 2007;13:822-828.
20 Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006;130:1652-1660.
21 Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet 2002;359:558-563.
22 Schmidt LE, Larsen FS. Prognostic implications of hyperlactatemia, multiple organ failure, and systemic in fl ammatory response syndrome in patients with acetaminophen-induced acute liver failure. Crit Care Med 2006;34:337-343.
23 Chung PY, Sitrin MD, Te HS. Serum phosphorus levels predict clinical outcome in fulminant hepatic failure. Liver Transpl 2003;9:248-253.
24 Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP,Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology 2005;41:32-39.
25 Schiodt FV, Bondesen S, Petersen I, Dalhoff K, Ott P, Tygstrup N. Admission levels of serum Gc-globulin: predictive value in fulminant hepatic failure. Hepatology 1996;23:713-718.
26 Schiodt FV, Bangert K, Shakil AO, McCashland T, Murray N, Hay JE, et al. Predictive value of actin-free Gc-globulin in acute liver failure. Liver Transpl 2007;13:1324-1329.
27 Antoniades CG, Berry PA, Bruce M, Cross TJ, Portal AJ,Hussain MJ, et al. Actin-free Gc globulin: a rapidly assessed biomarker of organ dysfunction in acute liver failure and cirrhosis. Liver Transpl 2007;13:1254-1261.
28 Baquerizo A, Anselmo D, Shackleton C, Chen TW, Cao C,Weaver M, et al. Phosphorus ans an early predictive factor in patients with acute liver failure. Transplantation 2003;75:2007-2014.
29 Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology 2002;36:659-665.
30 Bhatia V, Singh R, Acharya SK. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut 2006;55:98-104.
31 Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E,Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology 2007;46:1844-1852.
32 Davern TJ. Predicting prognosis in acute liver failure:Ammonia and the risk of cerebral edema. Hepatology 2007;46:1679-1681.
33 Murray-Lyon IM, Orr AH, Gazzard B, Kohn J, Williams R.Prognostic value of serum alpha-fetoprotein in fulminant hepatic failure including patients treated by charcoal haemoperfusion. Gut 1976;17:576-580.
34 Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology 2005;41:26-31.
35 Schiodt FV, Ostapowicz G, Murray N, Satyanarana R, Zaman A, Munoz S, et al. Alpha-fetoprotein and prognosis in acute liver failure. Liver Transpl 2006;12:1776-1781.
36 Yang SS, Cheng KS, Lai YC, Wu CH, Chen TK, Lee CL, et al.Decreasing serum alpha-fetoprotein levels in predicting poor prognosis of acute hepatic failure in patients with chronic hepatitis B. J Gastroenterol 2002;37:626-632.
37 Harrison PM, O'Grady JG, Keays RT, Alexander GJ, Williams R. Serial prothrombin time as prognostic indicator in paracetamol induced fulminant hepatic failure. BMJ 1990;301:964-966.
38 Donaldson BW, Gopinath R, Wanless IR, Phillips MJ,Cameron R, Roberts EA, et al. The role of transjugular liver biopsy in fulminant liver failure: relation to other prognostic indicators. Hepatology 1993;18:1370-1376.
39 Kamath PS, Wiesner RH, Malinchoc M, Kremers W,Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-470.
40 Said A, Williams J, Holden J, Remington P, Gangnon R, Musat A, et al. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J Hepatol 2004;40:897-903.
41 Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91-96.
42 Trotter JF, Olson J, Lefkowitz J, Smith AD, Arjal R, Kenison J.Changes in international normalized ratio (INR) and model for endstage liver disease (MELD) based on selection of clinical laboratory. Am J Transplant 2007;7:1624-1628.
43 Cholongitas E, Marelli L, Kerry A, Senzolo M, Goodier DW,Nair D, et al. Different methods of creatinine measurement signi fi cantly affect MELD scores. Liver Transpl 2007;13:523-529.
44 Trotter JF, Brimhall B, Arjal R, Phillips C. Speci fi c laboratory methodologies achieve higher model for endstage liver disease (MELD) scores for patients listed for liver transplantation. Liver Transpl 2004;10:995-1000.
45 Xiol X, Gines P, Castells L, Twose J, Ribalta A, Fuentes-Arderiu X, et al. Clinically relevant differences in the model for end-stage liver disease and model for end-stage liver disease-sodium scores determined at three university-based laboratories of the same area. Liver Transpl 2009;15:300-305.46 Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS.Re-weighting the model for end-stage liver disease score components. Gastroenterology 2008;135:1575-1581.
47 Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K,Patch D, et al. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl 2006;12:1049-1061.
48 Schiodt FV. Gc-globulin in liver disease. Dan Med Bull 2008;55:131-146.
49 Gressner OA, Lahme B, Gressner AM. Gc-globulin (vitamin D binding protein) is synthesized and secreted by hepatocytes and internalized by hepatic stellate cells through Ca(2+)-dependent interaction with the megalin/gp330 receptor. Clin Chim Acta 2008;390:28-37.
50 Meier U, Gressner O, Lammert F, Gressner AM. Gc-globulin:roles in response to injury. Clin Chem 2006;52:1247-1253.
51 Schiodt FV, Bondesen S, Tygstrup N. Serial measurements of serum Gc-globulin in acetaminophen intoxication. Eur J Gastroenterol Hepatol 1995;7:635-640.
52 Schiodt FV, Ott P, Bondesen S, Tygstrup N. Reduced serum Gc-globulin concentrations in patients with fulminant hepatic failure: association with multiple organ failure. Crit Care Med 1997;25:1366-1370.
53 Riordan SM, Williams R. Blood lactate and outcome of paracetamol-induced acute liver failure. Lancet 2002;360:573-574.
54 Murphy ND, Kodakat SK, Wendon JA, Jooste CA, Muiesan P, Rela M, et al. Liver and intestinal lactate metabolism in patients with acute hepatic failure undergoing liver transplantation. Crit Care Med 2001;29:2111-2118.
55 Cholongitas E, O'Beirne J, Betrossian A, Senzolo M, Shaw S, Patch D, et al. Prognostic impact of lactate in acute liver failure. Liver Transpl 2008;14:121-123.
56 Schmidt LE, Larsen FS. Blood lactate as a prognostic marker in acetaminophen-induced acute liver failure. Hepatology 2003;37:1199-1201.
57 Bernardi M, Tacconi C, Somaroli M, Gasbarrini G,Mazziotti A. Hyperammonemic, ammonia-independent coma in experimental acute liver failure induced in the pig.Gastroenterology 1981;81:191-192.
58 Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 1992;15:449-453.
59 Quirós-Tejeira RE, Molina RA, Katzir L, Lie A, Vargas JH, Ament ME, et al. Resolution of hypophosphatemia is associated with recovery of hepatic function in children with fulminant hepatic failure. Transpl Int 2005;18:1061-1066.
60 Scotto J, Opolon P, Etévé J, Vergoz D, Thomas M, Caroli J. Liver biopsy and prognosis in acute liver failure. Gut 1973;14:927-933.
61 Yamagishi Y, Saito H, Ebinuma H, Kikuchi M, Ojiro K,Kanamori H, et al. A new prognostic formula for adult acute liver failure using computer tomography-derived hepatic volumetric analysis. J Gastroenterol 2009;44:615-623.
62 Perkins JD. Another formula to determine the prognosis of patients with acute liver failure. Liver Transpl 2009;15:986-991.
63 Shakil AO, Jones BC, Lee RG, Federle MP, Fung JJ, Rakela J. Prognostic value of abdominal CT scanning and hepatic histopathology in patients with acute liver failure. Dig Dis Sci 2000;45:334-339.
64 Sato Y, Watanabe H, Kameyama H, Kobayashi T, Yamamoto S,Takeishi T, et al. Serum LECT2 level as a prognostic indicator in acute liver failure. Transplant Proc 2004;36:2359-2361.
65 Shneider BL, Rinaldo P, Emre S, Bucuvalas J, Squires R,Narkewicz M, et al. Abnormal concentrations of esteri fi ed carnitine in bile: a feature of pediatric acute liver failure with poor prognosis. Hepatology 2005;41:717-721.
BACKGROUND: Acute liver failure (ALF) remains a dramatic and unpredictable disease with high morbidity and mortality.Early and accurate prognostic assessment of patients with ALF is critically important for optimum clinical pathway.
DATA SOURCES: Five English-language medical databases,MEDLINE, ScienceDirect, OVID, Springer Link and Wiley Interscience were searched for articles on "acute liver failure","prognosis", and relat
ed topics.
RESULTS: Multi-variable prognostic models including the King's College Hospital criteria and the model for end-stage liver disease score have been widely used in determination of the prognosis of ALF, but the results are far from satisfactory.Other prognostic indicators including serum Gc-globulin,arterial blood lactate, serum phosphate, arterial blood ammonia, and serum alpha-fetoprotein are promising but await further assessement.
CONCLUSIONS: A reliable prognostic model to be developed in the future should not only have predictive value for poor outcome but also help to predict the survival of patients without a liver transplantation. Further studies are necessary to assess the prognostic accuracy of any new models.
Author Af fi liations: State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, First Af fi liated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Du WB, Pan XP and Li LJ)
Lan-Juan Li, MD, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, First Af fi liated Hospital,Zhejiang University School of Medicine, Hangzhou 310003, China (Tel:86-571-87236759; Fax: 86-571 87236759; Email: ljli@zju.edu.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
December 7, 2009
Accepted after revision March 15, 2010
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Delayed hepatocarcinogenesis through antiangiogenic intervention in the nuclear factor-kappa B activation pathway in rats
- Effect of blueberry on hepatic and immunological functions in mice
- Small-duct primary sclerosing cholangitis with hepatocellular carcinoma requiring liver transplantation
- Helicobacter species and pathogenesis of gallbladder cancer
- Proteomic analysis of differentially expressed proteins involving in liver metastasis of human colorectal carcinoma
- Protective effects of MCP-1 inhibitor on a rat model of severe acute pancreatitis
