低温加氢催化剂的设计:理论与实践
2010-12-11陈经广齐随涛HUMBERTMichaelMENNINGCarl朱月香
陈经广 齐随涛 HUMBERT Michael P MENNING Carl A 朱月香
(1Department of Chemical Engineering,Center for Catalytic Science and Technology(CCST),University of Delaware,Newark, DE 19716,USA;2西安交通大学化工系,西安 710049;3北京大学化学与分子工程学院,北京分子科学国家实验室,分子动态与稳态结构国家重点实验室,北京 100871)
低温加氢催化剂的设计:理论与实践
陈经广1,*齐随涛1,2HUMBERT Michael P1MENNING Carl A1朱月香3
(1Department of Chemical Engineering,Center for Catalytic Science and Technology(CCST),University of Delaware,Newark, DE 19716,USA;2西安交通大学化工系,西安 710049;3北京大学化学与分子工程学院,北京分子科学国家实验室,分子动态与稳态结构国家重点实验室,北京 100871)
简要总结了我们在C=C及C=O双键低温加氢双金属催化剂方面的最新研究成果.首先,我们以环己烯加氢为探针反应,证明了平行使用多种研究手段的重要性,包括单晶表面的基础研究与DFT计算,多晶表面的合成与表征,负载型催化剂的制备与性能测试等.其次,总结了双金属催化剂在其他加氢反应,如丙烯醛C=O双键的选择性加氢,苯的低温加氢,以及乙炔的选择性加氢等反应中的应用.最后,讨论了利用金属碳化物代替贵金属Pt以减少双金属催化剂中Pt用量的可能性.
*Corresponding author.Email:jgchen@udel.edu.
We acknowledge financial support from the United States Department of Energy,Office of Basic Energy Sciences(DE-FG02-00ER15104).Suitao Qi acknowledges support from the CSC(China Scholarship Council)for the Visiting Professor Program.
陈经广,北京大学客座教授.
加氢;双金属催化剂;金属碳化物;DFT计算
The field of heterogeneous catalysis,specifically catalysis on bimetallic alloys,has seen many advances over the past few decades.One of the main goals of the catalysis industry is to develop new materials that have novel catalytic properties.Bimetallic catalysts,which often show electronic and chemical properties that are distinctly different from those of the parent metals,offer the opportunity to design new catalytic materials with enhanced activity,selectivity,and stability[1-2].Currently bimetallic catalysts are widely utilized in many heterogeneous catalysis[3]and electrocatalysis[4]applications.
In order to understand the origins of the novel catalytic properties,bimetallic surfaces have been the subject of many experimental and theoretical studies,as summarized in several reviews[5-7].It is now well established that bimetallic surfaces often show novel properties that are not present on either of the parent metal surfaces[5-16].The modification effect is especially important when the admetal coverage is in the submonolayer to monolayer regime.However,it is difficult to know a priori how the electronic and chemical properties of a particular bimetallic surface will be modified relative to the parent metals.For this reason, the study of bimetallic surfaces in the field of catalysis has gained considerable interest.There are two critical factors that contribute to the modification of the electronic and chemical properties of a metal in a bimetallic surface.First,the formation of the hetero-atom bonds changes the electronic environment of the metal surface,giving rise to modifications of its electronic structure through the ligand effect.Second,the geometry of the bimetallic structure is typically different from that of the parent metals,e.g.the average metal-metal bond lengths change.This lattice mismatch leads to the strain effect that is known to modify the electronic structure of the metal through changes in orbital overlap[16].
While studies on model bimetallic surfaces provide fundamental insights into the novel properties,in an industrially relevant supported catalyst the active metal will be present in the form of nanoparticles.As shown in Fig.1,research efforts in our group involve three parallel approaches,with the goals being to bridge the“materials gap”and“pressure gap”between fundamental surface science studies and real world catalysis.In the current review we will utilize hydrogenation reactions as examples to demonstrate how the utilization of these three parallel approaches can lead to the rational design of bimetallic catalysts with novel low-temperature hydrogenation activities.
Catalytic hydrogenations are among the most commonly practiced catalytic processes,ranging from common steps in organic synthesis,to batch processes in pharmaceutical production,to stabilization of edible oils,and to petroleum upgrading processes.Because hydrogenation reactions are typically exothermic,it is advantageous to carry out these reactions at low temperatures.In the current review we will first use the hydrogenation of cyclohexene to demonstrate the feasibility of increasing the low-temperature hydrogenation activity by reducing the binding energies of atomic hydrogen and cyclohexene,which can be achieved by designing bimetallic surfaces with specific surface structures.We will then discuss several other types of hydrogenation reactions to further illustrate the advantages of bimetallic catalysts in terms of both hydrogenation activity and selectivity.
1 Structures of bimetallic surfaces
In the current review we will focus mainly on bimetallic surfaces by depositing one monolayer of a 3d transition metal on either a Pt(111)single crystal or a polycrystalline Pt substrate.As shown in Fig.2,monolayer bimetallic surfaces can have three ideal configurations:a surface 3d-Pt-Pt(111)configuration,where the 3d monolayer grows epitaxially on the surface of the Pt substrate;an intermixed configuration,where the 3d atoms reside in the first two Pt layers to some varying degree;and the unique subsurface Pt-3d-Pt(111)configuration,where the first layer is comprised of Pt atoms and the second layer is occupied with the 3d metals.
Procedures for the preparation of bimetallic surface structures under ultra-high vacuum(UHV)conditions have been described in detail previously[5].For example,the Ni/Pt(111)bimetallic surfaces have been characterized using a wide range of experimental techniques and DFT modeling[17].When Ni is deposited with the Pt(111)surface held at 300 K,Ni atoms stay on the top-most layer to produce the Ni-Pt-Pt(111)surface configuration.If this surface is subsequently heated to 600 K,or if the monolayer deposition of Ni occurs with the Pt(111)substrate held at 600 K, most of the Ni atoms diffuse into the subsurface region to produce the Pt-Ni-Pt(111)subsurface structure.Similar surface and subsurface structures have been obtained for several other 3d metals on the Pt(111)substrate[5,18].
The ab initio calculations in the current review were performed using the Vienna Ab initio Simulation Package(VASP) version 4.6[19-20].The monolayer bimetallic systems were modeled on the closed-packed Pt(111)substrate.The PW91 functional was used within the generalized gradient approximation with an energy cutoff on the basis set of 396 eV.The bimetallic systems were modeled using a periodic 2×2 or 3×3 unit cell with four metal layers,with the slabs being separated by 6 equivalent layers of vacuum in the epitaxial direction.The top two layers were allowed to relax to the lowest energy configuration while the third and fourth layers were frozen at the bulk Pt-Pt distance. More details about the DFT modeling procedures on monolayer bimetallic surfaces can be found in a recent review[5].

Fig.1 Parallel research approaches to bridge the“materials gap”and“pressure gap”

Fig.2 Idealized bimetallic surface structures with one monolayer of 3d metals on a Pt(111)substrate
2 Low-temperature hydrogenation of cyclohexene
Cyclohexene is used as a probe molecule to study the hydrogenation because cyclic hydrocarbons are important reaction intermediates in many refinery and petrochemical processes,in addition to serving as building blocks for many chemicals produced in the chemical industry.Furthermore,cyclohexene has several competitive reaction pathways,including decomposition, dehydrogenation,disproportionation(self-hydrogenation),and hydrogenation.Comparative studies of these reaction pathways provide an opportunity to determine how the hydrogenation activity and selectivity are affected by the formation of bimetallic surfaces.
2.1 DFT and experimental studies on single crystal surfaces
One hypothesis for promoting the low-temperature hydrogenation of alkene is that both reactants,atomic hydrogen and alkene,should bond relatively weakly on the catalyst surface to facilitate the hydrogenation steps.DFT calculations were performed to estimate the values of hydrogen binding energy (HBE)on several 3d-Pt-Pt(111)and Pt-3d-Pt(111)surfaces,as shown in Fig.3A[18].Fig.3A reveals that HBE is related to the position of the surface d-band center with respect to the Fermi level,in agreement with the trend observed in previous studies for other surfaces[5].In general,the addition of a 3d metal surface layer on Pt(111)moves the d-band center closer to the Fermi level as compared to the bulk 3d metals.This is primarily due to the tensile strain induced by the Pt lattice as the ligand effect is the weakest between late transition metal overlayers and the Pt(111) substrate[17].Conversely,subsurface 3d metals shift the surface d-band center of Pt away from the Fermi level as compared to that of Pt(111),mainly due to the electronic interaction of Pt and the subsurface 3d atoms[17].The comparison in Fig.3A demonstratesthatHBEtypicallyfollowsthetrendof3d-Pt-Pt(111)>Pt(111)> Pt-3d-Pt(111).In addition,the nearly linear correlation between HBE and the surface d-band center should enable one to predict HBE on other bimetallic surfaces based on the extensive database of d-band center values for many bimetallic surfaces[5].
In addition to the trend in the correlation of HBE with surface d-band center,the binding energies of unsaturated hydrocarbons,such as cyclohexene,follows the same trend as HBE.As shown in Fig.3B,DFT calculations reveal that the Pt-3d-Pt(111) subsurface structures bond to cyclohexene more weakly than Pt(111)and the corresponding 3d-Pt-Pt(111)surface structures[18]. For example,DFT results indicate that both cyclohexene and atomic hydrogen are more weakly bonded on Pt-Ni-Pt(111)than on Ni-Pt-Pt(111),Pt(111)and Ni(111),suggesting that the subsurface Pt-Ni-Pt(111)structure should be more effective in the hydrogenation of cyclohexene than the surface structure.This has been confirmed experimentally by comparing the hydrogenation activity of cyclohexene using temperature programmed desorption(TPD),as shown in Fig.4[18].As illustrated in the TPD peak area of the cyclohexane product,the subsurface Pt-Ni-Pt (111)structure shows the highest hydrogenation yield,with the desorption peak centered at a very low temperature of 203 K. Similar bimetallic surface structure can also be produced by depositing one monolayer of Pt on a Ni(111)substrate,which also possesses the novel low-temperature pathway for cyclohexene hydrogenation[21].

Fig.3 DFT calculations of binding energies of hydrogen and cyclohexene on Pt-3d-Pt(111)and 3d-Pt-Pt(111)surfaces[18]

Fig.4 TPD of hydrogenation of cyclohexene on Ni/Pt(111) monometallic and bimetallic surfaces[18]
The trend in the DFT calculations in Fig.3B also shows that the binding energy of cyclohexene on Pt-Co-Pt(111)and Pt-Fe-Pt(111)is even weaker than that on Pt-Ni-Pt(111).Although this might suggest that the former two surfaces would be more active toward the hydrogenation than Pt-Ni-Pt(111),one should keep in mind that the adsorption of cyclohexene needs to be strong enough for the hydrogenation to take place.One would therefore expect to observe a volcano relationship for the hydrogenation activity as the d-band center moves further away from the Fermi level,i.e.,when the adsorption of cyclohexene becomes too weak for the hydrogenation to occur.This is verified experimentally in the results shown in Fig.5.The hydrogenation yield from TPD measurements is the highest on Pt-Ni-Pt(111),but starts to decrease on the Pt-Co-Pt(111)and Pt-Fe-Pt(111)surfaces,where the binding of cyclohexene becomes too weak for hydrogenation to occur.On the other side of the volcano curve,the binding energies of cyclohexene on the 3d-Pt-Pt(111)surfaces are too strong,preventing the effective hydrogenation of cyclohexene[18].
2.2 Polycrystalline bimetallic surfaces

Fig.5 Volcano-type relationship between hydrogenation activity and surface d-band center value of 3d/Pt(111) surfaces
Industrial catalysts are often supported nanoparticles of varying shape and size.Polycrystalline bimetallic films provide a potential way to bridge the“materials gap”between single crystal surfaces and supported catalysts.As illustrated in Fig.6,it is possible to assume that the surface chemistry of the nanoparticle should be dominated primarily by the first few atomic layers.It is also reasonable to assume that the chemistry of the individual crystal facets on the nanoparticle(primarily(111)and(100)for an fcc nanoparticle)can be approximated by their respective single crystal extension[22].
With these assumptions we have investigated the chemical properties of 3d-Pt bimetallic structures prepared on a polycrystalline Pt film that contained mainly the(111)and(100)facets. Similar to Pt(111),monolayer Ni was deposit on a Pt foil at room temperature to produce the Ni-Pt-Pt surface structure,followed by annealing to higher temperatures to obtain the Pt-Ni-Pt subsurface structure[22].The TPD results of the hydrogenation of cyclohexene are shown in Fig.7.Similar to the corresponding single crystal surfaces,the subsurface Pt-Ni-Pt polycrystalline structure shows significantly higher hydrogenation activity than that from the polycrystalline Pt and Ni surfaces.These results confirm the assumption that the trend observed on single crystal bimetallic surfaces can be extended to the polycrystalline counterparts.
2.3 Thermodynamic stability of bimetallic surfaces under hydrogenation conditions

Fig.6 Schematic of the(100)and(111)regions of the supported bimetallic particles
Before extending the surface science results to supported catalysts,it is important to verify that the desirable Pt-Ni-Pt subsurface structure is the thermodynamically preferred configuration under hydrogenation conditions.As demonstrated in several re-cent studies,including single crystal surfaces[23-24],polycrystalline films[22,25]and supported catalysts[26],the thermodynamically preferred Pt-Ni bimetallic structure is directly related to the chemical environment present on the surface.Fig.8 shows the DFT predicted potential for segregation for a 3d metal atom to segregate from the subsurface to the surface of Pt(111).These values were calculated for the environments of vacuum,and with 0.5 monolayer(ML)atomic hydrogen and 0.5 ML atomic oxygen, usingproceduresdescribedpreviously[24].The thermodynamic potential for segregation is defined as follows:
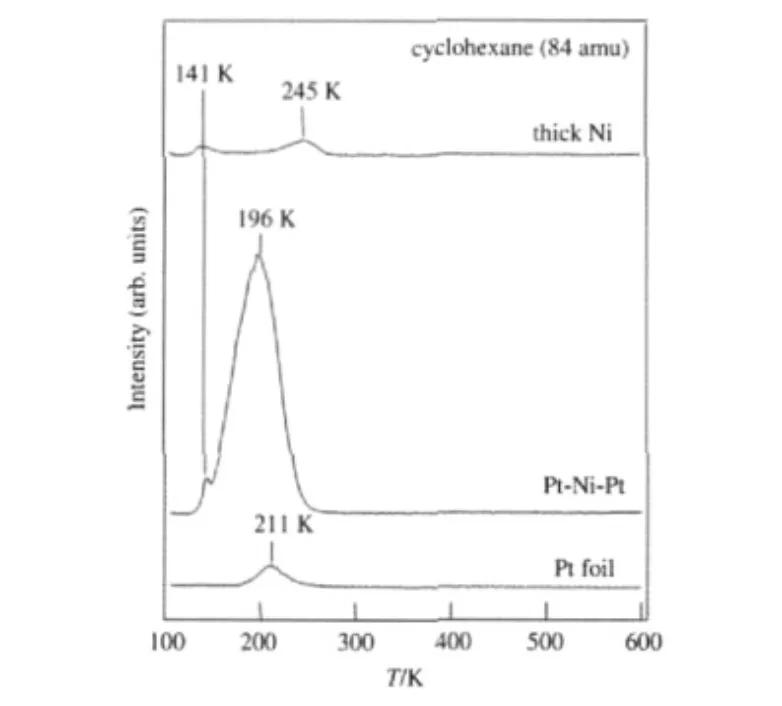
Fig.7 TPD of cyclohexene hydrogenation on polycrystalline Pt,Ni and Pt-Ni-Pt surfaces

where ΔEsegis the thermodynamic potential for segregation per Pt-3d pair,EA/3d-Pt-Ptis the total energy for the surface configuration with adsorbate A,EA/Pt-3d-Ptis the total energy for the subsurface configuration with adsorbate A,and M is the total number of Pt-3d pairs per unit cell.

Fig.8 DFT calculations of thermodynamic stability of 3d/ Pt(111)surfaces in vacuum,and with 0.5 ML of atomic hydrogen and oxygenPositive ΔEsegvalues indicate that the subsurface Pt-3d-Pt(111) structures are thermodynamically stable.
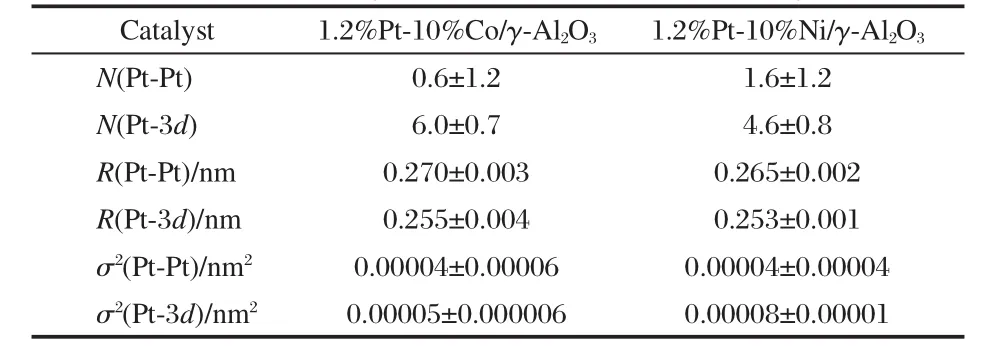
Table 1 Summary of EXAFS results of Pt LIII-edge fitting for 1.2%Pt-10%Co/γ-Al2O3and 1.2%Pt-10%Ni/γ-Al2O3[28]
As defined in a previous publication[22],a positive ΔEsegvalue indicates that the subsurface Pt-3d-Pt is more stable.The DFT results in Fig.8 predict that for the reducing environment of vacuum and 0.5 ML atomic hydrogen,the subsurface configuration is thermodynamically preferred,whereas in 0.5 ML atomic oxygen the surface configuration is preferred.There is a nearly linear trend between ΔEsegand the difference in d-band,Δεd,which leads to a generalized equation in predicting the thermodynamic stability of a wide range of bimetallic surfaces[24].Because the environment of hydrogenation reactions is similar to that of the reducing environment,with the bimetallic surface being partially covered by hydrogen,the results in Fig.8 suggest that the desirable subsurface Pt-Ni-Pt configuration should be thermodynamically stable,making it possible to extend model surfaces to supported catalysts for hydrogenation reactions.
2.4 Synthesis and evaluation of supported catalysts
Supported monometallic Pt and bimetallic Ni-Pt and Co-Pt catalysts were synthesized on γ-Al2O3using the incipient wetness method[27-28].The catalysts were characterized using a variety of techniques,including extended X-ray absorption fine structure(EXAFS).The utilization of EXAFS is critical in these studies because it provides direct information on the extent of bimetallic bond formation based on the coordination numbers of Ni-Pt and Co-Pt under in-situ reaction conditions.As summarized in Table 1,the detection of the Ni-Pt and Co-Pt nearest neighbors confirms that bimetallic bonds are indeed produced on the supported catalysts[28].

Fig.9 Batch reactor results following the hydrogenation of cyclohexene(r)on supported catalysts at 303 K
The supported catalysts were evaluated using both batch and flow reactors to determine the reaction kinetics of the hydrogenation of cyclohexene at a low temperature of 303 K[28].Fig.9 shows the production of cyclohexane from cyclohexene on Pt/γ-Al2O3,Co-Pt/γ-Al2O3,and Ni-Pt/γ-Al2O3,using a batch reactor equipped with Fourier transform infrared(FTIR)spectroscopy. The solid lines are fittings using the Langmuir-Hinshelwood model,resulting in a rate constant of 1.7,21 and 24 min-1for supported Pt,Co-Pt,and Ni-Pt,respectively[28].The trend observed in the rate constant of cyclohexene hydrogenation is consistent with that from the single crystal surfaces for the same reaction, Ni-Pt>Co-Pt>Pt,as shown earlier in the volcano curve in Fig.5. The observation of the similar trend between model surfaces and supported catalysts provides an important demonstration of the rational design of bimetallic catalysts from combined theoretical and experimental approaches.
3 Research opportunities in bimetallic catalysis
3.1 Low-temperature hydrogenation reactions
We have applied similar combined approaches for the design of bimetallic catalysts for the low-temperature hydrogenation of several types of hydrocarbon molecules.Below we will provide several examples of hydrogenation reactions that are of both fundamental and practical importance.
Hydrogenation of acrolein.Studies of the selective hydrogenation of unsaturated aldehydes,such as α,β-unsaturated aldehydes,have been of growing interest for the production of fine chemicalsandpharmaceuticalprecursors[29].Thehydrogenationof the C=C and/or C=O bonds in unsaturated aldehydes offers the possibility to improve both the hydrogenation activity and selectivity through the formation of bimetallic surfaces.Using the hydrogenation of acrolein as a probe reaction,we demonstrated that the selective hydrogenation of the C=O bond can be achieved through the formation of the subsurface Pt-Ni-Pt(111) and Pt-Co-Pt(111)bimetallic structures[30-31].
Hydrogenation of benzene.The hydrogenation of benzene to cyclohexane is of significant importance in the petroleum industry and for environmental protection.The process of benzene hydrogenation has been utilized commercially for the production of cyclohexane,which is one of the key intermediates in the synthesis of Nylon-6 and Nylon-66[32].We have identified Co-Pt bimetallic catalysts as promising materials for the hydrogenation of benzene at a relatively low temperature of 343 K[28,33].For example,Table 2 summarizes the batch and flow reactor results of benzene hydrogenation on several Co-based bimetallic catalysts. The Co-Pt catalyst shows the highest rate constant and lowestactivation barrier for the hydrogenation of benzene,which is consistent with the relatively weak binding energies of atomic hydrogen and benzene from DFT calculations[33].In addition,the catalyst support also plays a role in controlling the hydrogenation activity of Co-Pt catalysts[34]
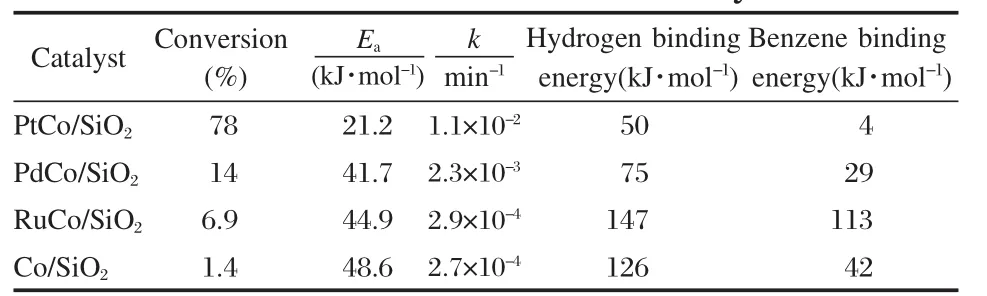
Table 2 Flow reactor measurements of conversion of benzene and apparent activation barrier(Ea),batch reactor determination of reaction rate constant(k),and DFT calculations of hydrogen and benzene binding energies on Co and several Co-based bimetallic catalysts[33]
Selective hydrogenation of acetylene in ethylene. The selective hydrogenation of acetylene in the presence of ethylene is an important reaction because acetylene poisons the catalysts in ethylene polymerization reactions[35-36].By supporting Pd-Ag bimetallic catalysts on ion-exchanged β-zeolites,we observed a synergistic effect that led to a higher selectivity for acetylene hydrogenation in the presence of excess ethylene[37]. The increase in the hydrogenation selectivity is attributed to a combination of an enhanced π-cation interaction between acetylene and zeolite at low temperatures and the ability of the Pd-Ag bimetallic catalysts to perform hydrogenation at such low temperatures[37].
3.2 Reducing bulk Pt in bimetallic catalysts with metal carbides
As demonstrated in Figs.3-5,the subsurface Pt-Ni-Pt structure is desirable to enhance the activity and selectivity of the hydrogenation of unsaturated hydrocarbons.However,if elevated temperatures are required for reactions,the subsurface Ni atoms start to diffuse into bulk Pt,leaving a monometallic Pt surface and therefore the disappearance of the enhanced bimetallic hydrogenation activity[17,22].In addition,as shown in Fig.8,adsorbates such as oxygen can cause the subsurface Ni atoms to segregate to the surface,forming the Ni-Pt-Pt surface that is not active for hydrogenation reactions.One idea to overcome such inherent instability of Pt-Ni-Pt is to replace the bulk Pt with an alternative substrate,such as transition metal carbides that often show catalytic properties similar to Pt[38-44].We have explored the utilization of tungsten monocarbide(WC)to produce the Pt-Ni-WC structure[45].As WC has been shown to be an effective diffusion barrier layer[46],thermal deactivation due to Ni diffusion will be alleviated.Furthermore,it is also possible that the WC substrate would anchor Ni by the formation of W—Ni or C—Ni bonds to prevent its segregation to the surface in an oxygen-rich environment.Fig.10 shows a comparison of the hydrogenation of cyclohexene from Pt-Ni-Pt and Pt-Ni-WC surfaces[45].The Pi-Ni-WC surface shows higher hydrogenation activity,which is consistent with parallel DFT calculations[45].The promising results in Fig.10 suggest the possibility to synthesize a more active and stable Pt-Ni-WC hydrogenation catalyst with much lower loading of Pt than that in Pt-Ni-Pt.
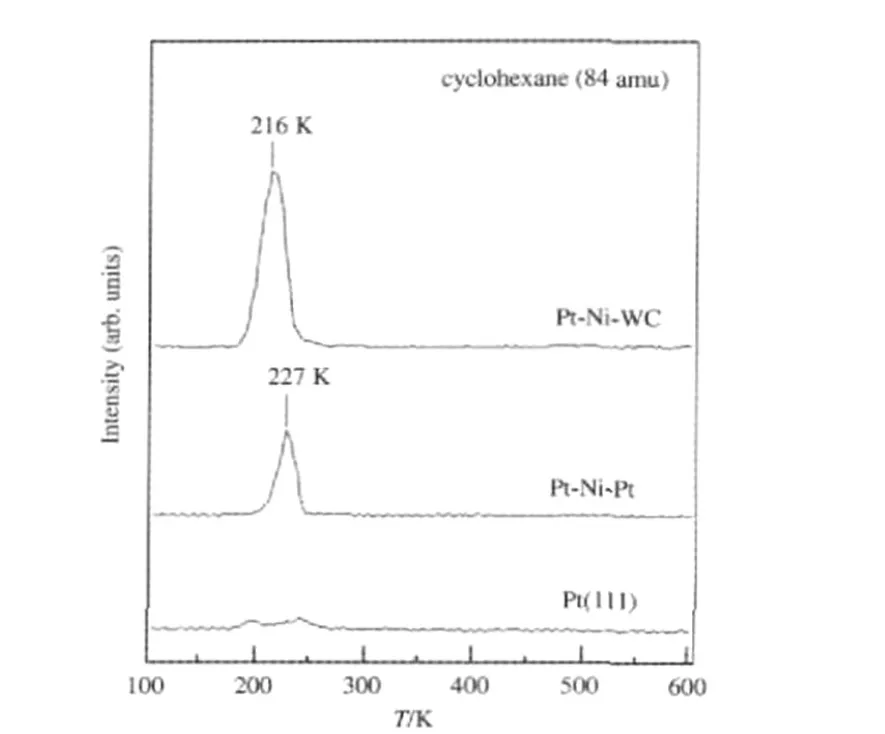
Fig.10 TPD of cyclohexene hydrogenation from Pt-Ni-WC
3.3 Production of hydrogen using bimetallic catalysts
All examples presented above are for hydrogenation reactions,which can be classified as hydrogen-consuming reactions and require catalysts to bond to atomic hydrogen and adsorbates relatively weakly.Using the surface d-band center argument in Fig.3,these reactions are preferred on bimetallic catalysts with surface d-band center away from the Fermi level,such as the Pt-3d-Pt subsurface structures.In contrast,for hydrogen-producing reactions,the desirable catalysts should be those that bond to hydrogen and adsorbates more strongly,i.e.,with d-band center closer to the Fermi level,such as the 3d-Pt-Pt surface structures. This hypothesis has been confirmed experimentally in our recent studies for the production of H2from the reforming of biomassderived molecules,including ethanol,ethylene glycol and glycerol on the Ni-Pt-Pt(111)surface[47].Similarly,the Ni-Pt-Pt(111) surface also shows a very high activity for H2production from thedecompositionofammonia[48].Wehavealsodemonstratedthat coking of the catalyst surfaces,a common deactivation mechanism in dehydrogenation reactions,can be reduced by the formation of bimetallic surfaces[49].These results further demonstrate the possibility of designing bimetallic catalysts from combined theoretical predictions and experimental verification.
4 Conclusions
The field of catalysis is undergoing a revolution in the selection process of catalytic materials,from the traditional“trialand-error”method to the“rational design”approach,with the latter requiring atomic level understanding of the catalyst structures and reaction mechanisms.In the current review we utilized several hydrogenation reactions to demonstrate the importance of combining theoretical and experimental approaches for designing bimetallic structures with desirable catalytic properties. In addition,the examples also illustrated the possibility to bridge the“materials gap”and“pressure gap”between fundamental studies on single crystal surfaces and catalytic evaluation of supported catalysts.Similar approaches can be adopted for the rational design of bimetallic catalysts beyond hydrogenation reactions.
1 Sinfelt,J.H.Acc.Chem.Res.,1977,10:15
2 Sinfelt,J.H.Bimetallic catalysts:discoveries,concepts and applications.New York:John Wiley and Sons,1983:1-10
3 Bartholomew,C.H.;Farrauto,R.J.Fundamentals of industrial catalytic processes.2nd ed.Hoboken,New Jersey:Wiley Interscience,2006:30-45
4 Stamenkovic,V.R.;Fowler,B.;Mun,B.S.;Wang,G.F.Ross,P. N.;Lucas,C.A.;Markovic,N.M.Science,2007,315:493
5 Chen,J.G.;Menning,C.A.;Zellner,M.B.Surf.Sci.Rep.,2008, 63:201
6 Campbell,C.T.Ann.Rev.Phys.Chem.,1990,41:775
7 Goodman,D.W.Ultramicroscopy,1990,34:1
8 Rodriguez,J.A.;Goodman,D.W.Science,1992,257:897
9 Rodriguez,J.A.;Goodman,D.W.J.Phys.Chem.,1991,95:4196
10 Neurock,M.;van Santen,R.A.J.Phys.Chem.B,2000,104: 11127
11 Hammer,B.;Morikawa,Y.;Nørskov,J.K.Phys.Rev.Lett.,1996, 76:2141
12 Pallassana,V.;Neurock,M.;Hansen,L.B.;Hammer,B.;Nørskov, J.K.Phys.Rev.B,1999,60:6146
13 Hwu,H.H.;Eng Jr.,J.;Chen,J.G.J.Am.Chem.Soc.,2002,124: 702
14 Khan,N.A.;Hwu,H.H.;Chen,J.G.J.Catal.,2002,205:259
15 Greeley,J.;Mavrikakis,M.Nature Materials,2004,3:810
16 Kitchin,J.R.;Norskov,J.K.;Barteau,M.A.;Chen,J.G.Phys. Rev.Lett.,2004,93:156801
17 Kitchin,J.R.;Khan,N.A.;Barteau,M.A.;Chen,J.G.;Madey,T. E.Surf.Sci.,2003,544:295
18 Humbert,M.P.;Chen,J.G.J.Catal.,2008,257:297
19 Kresse,G.;Furthmuller,J.Phys.Rev.B,1996,54:11169
20 Kresse,G.;Hafner,J.Phys.Rev.B,1993,47:558
21 Khan,N.A.;Zellner,M.B.;Chen,J.G.Surf.Sci.,2004,556:87
22 Menning,C.A.;Chen,J.G.J.Chem.Phys.,2008,128:164703
23 Menning,C.A.;Hwu,H.H.;Chen,J.G.J.Phys.Chem.B,2006, 110:15471
24 Menning,C.A.;Chen,J.G.J.Chem.Phys.,2009,130:174709
25 Menning,C.A.Chen,J.G.J.Power Sources,2010,195:3140
26 Mu,R.T.;Fu,Q.;Liu,H.Y.;Tan,D.L.;Zhai,R.S.;Bao,X.H. Appl.Surf.Sci.,2009,255:7296
27 Shu,Y.;Murillo,L.E.;Bosco,J.P.;Huang,W.;Frenkel,A.I.; Chen,J.G.Appl.Catal.A-Gen.,2008,339:169
28 Lu,S.;Lonergan,W.W.;Bosco,J.P.;Wang,S.;Zhu,Y.;Xie,Y.; Chen,J.G.J.Catal.,2008,259:260
29 Maki-Arvela,P.;Hajek,J.;Salmi,T.;Murzin,D.Y.Appl.Catal.AGen.,2005,292:1
30 Murillo,L.E.;Goda,A.M.;Chen,J.G.J.Am.Chem.Soc.,2007, 129:7101
31 Murillo,L.E.;Menning,C.A.;Chen,J.G.J.Catal.,2009,268: 335
32 Stanislaus,A.;Cooper,B.H.Catal.Rev.Sci.Eng.,1994,36:75
33 Lu,S.;Menning,C.A.;Zhu,Y.;Chen,J.G.ChemPhysChem, 2009,10:1763
34 Lu,S.;Lonergan,W.W.;Zhu,Y.;Chen,J.G.Appl.Catal.BEnviron.,2009,91:610
35 Borodzinki,A.Catal.Rev.Sci.Eng.,2006,48:91
36 Le Page,J.F.Applied heterogeneous catalysis:design, manufacture,use of solid catalysts.Paris:Technip,1987:291-292
37 Huang,W.;McCormick,J.R.;Lobo,R.F.;Chen,J.G.J.Catal., 2007,246:40
38 Levy,R.B.;Boudart,M.Science,1973,181:547
39 Oyama,S.T.The chemistry of transition metal carbides and nitrides.New York:Blackie Academics and Professional,1996: 123-125
40 Hwu,H.H.;Chen,J.G.Chem.Rev.,2005,105:185
41 Chen,J.G.;Weisel,M.D.;Liu,Z.M.;White,J.M.J.Am.Chem. Soc.,1993,115:8875
42 Fruhberger,B.Chen,J.G.J.Am.Chem.Soc.,1996,118:11599
43 Weigert,E.C.;Stottlemyer,A.L.;Zellner,M.B.Chen,J.G. J.Phys.Chem.C,2007,111:14617
44 Ji,N.;Zhang,T.;Zheng,M.;Wang,A.;Wang,H.;Wang,X.; Chen,J.G.Angew.Chem.Int.Edit.,2008,47:8510
45 Humbert,M.P.;Menning,C.A.;Chen,J.G.J.Catal.,2010, 271:132
46 Gouy-Pailler,P.;Pauleau,Y.J.Vac.Sci.Technol.A,1993,11:96
47 Skoplyak,O.Barteau,M.A.;Chen,J.G.ChemSusChem,2008,1: 524
48 Hansgen,D.A.;Vlachos,D.G.;Chen,J.G.Nature Chem.,2010, DOI:10.1038/NCHEM.626
49 Ren,H.;Humbert,M.P.;Menning,C.A.;Chen,J.G.;Shu,Y.; Singh,U.;Cheng,W.C.Appl.Catal.A,2010,375:303
November 30,2009;Revised:March 7,2010;Published on Web:March 12,2010.
Rational Design of Low-Temperature Hydrogenation Catalysts: Theoretical Predictions and Experimental Verification
CHEN Jingguang G1,*QI Sui-Tao1,2HUMBERT Michael P1MENNING Carl A1ZHU Yue-Xiang3
(1Department of Chemical Engineering,Center for Catalytic Science and Technology(CCST),University of Delaware,Newark,DE 19716,USA;2Department of Chemical Engineering,Xi′an Jiaotong University,Xi′an 710049,P.R.China;3Beijing National Laboratory for Molecular Sciences,State Key Laboratory for Structural Chemistry of Unstable and Stable Species, College of Chemistry and Molecular Engineering,Peking University,Beijing 100871,P.R.China)
In this review,we will provide a brief review of our recent theoretical and experimental studies of bimetallic surfaces and catalysts for the low-temperature hydrogenation of unsaturated C=C and C=O bonds.We will first use the hydrogenation of cyclohexene as a probe reaction to demonstrate the importance of using several parallel approaches,includingfundamentalsurfacescienceanddensityfunctionaltheory(DFT)studies on single crystal surfaces, synthesis,and characterization of polycrystalline surfaces and supported catalysts,and reactor evaluation of supported catalysts.We will then provide a summary of applications of bimetallic catalysts for other hydrogenation reactions, including the selective hydrogenation of the C=O bond in acrolein,the low-temperature hydrogenation of benzene, and the selective hydrogenation of acetylene in the presence of ethylene.Finally,we will discuss the possibility of replacing the platinum(Pt)component with metal carbides to reduce the loading of Pt in bimetallic catalysts.
Hydrogenation; Bimetallic catalyst;Metal carbide;Density functional calculation
O643
