基于柔性环己烷六酸配体的两个碱土金属配位聚合物的水热合成及结构表征
2010-11-09刘兆清
王 静 刘兆清
(广州大学化学化工学院,广州 510006)
基于柔性环己烷六酸配体的两个碱土金属配位聚合物的水热合成及结构表征
王 静*刘兆清
(广州大学化学化工学院,广州 510006)
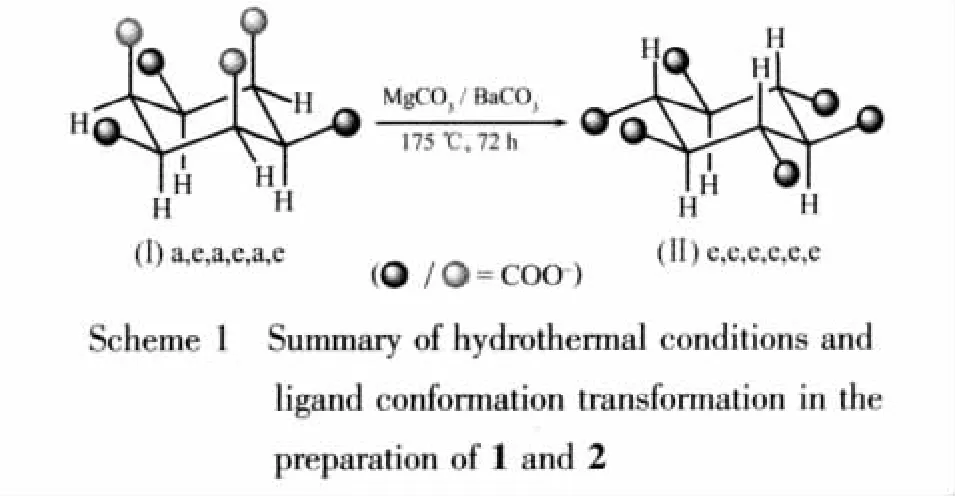
水热反应条件下,碳酸锰和碳酸钡分别与水合环己烷六酸(H6LⅠ·H2O)(顺式椅式构型 LⅠ:a,e,a,e,a,e)反应生成 2个三维的配位聚合物 [Mg3(LⅡ)(H2O)6](1)和[Ba2(H2LⅡ)(μ2-H2O)2](2)(反式椅式构型 LⅡ:e,e,e,e,e,e),通过元素分析和红外光谱对这 2个配位聚合物进行了表征。X射线单晶衍射分析表明配合物1属于三方晶系,R3空间群,晶胞参数为:a=1.439 3(2)nm,c=1.4597(4)nm,β=120.00°,V=2.6187(10)nm3,Z=18;配合物 2 结构属于单斜晶系,C2/c 空间群,晶胞参数为:a=1.6764(2)nm,b=0.9095(1)nm,c=1.0001(1)nm,β=105.991(2)°,V=1.4659(2)nm3,Z=4。 配合物 1是由反式椅式构型 LⅡ配体桥连形成的高对称性的三维配位网络。在配合物2中,顺式的H6LⅠ配体也发生构型转变并脱去部分羧基质子形成H2LⅡ配体,将Ba离子连接成一个具有一维孔道的三维有孔框架结构。在这2个配合物中,环己烷六酸配体均采取反式椅式LⅡ构型,证明了碱土金属离子Mg(Ⅱ)及Ba(Ⅱ)在水热条件下通过配位作用可以稳定环己烷六酸配体的这种反式椅式构型。
配位聚合物;碱土金属离子;环己烷六酸;水热合成
0 Introduction
In the rational design and synthesis of metalorganic coordination polymers,rigid polycarboxylates,for example benzenepolycarboxylates and pyridinepolycarboxylates,have been extensively employed to produce various extended structures[1-5].Nevertheless,only a few coordination polymers based on ligands with flexible conformations have been reported so far.It is probably due to the flexibility of the ligand backbones,which makes them more difficult to predict and control the final coordination networks[6-8].Many investigations have been focused on the flexible 1,4-cyclohexanedicarboxylic acid (1,4-H2chdc)for its cis/trans-chair conformations[6-7].1,2,3,4,5,6-cyclohexanehexacarboxylic acid (H6L,L stands for the ligand with different conformations),which is characteristic of multiple binding sites and pH-dependent coordination fashions and has versatile flexible conformations,has attracted our great interest in studying their conformational transformations and the use in constructing metalorganic frameworks[9-13].In our recent work on H6L,we have observed that the starting material 1,2,3,4,5,6-cyclohexanehexacarboxylic acid hydrate (H6LⅠ·H2O,LⅠ:a,e,a,e,a,e),adopting the all-cis-chair conformation with rich hydrogen bonds,can in situ convert to the trans-chair form or cis,trans-mixed-chair forms under different hydrothermal conditions.In our investigation,the L ligand in silver coordination polymers adopts the cis-chair conformation[9],in the transition metal coordination polymers such iron,cobalt,manganese and nickel adopts the trans-chair form[10,12],and the cis,trans-mixed-chairformscan be observed in the cadmium coordination polymers[11-12].Meanwhile,we also trapped the intermediates in crystalline states via the coordination with the relatively reactiveand catalytically active metal ion Cu(Ⅱ),and then found out the reaction mechanism with structural clues[12].
Compared to the d-block transition metal ions,the s-block alkaline earth ions size varies considerably on moving down the group,which makes a rapidly developing study of these metal coordination polymers[14].As our continuing investigation on this interesting metal-H6L system,we employed the alkaline earth ions to react with the H6L ligand to investigate the ligand flexible conformations.Herein,we report two alkaline earth coordination polymers,three-dimensional(3D)[Mg3(LⅡ)(H2O)6](1)and[Ba2(H2LⅡ)(μ2-H2O)2](2),in both of which the L ligands adopt the trans-chair LⅡconformation(LⅡ:e,e,e,e,e,e)(Scheme 1).
1 Experimental section
1.1 Materials and physical measurements
The starting material cis,cis,cis,cis,cis-1,2,3,4,5,6-cyclohexanehexacarboxylic acid hydrate(H6LⅠ·H2O)employed was commercially available and used as received without further purification.The C and H microanalyses were carried out with an Elementar Vario-EL CHNS elemental analyzer.The FTIR spectra were recorded from KBr tablets in the range 4 000~400 cm-1on a Bio-Rad FTS-7 spectrometer.
1.2 Hydrothermal synthesis
[Mg3(LⅡ)(H2O)6](1):A mixture of H6LⅠ·H2O(0.087 g,0.25 mmol)and magnesium carbonate(0.063 g,0.75 mmol)in H2O (15 mL)were placed to a 25 mL Teflon reactor and heated in an oven to 175 ℃ for 72 h.After being cooled at a rate of ca.5℃·h-1,the colorless crystals of 1 in single phase(in ca.18%yield based on H6LⅠ)were obtained,isolated by filtration and washed with water.Elemental analysis calcd for C4H6MgO6(%):C 27.55,H 3.47;found(%):C 28.22,H 3.05.IR(KBr,cm-1):3400(s),2366(m),1 620(vs),1 574(vs),1 420(vs),1 384(vs),1 258(w),1 080(w),1 021(w),937(w),760(w),681(w),517(w).
[Ba2(H2LⅡ)(μ2-H2O)2](2):Similar to the synthesis of 1,barium carbonate (0.145 mg,0.75 mmol)instead of magnesium carbonate reacted with H6LⅠ·H2O(0.087 g,0.25 mmol)at 175 ℃ for 72 h.The colorless crystals of 2 in single phase(in ca.10%yield based on H6LⅠ)were obtained,isolated by filtration and washed with water.Elemental analysis calcd for C12H10Ba2O13(%):C 22.63,H 1.58;found(%):C 22.09,H 1.79.IR(KBr,cm-1):3 412(s),2 908(w),1 715(m),1 635(vs),1 586(s),1 432(vs),1 402(s),1 273(w),1 071(w),1 012(w),935(w),728(w),629(w),566(w),497(w).
1.3 Crystal structure determination
Data collections of complexes 1 and 2 were performed on a Bruker Smart Apex CCD diffractometer with Mo Kα radiation(λ=0.071 073 nm)at 150(2)and 293(2)K.The raw data frames were integrated with SAINT+,and the corrections were applied for Lorentz and polarization effects.Absorption correction was applied by using the multiscan program SADABS[15].The structures were solved by direct methods,and all non-hydrogen atoms were refined anisotropically by least-squares on F2using the SHELXTL program[16].Hydrogen atoms on organic ligands were generated by the riding mode(C-H 0.093 nm).Crystal data as well as details of data collections and refinements for complexes 1 and 2 are summarized in Table 1.Selected bond distances and bond angles are listed in Table 2.
CCDC:755551,1;755552,2.
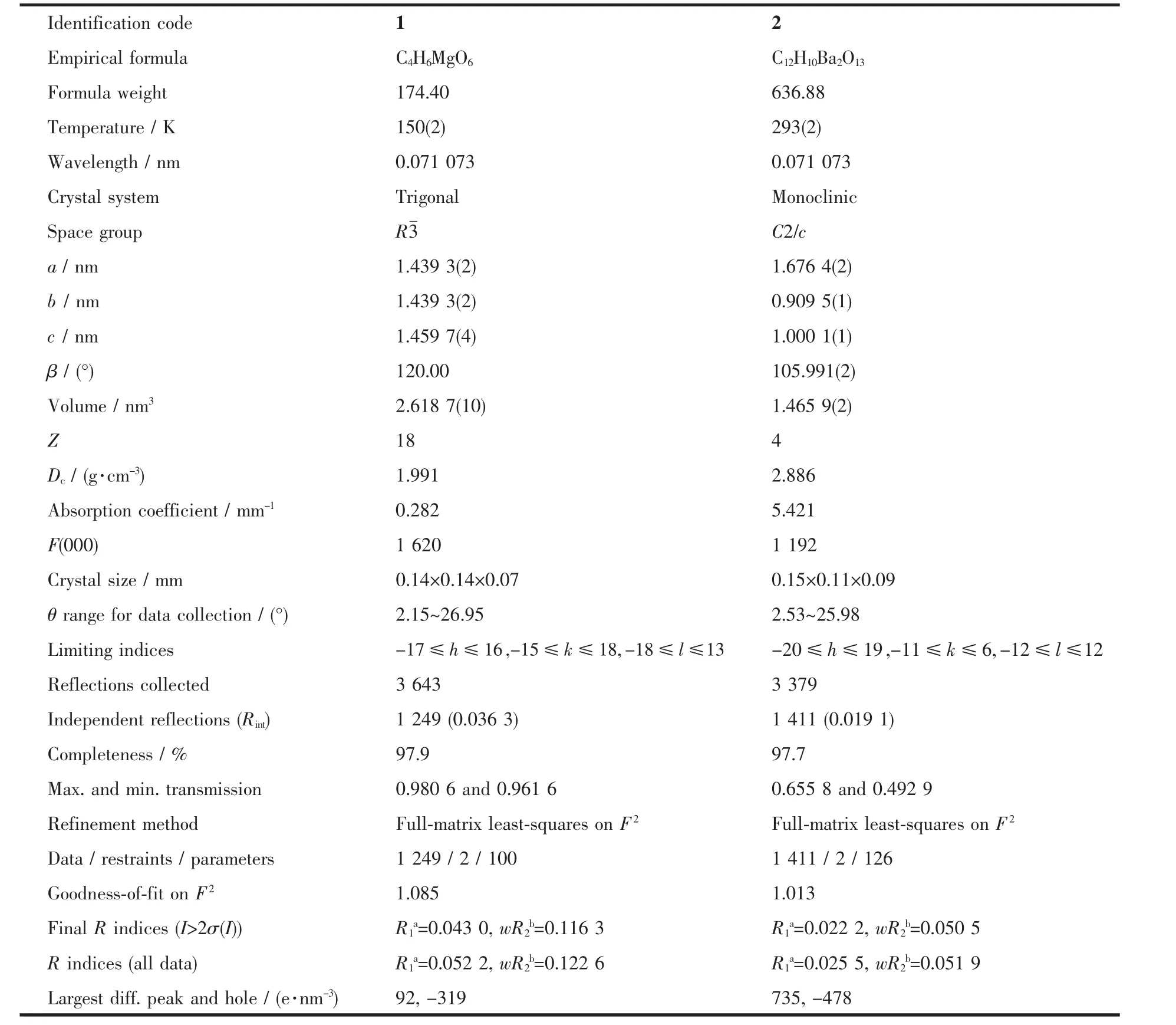
Table 1 Crystal data and structure parameters for 1 and 2
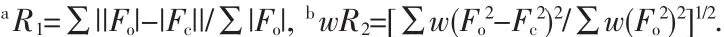
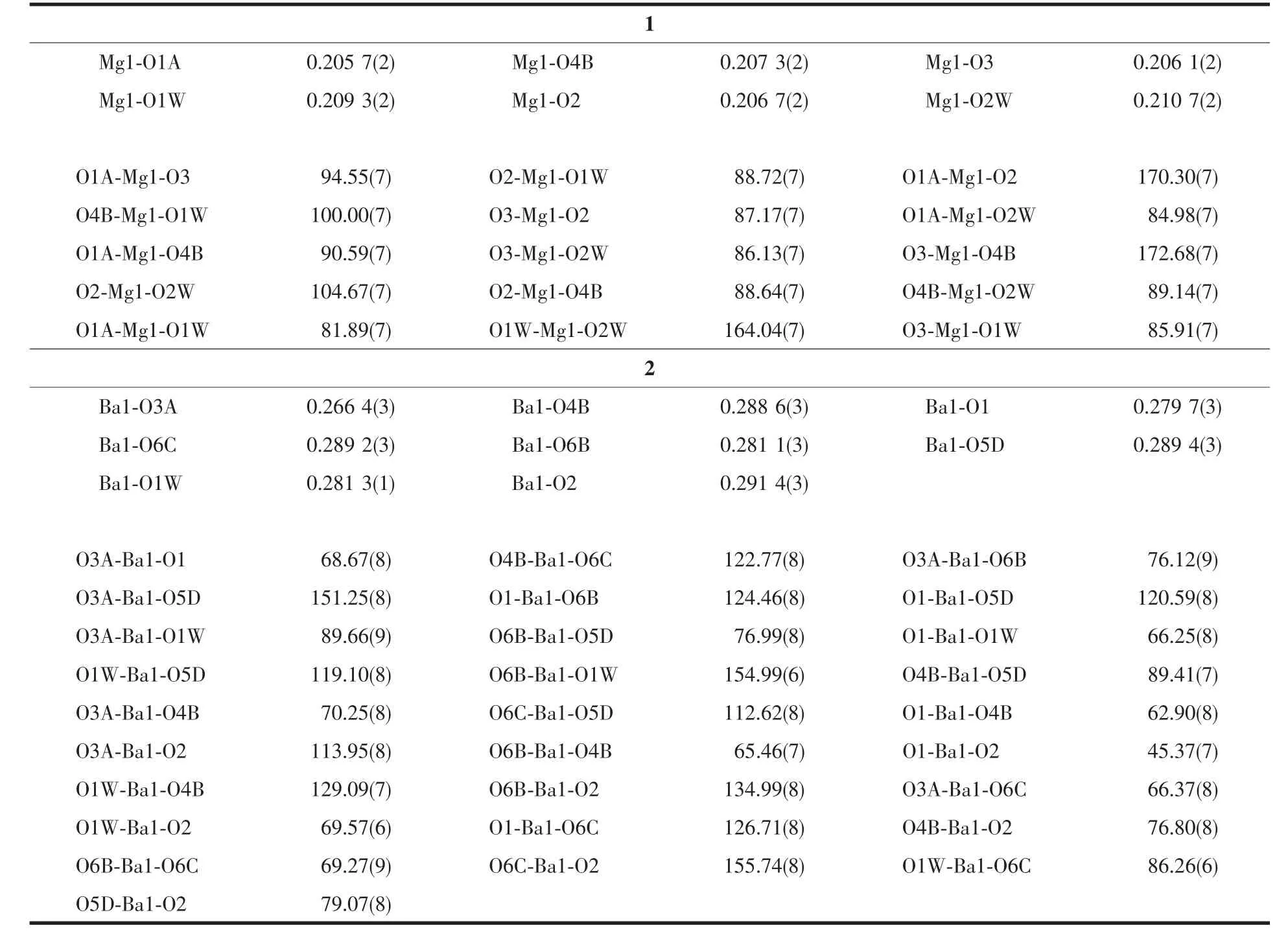
Table 2 Bond lengths(nm)and angles(°)for 1 and 2
2 Results and discussion
2.1 Synthesis
Asiswellknown,there are a variety of hydrothermal parameters such as time,temperature,pH value,and molar ratio of reactants,and small changes in one or more of the parameters can have a profound influence on the final reaction outcome[17-18].Meanwhile,the metal ion size[19-20]as well as the ligand conformation[9-13]also play an important role in the construction of coordination polymers.In our previous work,three different conformations can be trapped in the cadmium coordination polymers by the Cd(Ⅱ)ions,but the cischair form can only be found in the silver complexes,and the trans-chair form can be found in the iron,cobalt,manganese and nickel complexes[10,12].Considering the characters of alkaline earth ions,we employed Mg(Ⅱ) and Ba(Ⅱ) with different radii,attempting to trap the L ligand conformations.We carried out the reactions of MgCO3/BaCO3with H6LⅠ·H2O under 175 ℃hydrothermal conditions(Scheme 1).As a result,only the trans-chair LⅡin situ transformed from the cis-chair form LⅠin the starting material can be observed in the final crystal complexes 1 and 2,which may be because LⅡis a more stable conformation according to the theoretical study[12,21]and the cation size is not suitable to trap other conformations.The resulting structures illustrated that the alkaline earth ions Mg(Ⅱ)and Ba(Ⅱ) can stabilize the LⅡconformation by coordination bonding in their complexes.
2.2 Structure of[Mg3(LⅡ)(H2O)6](1)
X-ray diffraction crystal structure analysis reveals that 1 crystallizes in Rspace group and is isostructural to our recently reported cobalt,iron,manganese and nickel coordination polymers[10,12].Herein,the structure will be simply discussed.The asymmetric unit consists of one crystallographically unique Mg atom on a general position,one unique LⅡligand lying across a 3-fold axis transformed from the starting material cis-chair LⅠ,and two coordinated water molecules.Each Mg atom is coordinated in an octahedralgeometry by four carboxylate oxygen atoms from three LⅡligands and two water molecules(Mg-O 0.205 7(2)~0.210 7(2)nm,OMg-O 81.89(7)°~172.68(7)°).Each LⅡligand connects nine Mg atoms through its six carboxylate groups in a syn-anti bridging mode (Fig.1a).A 3D metal-organic framework is therefore generated by the Mg-carboxylate coordination(Fig.1b).
2.3 Structure of[Ba2(H2LⅡ)(μ2-H2O)2](2)

When the bigger alkaline earth Ba(Ⅱ)was used instead of Mg(Ⅱ)ions,a novel 3D framework was formed in the similar reaction condition.X-ray diffraction crystal structure analysis reveals that complex2 contains one crystallographically unique Ba atom,one partly deprotoned H2LⅡligand lying on a 2-fold axis transformed from the starting material cis-LⅠ,and one coordinated water molecule (Fig.2a).Each Ba atom adoptsaheavilydistorted coordination geometry,coordinated by seven O atoms from different LⅡligands and one water molecule(Ba-O 0.266 4(3)~0.291 4(3)nm,O-Ba-O 45.37(7)°~155.74(8)°).The partly deprotoned H2LⅡ ligand adopts μ10-bridging mode connecting ten Ba atoms through its six bridged μ-η1∶η1,μ-η1∶η2and chelatedcarboxylate groups(Fig.2b).
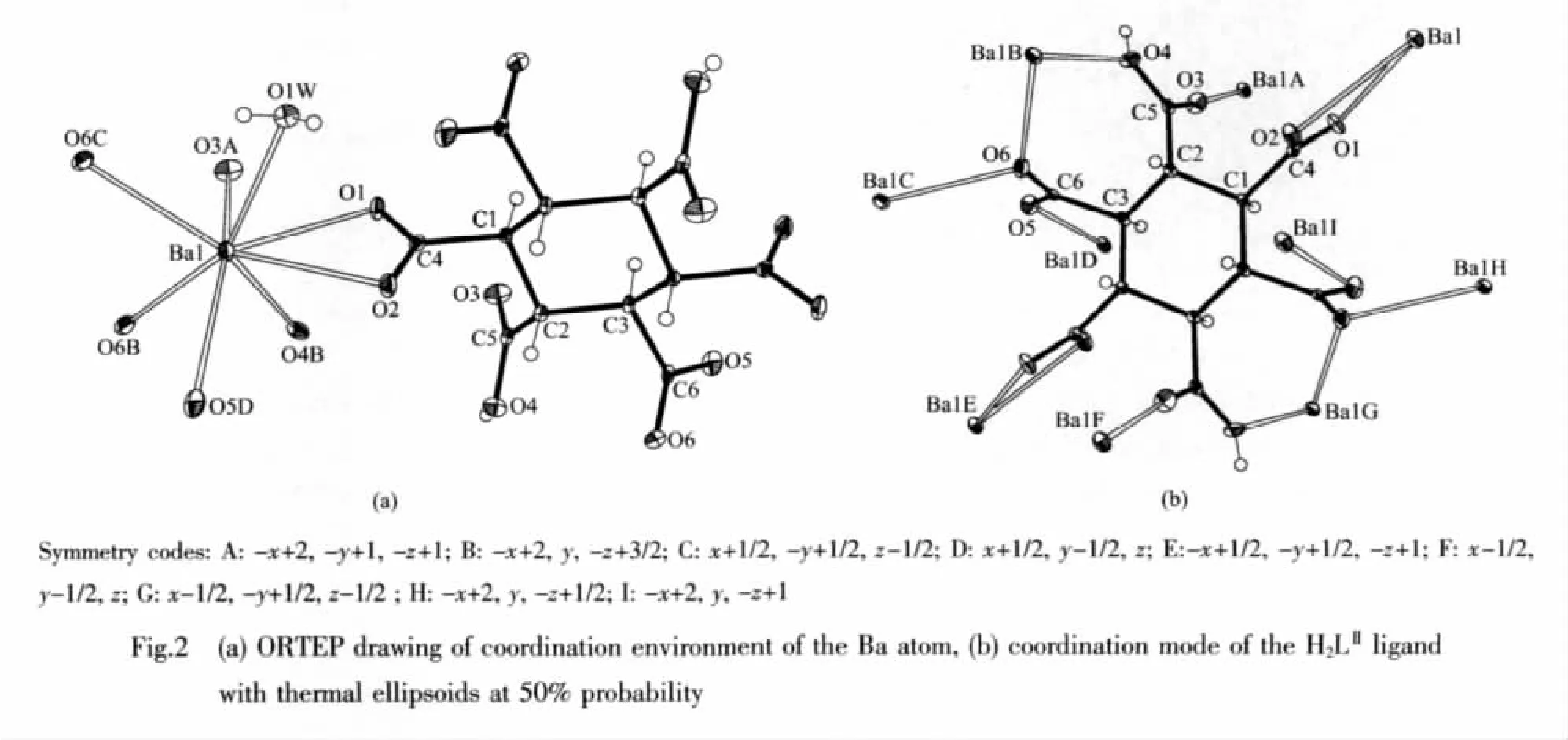
Interestingly,two Ba atoms was bridged by two μ2-O6 from two carboxylate groups to form a four-member ring through two Ba-O bonds with different distances 0.281 1(3)and 0.289 2(3)nm.The four-member rings are further connected to form a 1D(COO)2-Ba2-H2O chain by the μ2-H2O bridges(Fig.3a).Adjacent chains are linked to generate a 3D coordination framework by the μ10-H2LⅡligands(Fig.3b).The 3D framework has 1D distorted quadrangular channels along thecaxis filled with the coordinated water molecules(Fig.3c).In the channels,there are existing hydrogen bonds between the O atoms of carboxylate groups and coordinated water molecules(O4…O1A 0.2498 nm,O4-H4…O1A 176.10°;O1W…O5B 0.282 0 nm,O1W-H1W…O5B 137.34°;symmetry codes:A:x,-y+1,z+1/2;B:x+1/2,-y+1/2,z-1/2).

[1]Li H,Eddaoudi M,O′Keeffe M,et al.Nature,1999,402:276-279
[2]Chui S S Y,Lo S M F,Charmant J P H,et al.Science,1999,283:1148-1150
[3]Humphrey S M,Wood P T.J.Am.Chem.Soc.,2004,126:13236-13237
[4]Tong M L,Kitagawa S,Chang H C,et al.Chem.Commun.,2004:418-419
[5]PENG Meng-Xia(彭梦侠),CHEN Zi-Yun(陈梓云).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(6):1055-1061
[6]Kim Y J,Jung D Y.Chem.Commun.,2002:908-909
[7]Bi W,Cao R,Sun D,et al.Chem.Commun.,2004:2104-2105
[8]Kitagawa S,Uemura K.Chem.Soc.Rev.,2005,34:109-119
[9]Wang J,Hu S,Tong M L.Eur.J.Inorg.Chem.,2006:2069-2077
[10]Wang J,Zheng L L,Li C J,et al.Cryst.Growth Des.,2006,6:357-359
[11]Wang J,Zhang Y H,Tong M L.Chem.Commun.,2006:3166-3168
[12]Wang J,Lin Z J,Ou Y C.Chem.Eur.J.,2008,24:7218-7235
[13]Wang J,Ou Y C,Shen Y,et al.Cryst.Growth Des.,2009,9:2442-2450
[14]Murugavel R,Korah R.Inorg.Chem.,2007,46:11048-11062
[15]Sheldrick G M.SADABS2.05,University of Göttingen,2000.
[16]SHELXTL6.10,Bruker Analytical Instrumentation,Madison,Wisconsin,USA,2000.
[17]Forster P M,Stock N,Cheetham A K.Angew.Chem.,Int.Ed.,2005,44:7608-7611
[18]Tong M L,Hu S,Wang J,et al.Cryst.Growth Des.,2005,5:837-839
[19]Falcão E H L,Naraso,Feller R K,et al.Inorg.Chem.,2008,47:8336-8342
[20]Côté A P,Shimizu G K H.Chem.Eur.J.,2003,9:5361-5170
[21]Eliel E L,Allinger N L,Angyal S J,et al.Conformational Analysis.New York:John Wiley,1996.
Hydrothermal Syntheses and Crystal Structures of Two 3D Alkaline Earth Metal Coordination Polymers with Conformation-Flexible Cyclohexane-1,2,3,4,5,6-hexacarboxylate Ligand
WANG Jing*LIU Zhao-Qing
(School of Chemistry and Chemical Engineering,Guangzhou University,Guangzhou 510006)
Reactions of MgCO3/BaCO3and 1,2,3,4,5,6-cyclohexanehexacarboxylic acid hydrate (H6LⅠ·H2O)(cischair conformation LⅠ:a,e,a,e,a,e)resulted in formation of two three-dimensional coordination polymers[Mg3(LⅡ)(H2O)6](1)and[Ba2(H2LⅡ)(μ2-H2O)2](2)(trans-chair conformation LⅡ:e,e,e,e,e,e)under hydrothermal condition and were characterized by elemental analysis and IR.X-ray diffraction crystal structure analysis shows that 1 crystallizes in trigonal system,space group R3 with a=1.439 3(2)nm,c=1.459 7(4)nm,β=120.00°,V=2.618 7(10)nm3,Z=18;and 2 crystallizes in monoclinic system,space group C2/c with a=1.6764(2)nm,b=0.9095(1)nm,c=1.0001(1)nm,β=105.991(2)°,V=1.4659(2)nm3,Z=4.Complex 1 is a highly symmetrical network bridged by the trans-chair conformation LⅡligand.In complex 2,the cis-chair H6LⅠligand transformed to the partly deprotoned trans-chair H2LⅡligand,which bridged the Ba atoms to generate a porous 3D framework with 1D channels,which are filled with the coordinated water molecules.In the two complexes,both of the flexible cyclohexanehexacarboxylate ligands adopt the trans-chair conformation LⅡ,illustrating that the conformation LⅡcan be stabilized by the coordination with alkaline earth Mg(Ⅱ)/Ba(Ⅱ) through hydrothermal syntheses.CCDC:755551,1;755552,2.
coordination polymer;alkaline earth ions;cyclohexanehexacarboxylate;hydrothermal synthesis
O614.22;O614.23+3
A
1001-4861(2010)11-2077-06
2010-03-01。收修改稿日期:2010-08-10。
国家自然科学青年基金(No.20901018)和广东省自然科学基金(No.9451009101003177)资助项目。
*通讯联系人。 E-mail:wangjgzhu@yahoo.com.cn
王 静,女,29岁,博士,讲师;研究方向:金属有机配位聚合物化学。
