自制载体冷冻小鼠原核期胚胎效果分析
2010-09-09陆文昊程晋王维曹祖兵李运生曹鸿国刘亚陶勇章孝荣张运海
陆文昊,程晋,王维,曹祖兵,李运生,曹鸿国,刘亚,陶勇,章孝荣,张运海
(安徽农业大学动物科技学院,合肥 230036)
研究报告
自制载体冷冻小鼠原核期胚胎效果分析
陆文昊,程晋,王维,曹祖兵,李运生,曹鸿国,刘亚,陶勇,章孝荣,张运海
(安徽农业大学动物科技学院,合肥 230036)
目的探讨自制冷冻载体冷冻保存昆明小鼠体内原核期胚胎的可行性。方法首先,比较了两种流行的商业化载体:开放式拉长麦管(open pulled straw,OPS)和冷冻帽(cryotop)开展小鼠原核胚玻璃化冷冻保存效果。其次,以cryotop为对照,利用自制简易载体(cryotip)开展小鼠原核期胚胎的玻璃化冷冻保存。之后,利用ANOVA对各组胚胎在复苏后的体外培养卵裂率、囊胚率进行统计分析。结果OPS和cryotop两组之间,胚胎在玻璃化冷冻/复苏后发育的2-细胞率、4-细胞率和囊胚率差异均无显著性(P>0.05),但cryotop冷冻效果更接近对照组;cryotip玻璃化冷冻载体与cryotop相比,胚胎复苏后各组差异均无显著性(P>0.05),数值上除了2-细胞发育率外,cryotip其他几项结果都稍微高于cryotop组。结论OPS,cryotop,cryotip冷冻保存昆明小鼠体内原核期胚胎均是可行的;cryotop在冷冻效果上要优于OPS,笔者自制的cryotip因其成本低,制作简单,操作安全可靠,在实验中替代昂贵的商业化载体OPS和cryotop是可行的。
昆明小鼠;原核胚;不同冷冻载体;玻璃化冷冻
小鼠卵母细胞和胚胎冷冻已经广泛应用于医学、动物学和生物学基础研究等方面,为动物胚胎工程如体外受精、体细胞克隆等的发展做出了巨大的贡献,更为人类辅助生殖临床质控及应用基础研究提供了重要手段。而配子和胚胎的玻璃化冷冻相对于常规冷冻技术,具有操作简单,耗时较短,仪器低廉,冷冻效果较好的特点,越来越被研究工作者广泛运用。
尽管人们在小鼠胚胎的玻璃化冷冻上已取得很大的研究进展,但这些进展主要集中在8-细胞、16-细胞、桑葚胚和囊胚阶段。小鼠原核胚分化程度较低、对低温较敏感,造成了原核胚的冷冻保存较其他发育阶段的胚胎难度大,所以到目前为止,国内外关于原核胚的玻璃化冷冻的研究报道相对较少。
在卵母细胞和胚胎的玻璃化冷冻操作中,冷冻载体的选择非常关键。载体不同,所承载的玻璃化液的液量不同,而玻璃化液液量又决定着冷冻降温速度的快慢,降温速度快慢则是影响细胞冷冻效果的关键因素。迄今,在哺乳动物配子和胚胎冷冻保存实践中,电镜铜网(electron m icroscopeic grid,EMC)[1]、开放式拉长麦管(open pulled straw,OPS)[2]、冷冻帽(cryotop)[3]、玻璃微管(glass micropipette,GMP)[4]、金属表面积法(solid-surface vitrification,SSV)[5]、冷冻环(cryoloop)[6]等冷冻载体均有获得成功的报道。
回顾文献报道发现,近十余年以来,在玻璃化冷冻的实践中,以开放式拉长麦管(open pulled straw,OPS)和冷冻帽(cryotop)运用较为广泛。OPS是由Vajta于1997年冷冻牛卵母细胞中发明的,之后相继在其他物种上得到了推广,如人[7],山羊[8-9],马[10],小鼠[11-12],猪[13-15],蓝狐[16]以及绵羊[17]的胚胎或卵母细胞玻璃化冷冻。而cryotop自问世以来,在人、牛[3,18]M II期卵母细胞玻璃化冷冻上效果甚佳,近来则又在猪[19]、马[20]、羊[21]的卵母细胞冷冻中获得了成功。
可见,合适的载体成就冷冻的好坏。然而,上述玻璃化冷冻所用载体由于制备比较复杂,且多需从国外进口,价格昂贵,重复使用还会出现交叉污染,也在一定程度上影响了玻璃化冷冻技术在国内的进一步推广。因此,本文尝试利用笔者自制的一种新型载体cryotip,以昆明小鼠为实验材料,与进口的商业化载体比较,探讨利用其开展原核胚胎玻璃化冷冻的可行性,为小鼠乃至家畜卵母细胞及胚胎的玻璃化冷冻提供参考。
1 材料与方法
1.1 实验动物
清洁级昆明小鼠【SCXK(苏)2007-0001】购自于扬州大学比较医学中心,雌性小鼠为5~8周龄,体重20~25 g;雄鼠10~16周龄,体重30~35 g。小鼠在温度18~25℃控光(7:00 am~19:00 pm光照,19:00 pm~7:00 am黑暗),自由饮水采食。
1.2 超数排卵
选择处于发情初期的健康雌性昆明小鼠,腹腔注射10 IU PMSG(pregnant mare serum gonadotrophin,宁波第二激素制品厂),48h后注射10 IU hCG(human chorionic gonadotropin,宁波第二激素制品厂),然后与雄鼠1∶1合笼交配,第2天上午8:00前检查阴道栓。
1.3 冷冻载体
1.3.1 开放式拉长塑料细管法(open pulled straws,OPS):由0.25 m L细管加热变软后拉成,其内径为0.8 mm。管壁厚度为0.07 mm,冷冻后将OPS的细小端浸入含有胚胎的冷冻液小滴1~2 μL,利用虹吸效应将胚胎以及冷冻液吸入OPS,然后直接投入液氮保存。
1.3.2 冷冻帽(cryotop):购于日本Kitazato公司,前端是透明且成薄膜状的载体,并有塑料材质的把柄,可将胚胎置放在上,且体积仅为0.1 μL,然后迅速投入液氮中保存。
1.3.3 自制的载体(cryotip):用解剖剪刀在0.25 m L精液冷冻管的开口端剪成长2 cm、宽1~1.5 mm的矩形薄片(制备方法见图1),可将胚胎置放在上,且体积仅为0.1 μL,然后迅速投入液氮中保存。
1.4 冷冻液与解冻液
操作液(handling medium,HM):Hepes/TCM199 (货号:M7528)+20%FBS;平衡液(equilibrium solution,ES):HM+7.5%EG+7.5%DMSO;冷冻液(vitrification solution,VS):HM+15%EG+15% DMSO+1.0 mol/L蔗糖;解冻液(thawing solution,TS):HM+1.0 mol/L蔗糖;稀释液(dilution solution,DS):HM+0.5 mol/L蔗糖。除特别说明外,本文所有化学试剂均购自Sigma公司(St. Louis,MO,USA)。
1.5 胚胎收集及培养

注①如图B所示,按箭头方向沿实线剪开(A为精液冷冻管);②如图B所示,按箭头方向沿实线剪开,保留虚线部分;③:如图C所示,按箭头方向沿虚线剪开,所得结果D(自制载体cryotip)。图1 cryotip制备示意图Note①As shown in Figure B,according to the direction of the arrow cut along the solid lines(A:semen frozen pipes).②As shown in Figure B,according to the direction of the arrow along the solid line cut,retaining dotted line.③As shown in Figure C,according to the direction of the arrow cut along the dotted line,the results of D[self-made loading carrier Cryotip].Fig.1 Schematic illustration of the preparation of cryotip
于hCG注射后22~24 h内采取颈部脱臼法处死见栓雌鼠,无菌取出输卵管并除去其上附着的脂肪,放入小鼠胚胎操作液(M2)中,用1 m L注射器针头撕开输卵管膨大部释放出原核胚团块,移入含1%(W/V)透明质酸酶的0.9%生理盐水中,移液器轻轻吹打几次去除卵丘细胞,并用M2清洗3次,在体视显微镜挑选形态正常的胚胎进行冷冻解冻后,移入不同小鼠胚胎培养液培养5 d,观察其体外发育情况。
1.6 实验设计
1.6.1 实验1:将自然受精后的原核期胚胎随机分为两组,胚胎在M2中洗2~3遍后,移入HM中3~5 min,经过7.5%EG+7.5%DMSO处理3 min后,放入15%EG+15%DMSO+1.0 mol/L蔗糖液20~30 s,分别用OPS与cryotop两种载体冷冻,然后直接投入液氮盒中;解冻时,先把原核胚在含1.0 mol/L蔗糖的TS中解冻液中复苏1 m in,然后放入含0.5 mol/L的蔗糖的DS中处理3 min,最后用HM洗涤3次5~10 min,然后以10~20枚/50 μL液滴的培养密度将胚胎进行体外培养(冷冻和解冻均在37℃下进行)。对照组的原核期胚胎不进行玻璃化冷冻直接培养,培养密度与实验组相同。实验统计各组胚胎的2-细胞率、4-细胞率和囊胚率。
1.6.2 实验2:冷冻方法和解冻方法以及胚胎培养方法和实验1相同,冷冻载体运用cryotop(对照组)和自制cryotip(实验组),然后胚胎进行体外培养,统计胚胎的2-细胞率、4-细胞率、桑葚胚率和囊胚率。解冻后体外培养不同发育阶段的的胚胎观察见图2所示。
1.7 统计分析
每组实验均重复5~7次,实验数据以平均数±标准误表示,不同阶段发育率以及不同比例分组数据都根据SPSS 13.0软件one-way ANOVA分析,组间差异采用LDS检验。P<0.05认为存在显著差异。
2 结果
2.1 OPS与C ryotop冷冻效果的比较
实验结果(表1)结果显示:OPS、cryotop与对照组之间在2-细胞率、4-细胞率和囊胚率差异不显著(P>0.05),但是在4细胞和囊胚发育率数值上OPS组明显低于cryotop组和对照组。结果表明: OPS与cryotop对小鼠原核期胚胎冷冻均是可行的,用cryotop冷冻胚胎效果比OPS较好。
2.2 C ryotop与自制cryotip冷冻效果的比较
实验结果(表2)显示:我们自制的cryotip与成品相比,2-细胞率、4-细胞率、桑葚胚率和囊胚率差异都无显著性(P>0.05),但从数值上看,在自制cryotip载体冷冻结果中,除了2-细胞发育率外,其他几项结果都稍微高于成品组。结果表明:自制cryotip冷冻小鼠原核期胚胎是可行的,与成品cryotop相比具有更好的冷冻效果。
3 讨论
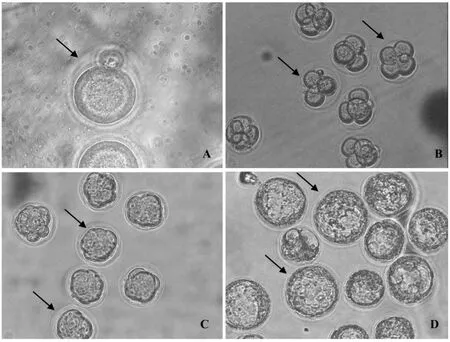
注:A:1-细胞胚胎,箭头所指;B:3-4细胞胚胎,箭头所指,培养48 h;C:桑葚胚,箭头所指,培养72 h;D:囊胚,箭头所指,培养96 h。图2冷冻解冻后体外不同发育阶段的小鼠胚胎Note:A:The arrow shows the 1-cell embryo;B:The arrows show the 3/4-cell embryo;48 h after IVC; C:The arrows show the morulae;72 h after IVC;D:The arrows show the blastocyst,96 h after IVC.Fig.2 The mouse embryos at different development stage after vitrification
表1 OPS与cryotop对小鼠原核期胚胎冷冻效果的影响(±s,%)Tab.1 The effect of different commercial loading devices on in vitro development ability of pronuclear embryos after vitrification and thawing(±s,%)

表1 OPS与cryotop对小鼠原核期胚胎冷冻效果的影响(±s,%)Tab.1 The effect of different commercial loading devices on in vitro development ability of pronuclear embryos after vitrification and thawing(±s,%)
注:2-细胞率=2-细胞胚胎数/原核胚胎数;4-细胞率=4-细胞胚胎数/2-细胞胚胎数;囊胚率=囊胚数/2-细胞胚胎数。同一列中含有相邻不同字母上标的表示差异有显著性(P<0.05)。Notes:The rate of 2-cell embryos=number of 2-cell embryos/number of 1-cell embryos;The rate of 4-cell embryos=number of 4-cell embryos/number of 2-cell embryos;The rate of blastocyst formation=number of blastocysts/number of 2-cell embryos;Values with different superscripts in the same column indicate a significant difference(P<0.05).
组别Group原核胚数No.of 1-cell embryos 2-细胞率2-cell embryo rate 4-细胞率4-cell embryo rate囊胚率Blastocyst rate OPS 75 84.28±4.31 57.35±5.08 28.37±2.88 Cryotop 74 74.10±7.23 65.87±10.10 43.57±7.11对照组Control 70 81.19±5.28 64.25±5.50 40.04±8.80
表2 成品cryotop与自制cryotip对小鼠原核期胚胎冷冻效果的影响(±s,%)Tab.2 The effect of self-made loading device on preimplantation development of mouse pronuclear embryos after vitrification and thawing(±s,%)

表2 成品cryotop与自制cryotip对小鼠原核期胚胎冷冻效果的影响(±s,%)Tab.2 The effect of self-made loading device on preimplantation development of mouse pronuclear embryos after vitrification and thawing(±s,%)
注:2-细胞率=2-细胞胚胎数/原核胚胎数;4-细胞率=4-细胞胚胎数/2-细胞胚胎数;桑葚胚率=桑葚胚胎数/2-细胞胚胎数;囊胚率=囊胚数/ 2-细胞胚胎数。同一列中含有相邻不同字母上标的表示差异有显著性(P<0.05)。Note:The rate of 2-cell embryos=number of 2-cell embryos/number of 1-cell embryos.The rate of 4-cell embryos=number of 4-cell embryos/number of 2-cell embryos.The rate of morula=number of morulae/number of 2-cell embryos.The rate of blastocyst formation=number of blastocysts/number of 2-cell embryos.Values with different superscripts in the same column indicate a significant difference(P<0.05).
组别Group原核胚数No.of 1-cell embryos 2-细胞率2-cell embryo rate 4-细胞率4-cell embryo rate桑葚胚率Morula rate囊胚率Blastocyst rate Cryotop 80 71.25±3.50 74.01±5.89 63.84±6.41 58.48±5.39 Cryotip 80 67.50±3.13 75.60±3.82 66.29±4.30 62.72±4.32
玻璃化冷冻要求在极短的时间里完成冷冻降温程序。由于载体承载玻璃化液液量不同,降温速率也不尽相同。加之,冷冻保护剂浓度越高,对细胞伤害越大。因此,亟需在尽最大可能加快降温速率及减少冷冻保护剂的浓度之间寻找一个平衡点。我们在实验一中比较了OPS与cryotop这两种当前较为流行的商业化载体对小鼠原核胚玻璃化冷冻/复苏后体外发育的影响,发现OPS、cryotop与对照组之间虽然在2-细胞率、4-细胞率和囊胚率上差异不显著,但是4-细胞和囊胚发育率数值上OPS组明显低于cryotop组。1998年,Vajta等[22]发现OPS冷冻速率为20 000℃/min,而Masahige等发现crytop冷冻速率为23 000℃/min,说明cryotop与液氮瞬时接触面更广,从而降低了冷冻对胚胎的损伤,提高冻后的发育能力[23]。Liu等[24]发现使用cryotop与OPS冷冻猪卵母细胞,解冻后经孤雌处理,胚胎体外发育率前者高于后者。同时,Morato等[25]在研究牛卵母细胞冷冻时发现,cryotop与OPS相比,cryotop能够更好地用于牛卵母细胞冷冻保存,可得到更高的囊胚发育率。以上的研究结果和我们实验的结果是一致的,说明cryotop相对于OPS更适合卵母细胞或者胚胎的玻璃化冷冻。
本研究所获小鼠原核胚在玻璃化冷冻/复苏后囊胚发育率为28.37%~62.72%,而朱士恩[26]等在20℃室温条件下运用一步法和两步法冷冻小鼠原核胚,得到的囊胚发育率为47%和68%,而当室温升至25℃,二步法冷冻保存后原核胚的囊胚发育率高达77%。Bagis[27]等在研究小鼠原核胚玻璃化冷冻中得到58.3%和68.5%的囊胚率,而Zhao等[28]运用EFS30、EFS40、EDFS30、EDFS40四种冷冻液对小鼠原核胚进行冷冻,囊胚率分别为66.7%、66.0%、85.7%、76.9%。本文研究结果和上述文献中的结果基本是一致的,但与Zhao等运用EDFS30,EDFS40冷冻得到了85.7%和76.9%囊胚率还有差距。这可能和实验操作温度,冷冻和解冻的操作时间以及玻璃化液配方的不同而导致实验结果的不同。另外,小鼠品系和饲养环境,以及室验室环境也可能对实验造成一定的影响。
OPS是一种塑料型的冷冻载体,虽然在当前应用比较广泛,但在实际操作中,时常有如下几点局限性:第一,当塑料细管拉制到0.8 mm的时候,由于管壁较薄使得载体容易折断,不容易拉制成功。第二,冷冻和解冻时,由于细管自身的因素,会产生虹吸不畅和解冻受阻,不但严重影响操作时间,而且会出现卵母细胞或者胚胎丢失的现象,增加实验结果不稳定的几率。第三,OPS一般为透明状,投入液氮中蒸汽会导致寻找困难,影响实验结果。Cryotop目前都是需要花费许多外汇从国外进口的,虽然它不存在OPS上述的一些局限性,但它由于制备比较精密,价格不菲,且反复使用会导致折旧和交叉污染,在国内关于家畜或实验动物配子、胚胎玻璃化冷冻保存实践中的应用报道非常少。
为克服OPS、cryotop的上述局限性,笔者参考cryotop的设计原理,尝试利用精液冷冻常用的廉价细管,研制出了一种载体:cryotip,我们的实验也表明当利用它作为载体开展小鼠原核胚玻璃化冷冻时,可以获得与cryotop相近的结果。Cryotip制备非常简单、容易掌握,并具有以下优点:由于材料是价格低廉的牛精液冷冻的细管,大大降低进口cryotop的成本,节约外汇;牛精液冷冻管内壁呈现的螺旋纹结构相对于OPS和cryotop内壁的平滑结构,降低了胚胎丢失的可能性。
综上所述,我们的研究表明:Cryotip冷冻保存昆明小鼠体内受精原核期胚胎的是可行的,可以取代昂贵的商业化载体cryotop和OPS,降低成本,制作简单、易学,便于在科研实践和教学实验中推广。
(致谢:衷心感谢冯颖、随刘才、季索菲在实验取材方面提供的帮助。)
[1]Martino A,Songsasen N,Leibo SP.Development into blastocyts of bovine oocytes cryopreserved by ultra-rapid cooling[J].Biol Reprod,1996,54:1059-1069.
[2]Vajta G,Booth PJ,Holm P.Successful vitrification of early stake bovine in vitro produced embryos with the open pulled straw (OPS)method[J].Cryo Lett,1997,18:191-195.
[3]Kuwayama M,Kato.A ll-round vitrification method for human oocytes and emborys[J].Assist Reprod Genet,2000,17:477.
[4]Kong LK,Lea SI,Cho SG,et al.Comparison of open pulled straw(OPS)vs glass micropipette(GMP)vitrification in mouse blastocytes[J].Theriogenology,2000,53:1817-1826.
[5]Dinnvers A,Dai Y,Jung S,et al.High developmental rates of vitrified bovine oocytes following parthenogenetic activation,in vitro fertilization,and somatic cell nuclear transfer[J].Biol Reprod,2000,63(2):513-518.
[6]Mukaida T,Nakamura S,Tomiyama T,et al.Successful birth after transfer of vitrified human blastocysts with use of a cryoloop containerless technique[J].Fertil Steril,2001,76:618-620.
[7]Isachenko V,Montag M,Isachenko E,et al.Aseptic technology of vitrification of human pronuclear oocytes using open-pulled straws[J].Hum Reprod,2005,20(2):492-496.
[8]Hong QH,Tian SJ,Zhu SE,et al.Vitrification of boer goat morulae and early blastocysts by straw and open-pulled straw method[J].Reprod Domest Anim,2007,42(1):34-38.
[9]Bettencourt EM,Bettencourt CM,Silva JN,et al.Ultrastructural characterization of fresh and cryopreserved in vivo produced ovine embryos[J].Theriogenology,2009,71(6):947-958.
[10]Moussa M,Bersinger I,Doligez P,et al.In vitro comparisons of two cryopreservation techniques for equine embryos:slow-cooling and open pulled straw(OPS)vitrification[J].Theriogenology,2005,64(7):1619-1632
[11]Meng Q,Li X,Wu T,et al.Piezo-actuated zona-drilling improves the fertilisation of OPS vitrified mouse oocytes[J].ActaVet Hung,2007,55(3):369-378.
[12]Suo L,Zhou GB,Meng QG,et al.OPS vitrification of mouse immature oocytes before or after meiosis:the effect on cumulus cells maintenance and subsequent development[J].Zygote,2009,17(1):71-77.
[13]Shi WQ,Zhu SE,Zhang D,et al.Improved development by Taxol pretreatment after vitrification of in vitro matured porcine oocytes[J].Reprodution,2006,131(4):795-804.
[14]Berthelot F,Venturi E,Cognié J,et al.Development of OPS vitrified pig blastocysts:effects of size of the collected blastocysts,cryoprotectant concentration used for vitrification and number of blastocysts transferred[J].Theriogenology,2007,68 (2):178-185.
[15]Varga E,Gajdócsi E,Makkosné BP,et al.Vitrification of in vitro matured oocytes of Mangalica and large white pigs[J].Acta Vet Hung,2008,56(3):399-410.
[16]Zhou GB,Ma CB,Liu GS,et al.Vitrification of farmed blue fox oocytes in ethylene glycol and DMSO-based solutions using openpulled straw(OPS)[J].Cryo Letters,2009,30(2):112-118.
[17]Green RE,Santos BF,Sicherle CC,et al.Viability of OPS vitrified sheep embryos after direct transfer[J].Reprod Domest Anim,2009,44(3):406-410.
[18]Kuwayama M,Vajta G,Kato O,et al.Highly efficient vitrification method for cryopreservation of human oocytes[J]. Reprod Biomed,2005,11:300-308.
[19]Fujihira T,Nagai H,Fukui Y,et al.Relationship between equilibration times and the presence of cumulus cells,and effect of taxol treatment for vitrification of in vitro matured porcine oocytes[J].Cryobiology,2005,51(3):339-343.
[20]Bogliolo L,Ariu F,Rosati I,et al.Vitrification of imm ture and in vitro matured horse oocytes[J].Reprod Fertil,2006,18:149-150.
[21]Succu S,Bebbere D,Bogliolo L,et al.Vitrification of in vitro matured ovine oocytes affects in vitro pre-implantation development and mRNA abundance[J].Mol Reprod Dev,2008,75(3):538-546.
[22]Vajta G,Holm P,KuwayamaM,et al.Open pulled straw (OPS)vitrification:A new way to reduce cryo injuries of bovine ova and embryos[J].Mol Reprod Dev,1998,51(1):53-58.
[23]Kuwayama M,Cobo A,Vajta G.Vitrification of oocytes:general considerations and the use of the Cryotop method[J].Vitrificat Assisted Reprod,2007,7A:119-128.
[24]Liu Y,Du Y,Lin L,et al.Comparison of efficiency of open pulled straw(OPS)and Cryotop vitrification for cryopreservation of in vitro matured pig oocytes[J].Cryo Letters,2008,29(4): 315-320.
[25]Morato R,Izquierdo D,Paramio MT,et al.Cryotops versus open-pulled straws(OPS)as carriers for the cryopreservation of bovine oocytes:effects on spindle and chromosome configuration and embryo development[J].Cryobiology,2008,57(2):137-141.
[26]朱士恩,权国波,吴通义,等.小鼠原核胚玻璃化冷冻保存技术的研究[J].中国实验动物学报,2003,12,2:69-73.
[27]Bagis H,Sagirkaya H,Mercan HO,et al.Vitrification of pronuclear-stage mouse embryos on solid surface(SSV)versus in cryotube:comparison of the effect of equilibration time and different sugars in the vitrification solution[J].Mol Reprod Dev,2004,67(2):186-92.
[28]Zhao XM,Quan GB,Zhou GB,et al.Conventional freezing,straw,and open-pulled straw vitrification of mouse two pronuclear (2-PN)stage embryos[J].Anim Biotechnol,2007,18(3): 203-212.
Vitrification of M ouse Pronuclear Stage Em bryos:Effect of a New ly Self-M ade,Econom ical Loading Device
LU Wen-hao,CHENG Jin,WANG Wei,CAO Zu-bing,LI Yun-sheng,CAO Hong-guo,LIU Ya,TAO Yong,ZHANG Xiao-rong,ZHANG Yun-hai
(College of Animal Science and Technology,Anhui Agricultural University,Hefei 230036,China)
Objective To explore the possibility of using a new ly self-made loading device to replace expensive commercial carriers to vitrify Kunm ing white mouse pronuclear stage embryos derived by in vivo fertilization.M ethods Firstly,the effect of two popular and commercial carriers:open pulled straw(OPS)and Cryotop,on in vitro development of mouse in vivo derived pronuclear embryos,subjected to vitrification and thawing,was exam ined.Secondly,with the Cryotop as control,the feasibility of using self-made loading carrier(Cryotip)to vitrify mouse pronuclear embryos was evaluated.Data of cleavage and blastocyst formation were analyzed by ANOVA.ResultsThe results showed that the rates of 2-cell embryo,4-cell embryo,and blastocyst in both OPS and Cryotop groups were similar(P>0.05),but the rates of cleavage and blastocyst formation in the Cryotop group were likely to be closer to that in the control group.The rates of each group were not significantly different between Cryotip and Cryotop groups(P>0.05),although Cryotop tends to have a higher rate in 2-cell embryo rate.ConlusionIt is feasible to cryopreserve Kunming white mouse pronuclear stage embryos derived from in vivo fertilization with OPS,Cryotop,and Cryotip.The results of cryopreservation by Cryotop are likely better than that by OPS.In particular,our self-made novel loading device–Cryotip,is of good quality and lower cost,easier to make,may replace the expensive commercial carriers such as OPS and Cryotop,widely used in mouse embryo or oocyte vitrification practice.
Kunming white mouse;Pronuclear embryo;Different carriers;Vitrification
R-318.52
A
1005-4847(2010)04-0283-06
2009-12-11
国家“863”重点项目(2008AA101003);国家“十一五”科技支撑计划(08010301066)。
陆文昊(1985-),男,硕士研究生,研究方向:动物胚胎工程。E-mail:wenhaolu123@163.com;Tel:13965002951。
张运海(1973-),男,博士,副教授,硕士生导师,主要从事哺乳动物发育生物学研究。E-mail:yunhaizhang@ahau.edu.cn; Tel:0551-5786357;Fax:0551-5785543
