Profile of hepatocyte apoptosis and bile lakes before and after bile duct decompression in severe obstructive jaundice patients
2010-06-29ToarJMLalisangRadenSjamsuhidajatNurjatiSiregarandAkmalTaher
Toar JM Lalisang, Raden Sjamsuhidajat, Nurjati C Siregar and Akmal Taher
Jakarta, Indonesia
Original Article / Biliary
Profile of hepatocyte apoptosis and bile lakes before and after bile duct decompression in severe obstructive jaundice patients
Toar JM Lalisang, Raden Sjamsuhidajat, Nurjati C Siregar and Akmal Taher
Jakarta, Indonesia
BACKGROUND:Excessive hepatocyte apoptosis and bile lakes in severe obstructive jaundice might impair liver functions. Although decompression of the bile duct has been reported to improve liver functions in animal studies, the mechanism of obstruction differs from that in humans. This study aimed to determine the profiles of hepatocyte apoptosis and bile lakes following bile duct decompression in patients with severe obstructive jaundice in the clinical setting.
METHODS:We conducted a "before and after study" on severe obstructive jaundice patients as a model of inhibition of the excessive process by bile duct decompression. Specimens of liver biopsies were taken before and after decompression of the bile duct and then stained by terminal deoxynucleotide transferase-mediated dUTP nick end-labeling (TUNEL) to identify hepatocyte apoptosis and by hematoxilin-eosin (HE) to identify bile lakes. All measurements were independently done by 2 observers.
RESULTS:Twenty-one severe obstructive jaundice patients were included. In all patients, excessive hepatocyte apoptosis and bile lakes were apparent. After decompression, the hepatocyte apoptosis index decreased from 53.1 (SD 105) to 11.7 (SD 13.6) (P<0.05), and the bile lakes from 23.6 (SD 14.8) to 10.9 (SD 6.9) (P<0.05).
CONCLUSION:Bile duct decompression improves hepatocyte apoptosis and bile lakes in cases of severe obstructive jaundice, similar to the findings in animal studies.
(Hepatobiliary Pancreat Dis Int 2010; 9: 520-523)
hepatocyte apoptosis; bile lakes; TUNEL; obstructive jaundice; bile duct decompression
Introduction
Excessive hepatocyte apoptosis and bile lakes have been reported to be induced by the accumulation of bile acid,[1,2]which decreases the quantity of functional hepatocytes and leads to liver function impairment.[3]Decompression of the bile duct in obstructive jaundice in animal models has been reported to improve both morphology and physiology of the liver;[4,5]however, the improvement is not related to improvement of operative morbidity and mortality.[6-8]This discrepancy might be due to the limitation of the models used in previous studies.[9]Obstructive jaundice in those models occurs in an acute phase while in humans, obstructive jaundice always occurs in a chronic phase.
Since whether the results obtained from animal studies are representative of those in humans is not clear, we examined hepatocyte apoptosis and bile lake index in severe obstructive jaundice patients before and after decompression of the bile duct.
Methods
This study was conducted according to the ethical principles ofthe Declaration of Helsinkiand was approved by the Ethics Committee of the Faculty of Medicine of the University of Indonesia. We recruited all patients presenting with severe obstructive jaundice (serum total bilirubin level >10 mg/dl) due to tumor during the period of December 2007 to January 2009. Patients with active viral hepatitis, hepatocellular carcinoma, severe liver dysfunction with massive ascites, chronic liver failure, and terminal state of tumorwere excluded. Informed consent was obtained from all participants. We performed bile duct decompression by the open cholecystostomy technique. Liver specimens were obtained by open incisional biopsy from each patient before and after decompression. The specimens were then stained by terminal deoxynucleotide transferasemediated dUTP nick end-labeling (TUNEL) fluorescence to identify hepatocyte apoptosis and hematoxylin-eosin (HE) to assess bile lakes. Hepatocyte index (defined as total hepatocyte apoptosis on 10 high-power fields in Rappaport zone II) and bile lakes index (defined as total bile lakes on 10 randomly-selected high-power fields) were counted by two researchers independently. They were analyzed by paired Student'sttest using SPSS 16.0. APvalue less than 0.05 was considered statistically significant.
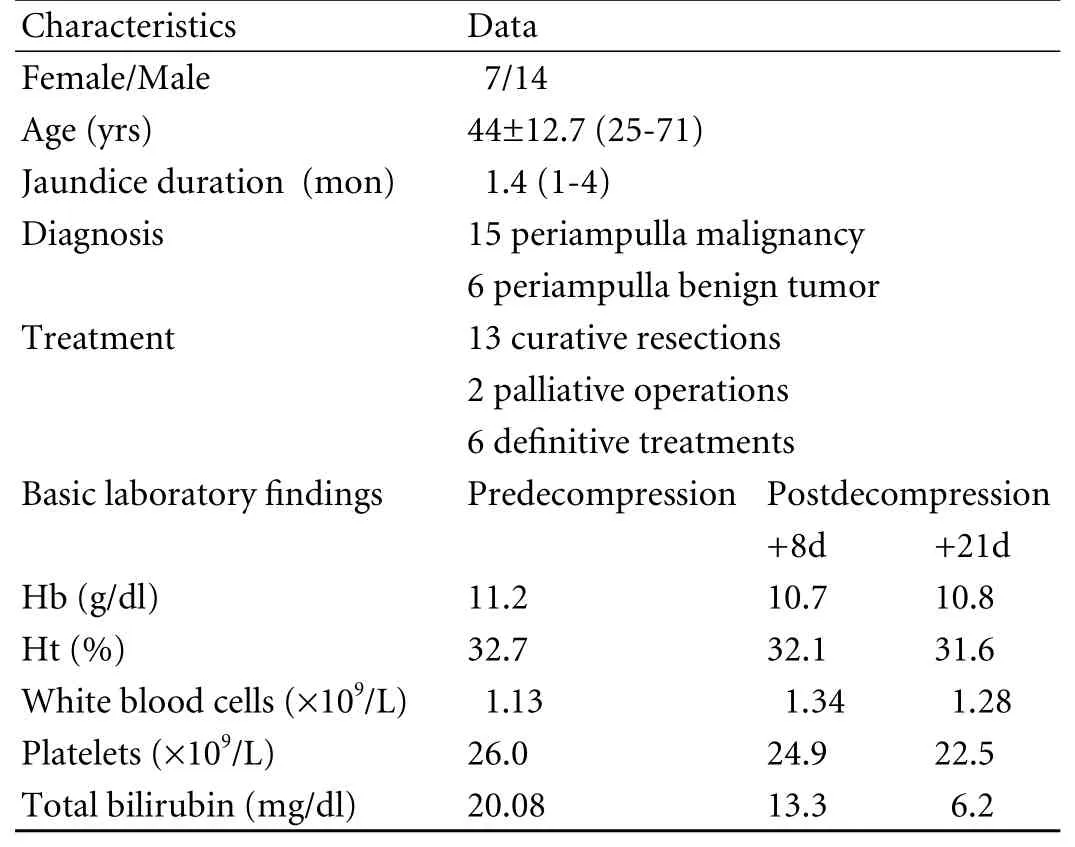
Table. Patients distribution
Results
During the period of December 2007 to January 2009, 38 eligible patients were recruited but only 21 were available for analysis. Eleven patients died before the second liver biopsy was done, 4 refused to continue the protocol, and 2 were considered not eligible for operation because of the nature of the tumor.
Patients distribution is shown in Table. Total mean bilirubin level before decompression was 20.8 mg/dl and after decompression 6.2 mg/dl. Biopsies were performed within an average of 21.3 days after the decompression.
Hepatocyte apoptosis index
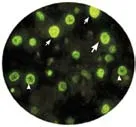
Fig. 1. A: Apoptosis on TUNEL fluorescent staining 400× HPF (white arrow: positive hepatocyte apoptosis; head arrow: negative hepatocyte apoptosis); B: Hepatocyte apoptosis index (1) before and (2) after decompression.
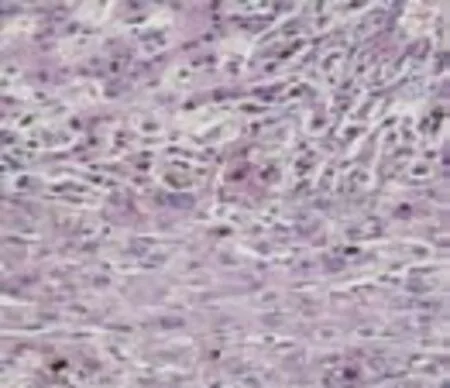
Fig. 2. A: Bile lakes on HE staining 400× HPF (bile lakes present as a brownish spot group on HE staining); B: Bile lakes index (1) before and (2) after decompression.
Hepatocyte apoptosis index increased in most patients (Fig. 1). In 10 patients, the index increased to less than 60 (normal: 5), in 7 to 60-150 (normal: 5) and in 2 to more than 900 (normal: 5). In 2 patients, however, the index did not increase. After decompression, the index decreased in 18 patients and increased in 3. On average, hepatocyte apoptosis decreased by 74.2% after bile duct decompression from 53.1 (SD 105) to 11.7 (SD 13.6) (P<0.05).
Bile lakes index
Bile lakes index varied between patients, i.e., from 3 to 69 (Fig. 2). On average, the index before decompression was 23.6. After decompression it decreased in 16 cases (12 to more than 50% and 4 to less than 50%). The index was unchanged in 1 patient and increased in 4. On average, bile lakes decreased by 53.8% from 23.6 (SD 14.8) to 10.9 (SD 6.9) (P<0.05).
Discussion
In this study, bile acid inhibition by surgical decompression resulted in improved hepatocyte apoptosis and bile lakes in patients with severe obstructive jaundice. HE staining is adequate to identify hepatocyte apoptosis morphology; however, the morphology itself does not show cell death because hepatocyte apoptotic bodiesare readily phagocyted by Kupffer cells.[10]On the other hand, TUNEL stains DNA fragmentation, a process that definitely leads to cell death. Hence, we preferred TUNEL to HE staining to assess the morphological changes of the liver due to the hepatocyte apoptosis cascade induced by bile acid accumulation.[11]
The hepatocyte apoptosis index was counted in Rappaport zone II because that zone has the highest hepatocyte density. About 300 hepatocytes were seen in this zone per high power field. To determine whether the zone of observation is crucial in studies using tissue specimens but not in the case where studies are performed on cultured cells, we used the TUNEL fluorescing method because it can differentiate clearly between bile pigment and apoptotic hepatocytes. When the TUNEL peroxidise method is used, the brown bile pigment is similar in color to hepatocyte apoptosis, thus confusing the counting.
In our study, the hepatocyte apoptosis index did increase in obstructive jaundice patients. Similar findings are also reported in animal studies showing higher levels of the hepatocyte apoptosis index.[4,12]The difference is due to different processes and rates of regeneration of hepatocytes and phagocytosis of apoptotic cell in humans and animals. In animals, the obstructive jaundice is induced acutely, whereas in our study all patients had chronic obstructive jaundice. Moreover in our study, high bilirubin levels might play a scavenger role, thus further lowering the index.[13]Kupffer cells might also be responsible for the lower index in our study which used liver tissue rather than the cultured hepatocytes inin vitrostudies.[14-17]Despite phagocyting apoptotic bodies, Kupffer cells also act as inducers of hepatocyte apoptosis.[17]They also interact with hepatocytes in modulating protein synthesis.[14-16]Obstructive jaundice might impair Kupffer cell activity and affect many liver regeneration factors. The hepatocyte apoptosis index should be perceived as liver compensation by intense proliferation to compensate for phagocytosis. The hepatocyte apoptosis index in our study increased only to a moderate level. Infection might explain the extreme index of apoptosis found in 2 patients of our study, as closer examination revealed they had hepatitis C and intraperitoneal infection.[18]
Excessive hepatocyte apoptosis would impair the balance of normal hepatocyte apoptosis and new hepatocyte regeneration. This imbalance would impair functional liver mass.[3]Suppressing hepatocyte apoptosis therefore would return the imbalance and restore liver functional capacity.
Many studies have addressed the suppression of hepatocyte apoptosis.[4,5,19]Wang et al[4]found in an animal study that decompression of the bile duct decreases hepatocyte apoptosis index significantly. Decompression depresses hepatocyte apoptosis by removal of bile acid, an hepatocyte apoptosis inducer, and also improves portal vein circulation, thus stimulating hepatocyte proliferation.[19]
In our study, we performed bile duct decompression by means of cholecystostomy instead of endoscopic retrograde cholangiopancreatography, transhepatic cholangiography, or even percutaneous cholecystostomy due to its availability during emergency and its affordibility. Our study supports the findings of Wang et al. Diminution of bile acid accumulation by decompression in humans also decreased the hepatocyte apoptosis index. Even though the index increased in three patients, this was due to external drainage tube infection.[18]Since decompression might improve the hepatocyte apoptosis index by inhibition of bile acid in the apoptosis cascade, we suggest it be performed in all patients presenting with severe obstructive jaundice.
To our knowledge, few clinical studies have addressed morphological changes of the liver after decompression of the bile duct in severe obstructive jaundice patients.[20]We studied liver morphology injury by identifying bile lakes. Bile lakes were reported to be formed in obstructive jaundice due to induction of bile acid accumulation and not in normal conditions.[21]Their appearance therefore characterized severe cholestasis. Moreover, in the chronic process, necrotic cells are rapidly removed and therefore are rarely found. Bile lakes are reported to be a good representative of necrosis in the chronic process, especially in chronic obstructive jaundice.
In our study bile lakes did increase. Their appearance in the periportal area indicates severe obstruction of the bile duct.[10]After decompression, bile lake decrement was not obvious in 9 of 21 patients. Apparently induction and inhibition of bile lakes occurred in the chronic phase. Evidence of bile lakes until the 21st day showed that 21 days of decompression did not remove them, although liver functional mass and functional liver capacity had been increased.[20]In our patients, decrement of bile lakes was due to decrement of bile acid as an inductor of necrosis following bile duct decompression. Liver functional capacity improved as a result of gradual replacement of necrotic hepatocytes with primary hepatocytes.
To date, there has been no histological classification of liver injury due to obstructive jaundice. The most commonly used has been the classification of Knodel or Metafir,[22]that is more specific for liver cirrhosis. Since bile lakes remain for a long time in obstructive jaundice, we suggest that it is more suitable and objective if we consider them in evaluating the degree of liver injury due to obstructive jaundice.
However, further studies are needed to construct a morphological classification of liver injury due to obstructive jaundice. These studies should address not only the quantity but also the quality of hepatocyte apoptosis, bile lakes, and fibrosis. The morphological classification generated should then become an objective tool to evaluate liver injury, in addition to its clinical manifestations and laboratory findings.
The primary weakness of our study lies in its design, which did not have true controls. The onegroup pretest-posttest design ("before and after design") might show weak evidence of a cause-effect relationship, but on the other hand explain the real mechanism of decompression in liver morphologic change. However, the design was acceptable since it was ethically unsound to not decompress patients just to obtain controls. It is also technically difficult to obtain appropriate controls in surgical research. Other limitations include a high "dropout rate" and no postmortem biopsy, so there was no comparison of variables in succumbing patients. The strength of this study is its real clinical setting in the treatment of severe obstructive patients who were decompressed to improve the perioperative condition.
In conclusion, our study supports the hypothesis that severe obstructive jaundice induces apoptosis of hepatocytes. Hepatocyte apoptosis index and bile lakes improve after decompression of bile duct in human, although less in humans than in animals. Decompression of the bile duct improves the liver morphological picture.
Funding:None.
Ethical approval:None.
Contributors:All authors contributed to the design and interpretation of the study and to further drafts. LTJM is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Eichhorst ST. Modulation of apoptosis as a target for liver disease. Expert Opin Ther Targets 2005;9:83-99.
2 Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol 1997;273:G1174-1188.
3 Black D, Lyman S, Heider TR, Behrns KE. Molecular and cellular features of hepatic regeneration. J Surg Res 2004;117: 306-315.
4 Wang DS, Dou KF, Li KZ, Gao ZQ, Song ZS, Liu ZC. Hepatocellular apoptosis after hepatectomy in obstructive jaundice in rats. World J Gastroenterol 2003;9:2737-2741.
5 Koyama K, Takagi Y, Ito K, Sato T. Experimental and clinical studies on the effect of biliary drainage in obstructive jaundice. Am J Surg 1981;142:293-299.
6 Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg 2002;236:17-27.
7 Clements WD, Diamond T, McCrory DC, Rowlands BJ. Biliary drainage in obstructive jaundice: experimental and clinical aspects. Br J Surg 1993;80:834-842.
8 McPherson GA, Benjamin IS, Hodgson HJ, Bowley NB, Allison DJ, Blumgart LH. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg 1984;71:371-375.
9 Hodul P, Creech S, Pickleman J, Aranha GV. The effect of preoperative biliary stenting on postoperative complications after pancreaticoduodenectomy. Am J Surg 2003;186:420-425.
10 Patel T. Apoptosis in hepatic pathophysiology. Clin Liver Dis 2000;4:295-317.
11 Rode HD, Eisel D, Frost I. Apoptosis, cell death and cell proliferation. 3rd ed. London: Roche Applied Science;2004.
12 Gonzalez B, Fisher C, Rosser BG. Glycochenodeoxycholic acid (GCDC) induced hepatocyte apoptosis is associated with early modulation of intracellular PKC activity. Mol Cell Biochem 2000;207:19-27.
13 Granato A, Gores G, Vilei MT, Tolando R, Ferraresso C, Muraca M. Bilirubin inhibits bile acid induced apoptosis in rat hepatocytes. Gut 2003;52:1774-1778.
14 Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol 2000;278:G992-999.
15 Nakeeb A, Pitt H. Biliary tract pathophysiology: pathophysiology of biliary tract obstruction. In: Blumgart L, Belghiti J, Jarnagin W, DeMatteo R, Chapman W, Buchler M, et al, ed. Surgery of the liver, biliary tract and pancreas, 4th ed. Philadelphia: Saunders; 2007:79-86.
16 Kilicoglu B, Gencay C, Kismet K, Serin Kilicoglu S, Erguder I, Erel S, et al. The ultrastructural research of liver in experimental obstructive jaundice and effect of honey. Am J Surg 2008;195:249-256.
17 Pagliara P, Carlà EC, Caforio S, Chionna A, Massa S, Abbro L, et al. Kupffer cells promote lead nitrate-induced hepatocyte apoptosis via oxidative stress. Comp Hepatol 2003;2:8.
18 Pisters PW, Hudec WA, Hess KR, Lee JE, Vauthey JN, Lahoti S, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg 2001;234:47-55.
19 Yokoyama Y, Nagino M, Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepatobiliary Pancreat Surg 2007;14:159-166.
20 Desmet VJ. Cholestasis: extrahepatic obstruction and secondary biliary cirrhosis. In: Macsween RNM, Anthony PP, Scheuer PJ, Burt AD, Portmann BC, eds. Pathology of the liver. Edinburgh: Churchill Livingstone; 1994:425-476.
21 Kanel G, Korula J. Cholestasis and biliary disorders. In: Atlas of liver pathology. 2nd ed. Philadelphia: Saunders;2005:44-62.
22 Calabrese F, Pontisso P, Pettenazzo E, Benvegnù L, Vario A, Chemello L, et al. Liver cell apoptosis in chronic hepatitis C correlates with histological but not biochemical activity or serum HCV-RNA levels. Hepatology 2000;31:1153-1159.
May 22, 2010
Accepted after revision August 13, 2010
Author Affiliations: Department of Surgery (Lalisang TJM and Sjamsuhidajat R) and Department of Anatomical Pathology (Siregar NC), Faculty of Medicine, University of Indonesia; Department of Urology, Cipto Mangunkusumo Hospital (Taher A), Jakarta, Indonesia
Toar JM Lalisang, PhD, Digestive Surgery Division, Department of Surgery, University of Indonesia, Cipto Mangunkusumo Hospital Jakarta, Jl. Diponegoro 71, Jakarta 10430, Indonesia (Tel/Fax: +62-21-3148705; Email: toar@doctor.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Life made easy: simplifying reconstruction for dual portal veins in adult right lobe live donor liver transplantation
- Effect of Danshen on apoptosis and NF-кB protein expression of the intestinal mucosa of rats with severe acute pancreatitisor obstructive jaundice
- Roles of Smad3 and Smad7 in rat pancreatic stellate cells activated by transforming growth factor-beta 1
- Salmonella typhi and gallbladder cancer: report from an endemic region
