Serine protease HtrA1 expression in human hepatocellular carcinoma
2010-06-29FengZhuLeiJinTianPingLuoGuangHuaLuoYanTanandXiHuQin
Feng Zhu, Lei Jin, Tian-Ping Luo, Guang-Hua Luo, Yan Tan and Xi-Hu Qin
Changzhou, China
Serine protease HtrA1 expression in human hepatocellular carcinoma
Feng Zhu, Lei Jin, Tian-Ping Luo, Guang-Hua Luo, Yan Tan and Xi-Hu Qin
Changzhou, China
BACKGROUND:HtrA1, a serine protease, is down-regulated in various human solid tumors. Overexpression of HtrA1 in human cancer cells inhibits cell growth and proliferationin vitroandin vivo, suggesting its possible role as a tumor suppressor.
METHODS:Immunohistochemistry was used to determine the expression of HtrA1 in 50 hepatocellular carcinoma specimens and adjacent liver tissues. The correlation between the expression of HtrA1 and the clinico-pathologic data were analyzed.
RESULTS:The levels of HtrA1 were lower in tumor tissues than in their adjacent liver tissues. Moreover, an inverse relationship was found between HtrA1 expression and the differentiation of hepatocellular carcinoma. Loss of HtrA1 was more frequently found in tumors in Edmondson grade III-IV, especially in those with venous invasion, compared to tumors in Edmondson grade I-II. Most importantly, patients with higher HtrA1 expression had a better survival rate.
CONCLUSION:All these data suggest an important role of HtrA1 in hepatocellular carcinoma development and progression, which may be a new target for its treatment.
(Hepatobiliary Pancreat Dis Int 2010; 9: 508-512)
HtrA1; hepatocellular carcinoma; serine protease; apoptosis; metastasis
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths, with increasing incidence worldwide.[1,2]It is more prevalent in Central Africa and parts of Asia, among which half are reported from China. Most patients with HCC have a very poor prognosis, with an annual number of newly diagnosed cases almost equal to the number of deaths.[3]This tragic situation stems from a lack of early diagnosis, the deteriorated condition of the cirrhotic liver from which most HCC cases develop, and the high resistance of HCC to chemotherapy.[4]Identification of novel molecular mechanisms involved in the development and progression of HCC may provide new strategies for the diagnosis and treatment of this life-threatening disease.
The HtrA1 gene was initially identified as being repressed in SV40-transformed fibroblasts (Zum-brunn & Trueb, 1996).[5]It belongs to a family of trypsinlike proteases (the HtrA family).[6]These proteases are highly conserved and occur ubiquitously in microbes, plants, and animals, sharing a highly conserved trypsinlike serine protease (SP) domain and one or two C-terminal PDZ domains. Prokaryotic HtrA proteins are well characterized in their dual roles as chaperones and proteases that degrade misfolded proteins in the cytoplasm and allow cell survival under high temperature conditions. The human HtrA family of serine proteases consists of four members: HtrA1 (L56 or PRSS11),[5,7]HtrA2/Omi,[8,9]HtrA3 (PRSP)[10]and HtrA4.[6]These members appear to be involved in several important biological processes in humans, such as growth, apoptosis, arthritis, embryogenesis, neurodegenerative and neuromuscular disorders, and cancer.[11]In particular, alterations of these proteins associated with specific tumor behavior have received increasing attention and may become new therapeutic targets in cancer.[12]
Several lines of evidence suggest that HtrA1 functions as a tumor suppressor gene in various solid tumors, such as ovarian cancer, melanoma, and lung cancer.[13-16]To our best knowledge, however, the role of HtrA1 inhepatic cancer has not been reported. In the present study, the expression of HtrA1 was investigated in depth, by means of immunohistochemistry, in a large group of human HCC specimens and their adjacent liver tissues. Correlations between the expression of HtrA1 and the clinico-pathologic data were analyzed to explore the role of HtrA1 in the development and progression of HCC.
Methods
Patients and tissue samples
Fifty patients were treated at the Third Affiliated Hospital of Soochow University (Changzhou, China) between 2003 and 2008. Their clinical data were obtained by retrospective chart review and survival was determined from the date of initial surgery. Follow-up was available for all patients. In the 50 patients who had undergone surgery were included, 42 were male (mean age 52.43±9.94 years) and 8 female (mean age 50.39±14.12 years). None of them underwent transcatheter arterial chemoembolization (TACE) or radiation as preoperative treatment. But after surgery, all patients underwent at least once TACE. The liver tissues adjacent to the tumor, 1-2 cm from its original margin, were also obtained for each patient. The same staging procedure was performed for all patients. The survival durations of the patients ranged from 1 to 72 months (mean 24.91±16.64 months). The use of these tissues in this study was approved by the Institutional Research Committee and the Ethics Committee. The clinical data of the patients are summarized in Table 1.
Histologic and laboratory data
Among the 50 patients, 42 (84%) had cirrhosis, which was proved by biopsy performed under ultrasonographic guidance, whereas 8 (16%) had no cirrhosis. Cirrhosis was caused by chronic infection with hepatitis B virus. Specimens were assessed blindly and independently by two pathologists. In case of interobserver disagreement, the final decision was reached by consensus. Cancer grade was determined histologically according to the Edmondson criteria.[17]We designated Edmondson grades I-II as low-grade HCC and grades III-IV as highgrade HCC. Histological grading was made in all patients. Tumors were graded as low-grade HCC in 66% (33/50), and as high grade HCC in 34% (17/50).
Serum α-fetoprotein (AFP) assay was performed in all patients. The AFP level was normal (≤20 μg/L) in 7 patients and abnormal (>20 μg/L) in the remaining 43 (range 43.7-4369.4 μg/L).
Immunohistochemistry
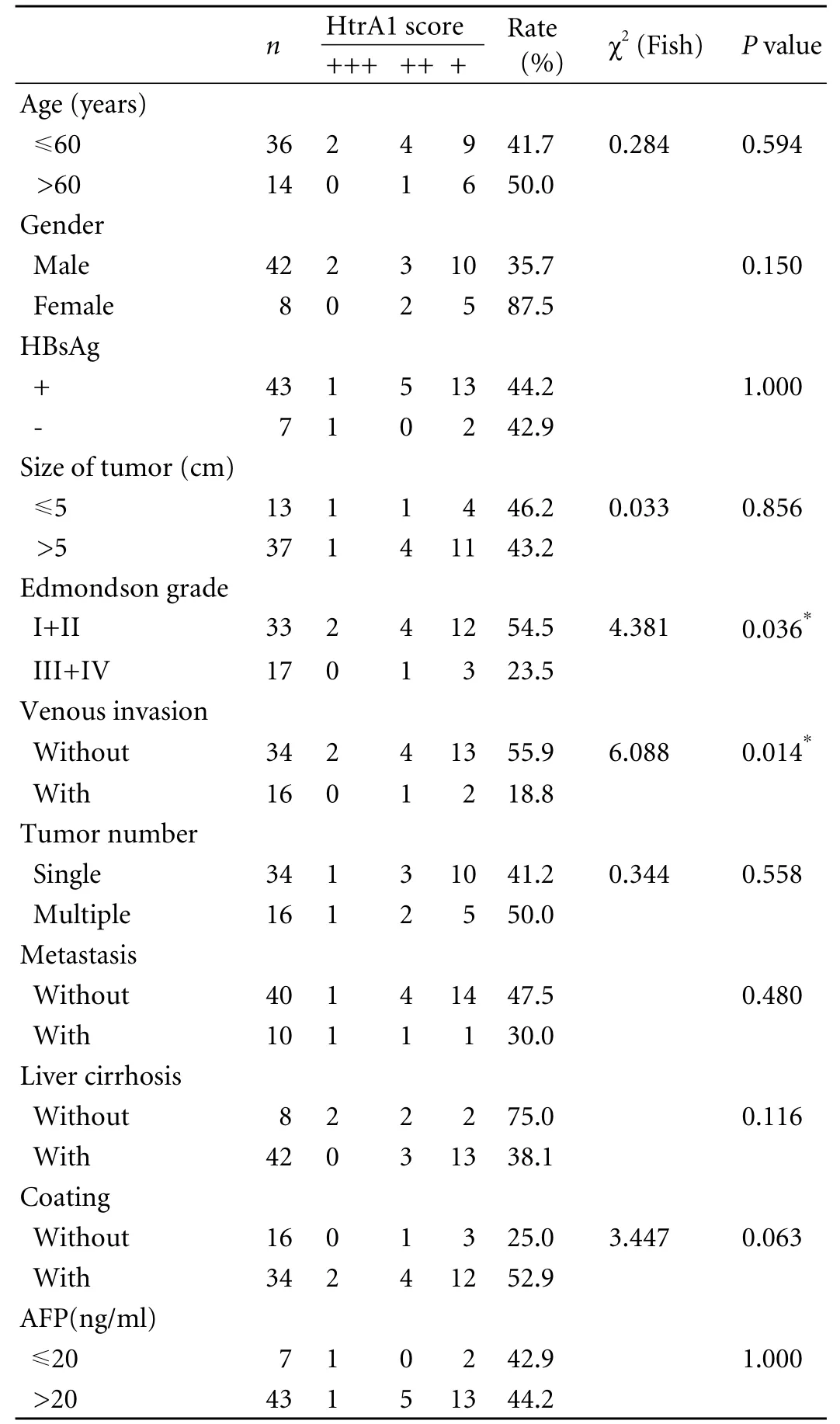
Table 1. Expression of HtrA1 in HCC and clinico-pathologic features
Tissue samples from the 50 patients with HCC were collected and fixed in 10% formalin before embedding in paraffin. Representative sections of each specimen were stained with hematoxylin-eosin. Immunohistochemistry was carried out essentially as described previously.[14]Briefly, the sections from each specimen embedded in paraffin were cut at 5-7 μm and then deparaffinized, rehydrated, and blocked with 6% nonfat milk-PBS solution for 1 hour at room temperature. Slides were incubated at 4 ℃ overnight with rabbit polyclonal HtrA1 antibody (Abcam, UK) at a 1∶50 dilution. On the next day, the slides were incubated with goat anti-rabbit biotinylated antibody (Vector Laboratories; Burlingame,CA) at a 1∶200 dilution for 1 hour. The slides were subsequently processed by the ABC method (Vector Laboratories) for 30 minutes at RT. Novared (Vector Laboratories) was used as the final chromogen and hematoxylin was used as the nuclear counterstain. The expression level of HtrA1-stained cells per field (×250) under a light microscope was calculated and compared in different specimens by two separate observers in a double-blind fashion and was described as a score of 0 (<1% positive cells), 1 (1%-10% positive cells), 2 (10%-20% positive cells), or 3 (>20% positive cells). An average of 22 fields were counted for each specimen.

Fig. 1. A: High cytoplasmic expression of HtrA1 in HCC without venous invasion (original magnification ×400).B:Low expression of HtrA1 in HCC with venous invasion (original magnification ×400). C: High cytoplasmic expression of HtrA1 in liver tissue adjacent to a tumor (original magnification ×400).
Statistical analysis
The Spearman's rank-order correlation test was used to assess the relationship between clinical parameters and immunohistochemistry data. Differences between the groups of patients were compared by the Mann-WhitneyUtest or the Chi-square test.Pvalues less than 0.05 were regarded as statistically significant in twotailed tests. SPSS software (version 16.0, SPSS, Chicago) was used for the statistical analysis. Life tables and the Kaplan-Meier analysis were used for survival analysis. A difference was considered statistically significant when thePvalue was less than 0.05.
Results
Immunohistochemical analysis of HtrA1 protein expression was made on 50 HCC specimens and adjacent liver tissues (Table 2). All of the neoplastic cells displayed cytoplasmic immunoreactivity. The positive-staining cells exhibited homogeneous brown staining. Part of the nuclei of HCC cells, vascular endothelial cells, and bile duct epithelial cells were also stained positive. Examples of positive immunostaining for HtrA1 are shown in Fig. 1. The expression of HtrA1 was analyzed with respect to the detailed clinico-pathologic information about the50 patients.
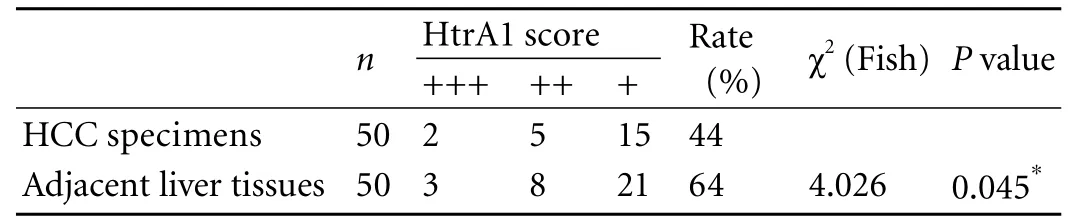
Table 2. Expression of HtrA1 in HCC specimens and adjacent liver tissues
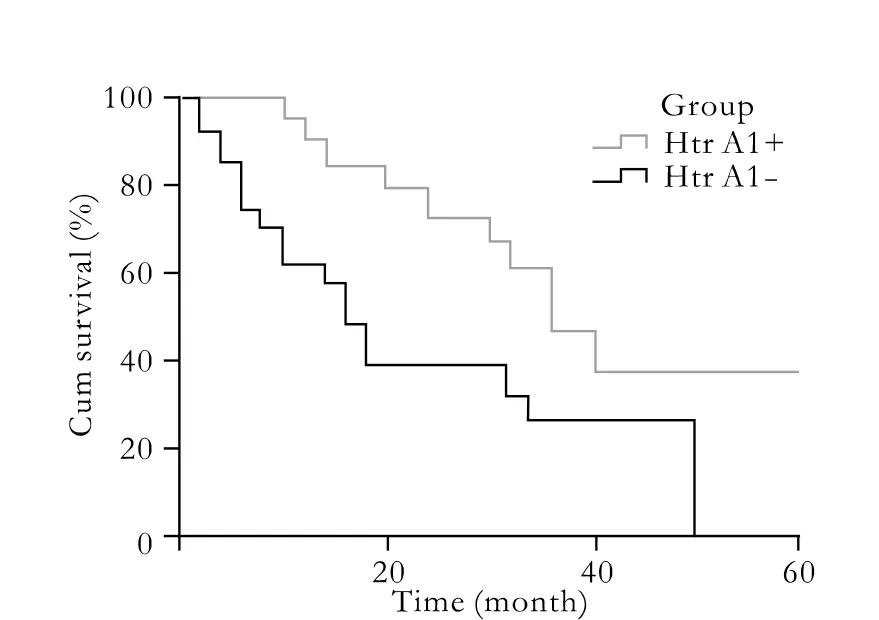
Fig. 2. Comparison of overall survival rates of patients with positive and negative staining for HtrA1 in HCC specimens.
Positive staining was observed in 44% (22/50) of HCC specimens, significantly lower than 64% (32/50) in adjacent liver tissues. Moreover, a significant correlation was recorded when comparing the HtrA1 immunohistochemical profiles between different Edmondson grades. HtrA1 expression was significantly lower in grade III+IV HCC specimens than in grade I+II samples. Notably, HtrA1 expression was also significantly lower in tumors with venous invasion than in those without venous invasion. The expression level of HtrA1 in HCC was inversely correlated with the degree of cell differentiation.
Most importantly, the data from long-term followup demonstrated a close relationship between HtrA1 expression and survival rate. In HtrA1-positive cases,the 3-year survival rate was 46%, with a median survival time of 35.5 months, while in HtrA1-negative cases, this rate was only 26%, with a median survival time of 15.6 months (Fig. 2).
However, no correlation was found between HtrA1 expression and other clinical features, such as age, gender, HBV infection, tumor number, tumor size, cirrhosis status, metastasis, or AFP level.
Discussion
In the present study, we evaluated the immunohistochemical expression of the serine protease HtrA1 in 50 HCC specimens in order to evaluate the possible impact of this protease in the process of tumor development and progression. The immunohistochemical method was chosen because it avoids the contamination by nonneoplastic cells that constantly affects both Western blot analyses and nucleic acid-based approaches.
Our results showed that HtrA1 expression was downregulated in tumor tissues compared to tissues adjacent to the tumor, suggesting loss of HtrA1 may be involved in the development of HCC. Similar to our results, HtrA1 is a down-regulated gene in most ovarian cancers. Down-regulation of HtrA1 by anti-sense transfection promotes anchorage-independent growth, while heterologous overexpression of HtrA1 in ovarian cell lines induces cell death.[13]Overexpression of HtrA1 in a highly invasive metastatic melanoma cell line inhibits proliferationin vitroand tumor growthin vivo.[14]Moreover, our data revealed an inverse relationship between HtrA1 expression and HCC differentiation, which suggests that HtrA1 down-regulation may also promote tumor progression in HCC. Notably, univariate analysis demonstrated a significant correlation between HtrA1 immunohistochemical profile and Edmondson grades of HCC. HtrA1 expression was significantly lower in Edmondson grade III+IV HCC specimens, especially in those with venous invasion. Consistently, HtrA1 expression has been found to be frequently downregulated in metastatic foci of various tumors compared to primary tumors, such as melanoma,[14]prostate cancer,[18,19]sarcoma,[20]and lung cancer.[16]All these data provide a strong evidence of the involvement of down-regulated HtrA1 in tumor metastasis, including hepatic carcinoma. Most importantly, our data showed that patients with higher HtrA1 expression had a better survival rate, indicating HtrA1 as a novel prognostic factor in HCC.
However, the mechanism of HtrA1 down-regulation during tumor development is still unknown. It has been shown that in primary ovarian cancer a decrease of HtrA1 expression is correlated with loss of heterozygosity of the HtrA1 gene.[13]In 2007, using fluorescencein situhybridization, Barbara Lipinska localized the Syrian hamster HtrA1 gene to chromosome 2, region qb3-4.[21]This region has been shown by Papa et al[22]to undergo a nonrandom deletion upon prolonged estradiol treatment. It is possible that the observed reduced level of HtrA1 gene expression could be due, at least partially, to chromosomal aberration. Epigenetic inactivation by hypermethylation is another possible mechanism which can contribute to the loss expression of HtrA1.
So far, the exact mechanism under which loss of HtrA1 regulates tumor development and progression is also largely unknown. A recent paper[23]has indicated a role of HtrA1 in inhibition of the TGF-beta pathway, while the role of this pathway during HCC progression is well documented.[24]Therefore, it is reasonable to speculate that down-regulation of HtrA1 leads to weakened inhibition of TGF-beta signaling, which finally contributes to the development and progression of HCC. However, this hypothesis needs further study. On the other hand, it has been documented that over-expression of HtrA1 in ovarian cancer cells induces apoptosis.[13]Altered regulation of apoptosis is one recognized event contributing to HCC progression.[25-28]Thus, loss of HtrA1 expression may result in deregulated apoptotic events which can also promote tumor development and progression.
In conclusion, our data strongly suggests an important role of loss of HtrA1 in the pathogenesis of HCC, and highlights its possible role as a novel prognostic factor for HCC. However, additional studies with large numbers of patients are required to underpin the prognostic impact of HtrA1 in HCC. Nevertheless, the crucial role of loss of HtrA1 in the development and progression of HCC supports the idea that restoring HtrA1 expression may serve as a new therapeutic strategy for HCC.In vitroandin vivostudies are currently under way in our laboratory to test this hypothesis.
Funding:None.
Ethical approval:Not needed.
Contributors:ZF proposed the study and wrote the first draft. JL analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZF is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trendsin worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol 2009;3:353-367.
2 Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int 2008;7: 237-257.
3 Koorey D. Hepatocellular carcinoma: prevention, detection and treatment in the real world. Intern Med J 2007;37:513-515.
4 Pleguezuelo M, Marelli L, Misseri M, Germani G, Calvaruso V, Xiruochakis E, et al. TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 2008;8: 1623-1641.
5 Zumbrunn J, Trueb B. Primary structure of a putative serine protease specific for IGF-binding proteins. FEBS Lett 1996;398: 187-192.
6 Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell 2002;10:443-455.
7 Hu SI, Carozza M, Klein M, Nantermet P, Luk D, Crowl RM. Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J Biol Chem 1998;273:34406-34412.
8 Faccio L, Fusco C, Chen A, Martinotti S, Bonventre JV, Zervos AS. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J Biol Chem 2000;275: 2581-2588.
9 Gray CW, Ward RV, Karran E, Turconi S, Rowles A, Viglienghi D, et al. Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur J Biochem 2000;267:5699-5710.
10 Nie GY, Hampton A, Li Y, Findlay JK, Salamonsen LA. Identification and cloning of two isoforms of human hightemperature requirement factor A3 (HtrA3), characterization of its genomic structure and comparison of its tissue distribution with HtrA1 and HtrA2. Biochem J 2003;371:39-48.
11 Canfield AE, Hadfield KD, Rock CF, Wylie EC, Wilkinson FL. HtrA1: a novel regulator of physiological and pathological matrix mineralization? Biochem Soc Trans 2007;35:669-671.
12 Chien J, Campioni M, Shridhar V, Baldi A. HtrA serine proteases as potential therapeutic targets in cancer. Curr Cancer Drug Targets 2009;9:451-468.
13 Chien J, Staub J, Hu SI, Erickson-Johnson MR, Couch FJ, Smith DI, et al. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene 2004;23:1636-1644.
14 Baldi A, De Luca A, Morini M, Battista T, Felsani A, Baldi F, et al. The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene 2002;21:6684-6688.
15 Shridhar V, Sen A, Chien J, Staub J, Avula R, Kovats S, et al. Identification of underexpressed genes in early- and latestage primary ovarian tumors by suppression subtraction hybridization. Cancer Res 2002;62:262-270.
16 Esposito V, Campioni M, De Luca A, Spugnini EP, Baldi F, Cassandro R, et al. Analysis of HtrA1 serine protease expression in human lung cancer. Anticancer Res 2006;26:3455-3459.
17 Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503.
18 Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol 2004;22:2790-2799.
19 Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 2005;8:393-406.
20 Segal NH, Pavlidis P, Noble WS, Antonescu CR, Viale A, Wesley UV, et al. Classification of clear-cell sarcoma as a subtype of melanoma by genomic profiling. J Clin Oncol 2003; 21:1775-1781.
21 Zurawa-Janicka D, Kobiela J, Stefaniak T, Wozniak A, Narkiewicz J, Wozniak M, et al. Changes in expression of serine proteases HtrA1 and HtrA2 during estrogen-induced oxidative stress and nephrocarcinogenesis in male Syrian hamster. Acta Biochim Pol 2008;55:9-19.
22 Papa D, Li SA, Li JJ. Comparative genomic hybridization of estrogen-induced ectopic uterine-like stem cell neoplasms in the hamster kidney: nonrandom chromosomal alterations. Mol Carcinog 2003;38:97-105.
23 Oka C, Tsujimoto R, Kajikawa M, Koshiba-Takeuchi K, Ina J, Yano M, et al. HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development 2004;131: 1041-1053.
24 Mazzocca A, Fransvea E, Lavezzari G, Antonaci S, Giannelli G. Inhibition of transforming growth factor beta receptorikinase blocks hepatocellular carcinoma growth through neoangiogenesis regulation. Hepatology 2009;50:1140-1151.
25 Haurie V, Ménard L, Nicou A, Touriol C, Metzler P, Fernandez J, et al. Adenosine triphosphatase pontin is overexpressed in hepatocellular carcinoma and coregulated with reptin through a new posttranslational mechanism. Hepatology 2009;50:1871-1883.
26 Zhang X, Frank AC, Gille CM, Daucher M, Kabat J, Becker S, et al. Altered regulation of extrinsic apoptosis pathway in HCV-infected HCC cells enhances susceptibility to mapatumumabinduced apoptosis. Hepatol Res 2009;39:1178-1189.
27 Shi YH, Ding ZB, Zhou J, Qiu SJ, Fan J. Prognostic significance of Beclin 1-dependent apoptotic activity in hepatocellular carcinoma. Autophagy 2009;5:380-382.
28 Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernández M, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its proapoptotic activity. J Hepatol 2008;49:965-976.
February 10, 2010
Accepted after revision May 3, 2010
Author Affiliations: Department of Hepatobiliary Surgery (Zhu F, Jin L, Luo TP and Qin XH), Comprehensive Laboratory (Luo GH), and Department of Pathology (Tan Y), Third Affiliated Hospital, Soochow University, Changzhou 210003, China
Feng Zhu, MD, PhD, Department of Hepatobiliary Surgery, Third Affiliated Hospital, Soochow University, Changzhou 210003, China (Tel: 86-519-86181348; Fax: 86-519-86180814; Email: zhufeng90med@sohu.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Advances in prognostic factors in acute pancreatitis: a mini-review
- Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma
- Simultaneous breast and ovarian metastasis from gallbladder carcinoma
- An eight-year journey of Hepatobiliary & Pancreatic Diseases International
- Interferon and lamivudine combination therapy versus lamivudine monotherapy for hepatitis B e antigen-negative hepatitis B treatment: a meta-analysis of randomized controlled trials
