High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure in patients with chronic hepatitis B
2010-06-29LuWenWangHuiChenandZuoJiongGong
Lu-Wen Wang, Hui Chen and Zuo-Jiong Gong
Wuhan, China
High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure in patients with chronic hepatitis B
Lu-Wen Wang, Hui Chen and Zuo-Jiong Gong
Wuhan, China
BACKGROUND:Liver failure in chronic hepatitis B (CHB) patients is a severe, life-threatening condition. Intestinal endotoxemia plays a significant role in the progress to liver failure. High mobility group box-1 (HMGB1) protein is involved in the process of endotoxemia. Regulatory T (Treg) cells maintain immune tolerance and contribute to the immunological hyporesponsiveness against HBV infection. However, the roles of HMGB1 and Treg cells in the pathogenesis of liver failure in CHB patients, and whether HMGB1 affects the immune activity of Treg cells are poorly known at present, and so were explored in this study.
METHODS:The levels of HMGB1 expression were detected by ELISA, real-time RT-PCR, and Western blotting, and the percentage of CD4+CD25+CD127lowTreg cells among CD4+cells was detected by flow cytometry in liver failure patients with chronic HBV infection, CHB patients, and healthy controls. Then, CD4+CD25+CD127lowTreg cells isolated from the peripheral blood mononuclear cells from CHB patients were stimulated with HMGB1 at different concentrations or at various intervals. The effect of HMGB1 on the immune activity of Treg cells was assessed by a suppression assay of the allogeneic mixed lymphocyte response. The levels of forkhead box P3 (Foxp3) expression in Treg cells treated with HMGB1 were detected by RT-PCR and Western blotting.
RESULTS:A higher level of HMGB1 expression and a lower percentage of Treg cells within the population of CD4+cells were found in liver failure patients than in CHB patients (82.6±20.1 μg/L vs. 34.2±13.7 μg/L; 4.55±1.34% vs. 9.52± 3.89%, respectively). The immune activity of Treg cells was significantly weakened and the levels of Foxp3 expression were reduced in a dose- or time-dependent manner when Treg cells were stimulated with HMGB1in vitro.CONCLUSIONS:The high level of HMGB1 and the low percentage of Treg cells play an important role in the pathogenesis of liver failure in patients with chronic HBV infection. Moreover, HMGB1 can weaken the immune activity of Treg cells. It is suggested that effectively inhibiting HMGB1 expression could be a feasible way to treat liver failure by suppressing endotoxemia and enhancing Treg cell activity.
(Hepatobiliary Pancreat Dis Int 2010; 9: 499-507)
high mobility group box-1 protein; regulatory T cells; chronic hepatitis B; liver failure
Introduction
HBV infection is a major public health problem worldwide, especially in China.[1-3]Some patients with chronic hepatitis B (CHB) progress towards acute or subacute-on-chronic liver failure, which is a severe life-threatening condition with a high fatality, and effective treatment is lacking.[4]A lot of evidences indicate that intestinal endotoxemia plays a significant role in the occurrence and progression of liver failure in CHB patients.[5-8]The gut is a vast pool of bacteria and endotoxin (lipopolysaccharide, LPS). When large quantities of endotoxin are produced by overgrowth of Gram-negative bacteria in the gut, and/or bacteria are translocated into the peritoneal cavity due to the high permeability of the intestinal wall, endotoxemia is generated because the levels of endotoxin in the portal vein surpass the hepatic capacity for endotoxin scavenging. Then, endotoxin spills over into the systemic circulation, which aggravates liver injury and induces liver failure.[5-7]
Previous studies have shown that high mobility group box-1 (HMGB1) protein, a late inflammatory mediator, is involved in the progression of endotoxemia.[9-12]HMGB1 was originally identified as a DNA-binding protein that functions as a structural co-factor criticalfor proper transcriptional regulation and nucleosome stabilization.[13-15]In addition, HMGB1 is released into the extracellular milieu from activated macrophages, immuno-stimulated gut epithelial cells, and necrotic or damaged cells in a delayed manner relative to the secretion of other proinflammatory mediators, such as TNF-α and IL-1, and contributes to the pathogenesis of diverse inflammatory and infectious disorders.[16-18]A growing number of studies indicate that HMGB1 is a successful therapeutic target in experimental models of ischemia-reperfusion injury, acute respiratory distress syndrome, rheumatoid arthritis, sepsis, and cancer.[19-23]Since intestinal endotoxemia is closely related to the progression of liver failure in CHB patients, it is hypothesized that HMGB1 as an important inflammatory mediator of endotoxemia likely plays a significant role in the pathogenesis of liver failure in CHB patients.
Regulatory T (Treg) cells are a specialized subpopulation of CD4+T cells that are responsible for the balance of immune responses, maintaining peripheral tolerance, preventing autoimmune diseases, and limiting chronic inflammatory diseases.[24-26]Several studies have shown that Treg cells play an important role in maintaining immune tolerance and contributing to immunological hyporesponsiveness against HBV infection.[27-30]Therefore, Treg cells may limit liver injury by controlling inflammation and reducing the incidence of liver failure in CHB patients. However, the roles of Treg cells in the pathogenesis of liver failure in CHB patients are poorly known at present.
In the present study, the levels of HMGB1 expression and the percentage of CD4+CD25+CD127lowTreg cells within the CD4+cell fraction were assessed in liver failure patients with chronic HBV infection to investigate the roles of HMGB1 and Treg cells in the pathogenesis of liver failure. In addition, by suppression assay of CD4+CD25+CD127lowTreg cells isolated from the peripheral blood mononuclear cells (PBMCs) of CHB patients after treatment with HMGB1 at various concentrations and times, the effects of HMGB1 on immune activity of Treg cells were also studied.
Methods
Isolation of PBMCs
Patients were diagnosed with CHB and liver failure according to the criteria from the program of prevention and cure for viral hepatitis amended at the Xi'an meeting held in September 2000 by the Chinese Society of Infectious Disease and Parasitology and the Chinese Society of Hepatology of the Chinese Medical Association.[31]The major diagnostic criteria included basic conditions, and serum bilirubin in the liver failure index ten times higher than normal (17.1 μmol/L), i.e. above 171.1 μmol/L. Two additional conditions were at least one liver failure index or more than one of the following indices: 1) prothrombin activity <40% or international normalized ratio ≥1.5; 2) hepatic encephalopathy; 3) ascites; 4) progressive reduction of liver size; and 5) hepatorenal syndrome. Patients coinfected with HIV, HAV, HCV, HDV, or HEV were excluded from this study. Patients and controls who were immunocompromised or pregnant and patients who had received antiviral or immunomodulatory treatment against HBV during the last 6 months before blood sampling were also excluded. Patients with autoimmune hepatitis, alcohol abuse (≥80 g ethanol/day), and HCC diagnosed within 3 months were not enrolled. Informed consent was obtained from the all participants before blood donation. The study protocol was approved by the local ethics committee.
Heparinized peripheral blood samples were obtained from 15 liver failure patients with chronic HBV infection, 20 CHB patients, and 10 healthy controls. PBMCs from all participants were obtained by density gradient centrifugation using Lydroxypropylmethyl Cellulose (Boster Biotechnology Co., Ltd., Wuhan, China) according to the manufacturer's protocols.
Detection of HMGB1 expression
The serum samples of 15 liver failure patients with chronic HBV infection, 20 CHB patients and 10 healthy controls were isolated with routine methods, and stored in aliquots at -80 ℃ in microfuge tubes until assay. Serum HMGB1 levels were measured using the HMGB1 ELISA Kit II (Shino-Test Corp., Kanagawa, Japan) according to the manufacturer's recommendations. In brief, 10 μl of standards, and samples or controls supplemented with 100 μl diluent were added to the wells in microtiter plates, and incubated for 24 hours at 37 ℃. After washing, 100 μl/well of antihuman HMGB1 peroxidase-conjugated monoclonal antibody was added to the plates and incubated at room temperature for 2 hours. After washing, the chromogen 3, 3', 5, 5'-tetramethylbenzidine was added to each well to perform enzyme reaction for 30 minutes at room temperature before stopping by the addition of stop solution (0.35 mol/L Na2SO4). The absorbance was read at 450 nm, and the results were calculated using a calibration curve prepared from standards.
HMGB1 mRNA levels in PBMC samples from enrolled patients and healthy controls were detected by real-time RT-PCR. Briefly, RNA was isolated from freshPBMCs using TRIzol reagent (Invitrogen Co., USA). Two micrograms total RNA was reverse-transcribed into cDNA using a random primer hexamer (Promega Corp., USA) and MultiScribeTMReverse Transcriptase (Applied Biosystems, USA). Gene expression was measured in real-time with the LightCycler (Roche, Switzerland) using primers and the QuantiTect SYBR green PCR Kit (Qiagen China Co., Ltd., Shanghai) according to the manufacturer's protocols. GAPDH was used as an internal control. The primers for HMGB1 were 5'-GCC TCC TTC GGC CTT CTT-3' and 5'-ACA GGC CAG GAT GTT CTC CTT T-3'; and for GAPDH were 5'-CCA CAT CGC TCA GAC ACC AT-3' and 5'-CCA GGC GCC CAA TAC G-3'. LightCycler collected data automatically and analyzed the value of threshold cycle (Ct). The fold change of HMGB1 mRNA expression in the patients with liver failure or CHB relative to control was detected using the 2-ΔΔCtmethod.[32]
HMGB1 protein levels in liver tissues were assayed by Western blotting. Briefly, liver samples were obtained from enrolled patients and controls by percutaneous Menghini needle extraction. Samples (100 mg) were crushed in a liquid nitrogen-cooled grinding bowl and then lysed in cold RIPA buffer (Pierce Biotechnology, Inc., USA) supplemented with HaltTMProtease Inhibitor Cocktail (Pierce Biotechnology). Protein extracts (50 μg) were subjected to 12% SDS-PAGE and then transferred to a Protran® nitrocellulose membrane (Schleicher & Schuell BioScience GmbH, Germany). The membrane was incubated with a rabbit anti-HMGB1 primary antibody (Abcam plc., UK), then with a secondary goat anti-rabbit horseradish peroxidase-conjugated antibody (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China), and finally assessed by chemiluminescence using the Enhanced NuGloTMChemiluminescent Substrate Kit (Alpha Diagnostic Intl. Inc., USA) followed by autoradiography and densitometric analysis. Membranes were also probed for β-actin as additional loading control.
Flow cytometric analysis of CD4+CD25+CD127lowTreg cells
PBMCs isolated from patients and controls were immediately frozen in RPMI-1640 (Gibco BRL, USA) containing 20% fetal calf serum (Gibco) and 10% dimethyl sulfoxide and stored at -135 ℃ until further use. PBMCs from different time points were tested simultaneously to avoid interassay variations in individual patients. The PBMCs (1×106) were incubated for 30 minutes at room temperature with fluorochromeconjugated antibodies specific for the surface markers CD4, CD25, and CD127 (anti-CD4-FITC, anti-CD25-PE-Cy5, and anti-CD127-PE, each 10 μl; BD Biosciences, USA). The cells were washed twice with phosphatebuffered saline (PBS), and made into a unicell suspension with a small amount of PBS. Flow cytometric analysis was performed using a FACSCaliburTMcytometer (BD Immunocytometry Systems, USA) as described elsewhere.[33,34]For CD25 and CD127 antibodies, isotypic matched control antibodies (IgG2a-PE-Cy5 and IgG1-PE; BD Biosciences) were used to determine the level of background staining. CD4+T cells were gated in PBMCs, and then CD25+CD127lowT lymphocytes, as a phenotype of Treg cells, were gated in CD4+T cells to analyze the proportion of Treg cells relative to CD4+T cells as described elsewhere.[35]
Isolation of CD4+CD25+CD127lowTreg cells
The PBMCs freshly isolated from CHB patients were enriched for CD4+T cells using a BD IMagTMHuman CD4 T Lymphocyte Enrichment Kit (BD Biosciences, USA). Briefly, a negative selection was performed using BD IMagTMstreptavidin particles with a cocktail of biotinylated monoclonal antibodies (mAbs) that recognizes antigens expressed on erythrocytes, platelets, granulocytes, monocytes, B cells, and CD8+T cells to enrich for the CD4+T lymphocytes. The CD4+T cells were then stained with fluorescence-labeled mAbs directed against human CD4, CD25, and CD127 (anti-CD4-FITC, anti-CD25-PE-Cy5, and anti-CD127-PE; BD Biosciences) for 30 minutes at 4 ℃. CD4+CD25+CD127lowand CD4+CD25lowCD127highsubsets were isolated using a BD FACSAriaTMcell sorter (BD Biosciences). The purity of FACS-sorted cell fractions was routinely 98%-99% for each T-cell fraction.
Stimulation of Treg cells with HMGB1
CD4+CD25+CD127lowTreg cells (1×106/ml) were seeded into 96-well plates coated with anti-CD3 (2 μg/ ml; Becton, Dickinson and Co., USA) in 10% FCS-RPMI 1640 complete culture medium (Gibco BRL, USA). Anti-CD28 (2 μg/ml; Becton, Dickinson and Company, USA) was added to the cells which were cultured for 24 hours, and then were stimulated with 100 μg/L HMGB1 for 12, 24, 36, or 48 hours, with various concentrations of HMGB1 (10, 50, 100, or 200 μg/L) for 24 hours. Every well had ten replicates and unstimulated cells were set as negative controls. Recombinant human HMGB1 was from Sigma-Aldrich Chemical Corp., USA at a greater purity more than 90%, dissolved in sterile PBS, and its concentration was confirmed by ELISA.
Suppression assay
A one-way mixed lymphocyte culture wasperformed in round-bottom 96-well microtiter plates. Briefly, 5×104CD4+CD25+CD127lowTreg cells treated with HMGB1 at different concentrations or at various intervals, 1×105autologous PBMCs from the same cell source as the CD4+CD25+CD127lowcells (responders), and 1×105allogeneic irradiated CD3-depleted PBMCs from a third party (stimulators) were incubated together for 7 days at 37 ℃ in 5% CO2. The allogeneic PBMCs were depleted of T cells using StemSep® Human CD3+T Cell Depletion (StemCell Technologies, Inc., Canada) according to the manufacturer's protocol and were irradiated with 40 Gy. Sixteen hours before the end of the incubation, 1 μCi3[H]-thymidine (Amersham GE, USA) was added to each well. Plates were harvested using a Tomtec® cell harvester (Tomtec Inc., USA) and3[H]-thymidine incorporation was measured using a MicroBeta® liquid scintillation counter (PerkinElmer Inc., USA), and the data were expressed as counts per minute (cpm). Experiments had ten replicates.
Detection of forkhead box P3 (Foxp3) expression
Foxp3 mRNA in Treg cells was detected by real-time RT-PCR. Briefly, RNA was isolated from CD4+CD25+CD127lowTreg cells stimulated with HMGB1 using TRIzol reagent. One microgram total RNA was reverse transcribed into cDNA using a random primer hexamer. Foxp3 mRNA expression was determined in real-time PCR with LightCycler using primers and a QuantiTect SYBR green PCR kit. The primers for Foxp3 were 5'-GAG AAG CTG AGT GCC ATG CA-3' and 5'-AGG AGC CCT TGT CGG ATG AT-3'. GAPDH served as an internal control. The 2-ΔΔCtmethod was used to analyze the fold change of Foxp3 mRNA expression relative to control.
Foxp3 protein in Treg cells was assayed by Western blotting. Briefly, CD4+CD25+CD127lowTreg cells stimulated with HMGB1 (1×106/ml) were lysed in RIPA buffer supplemented with protease inhibitor. Protein extracts (50 μg) were subjected to 12% SDS-PAGE and then transferred to a Protran® nitrocellulose membrane. The membrane was incubated with a rabbit anti-Foxp3 primary antibody (Abcam plc., UK), and then with a secondary goat anti-rabbit horseradish peroxidaseconjugated antibody after washing in TBST, and finally detected by chemiluminescence using a commercial kit. β-actin was used as additional loading control.
Statistical analysis
All data are presented as mean±SEM. Calculations were performed with SPSS version 13.0 (SPSS, Chicago, IL, USA). Differences among groups were assessed by unpaired Student'sttest and one-way ANOVA. APvalue less than 0.05 was considered to be statistically significant.
Results
Higher expression of HMGB1 in liver failure patients
To determine whether HMGB1 (a late inflammation mediator of endotoxemia) plays a role in the pathogenesis of liver failure caused by chronic HBV infection, the levels of HMGB1 in serum, PBMCs, and liver tissue from liver failure patients with chronic HBV infection, CHB patients, and healthy controls were evaluated by ELISA, real-time RT-PCR, and Western blotting. The results showed that the serum HMGB1 levels were 82.6±20.1 μg/L in liver failure patients, 34.2± 13.7 μg/L in CHB patients, and 27.8±6.5 μg/L in healthy controls. Serum HMGB1 levels were greater in the liver failure patients than in the CHB patients (t=8.48,P<0.01), and the serum HMGB1 levels did not differ between the CHB patients and healthy controls (t=1.73,P>0.05) (Fig. 1A). The results of RT-PCR and Western blotting furtherconfirmed a higher expression of HMGB1 mRNA in PBMCs and HMGB1 protein in the liver tissues of the liver failure patients than in the CHB patients (5.21±0.42 vs. 1.78±0.18,t=29.43,P<0.01; 4.86±1.07 vs. 1.43±0.35,t=13.46,P<0.01, respectively). HMGB1 expression levels of CHB patients were higher than healthy controls, but the difference was not statistically significant (Fig. 1B and 1C).
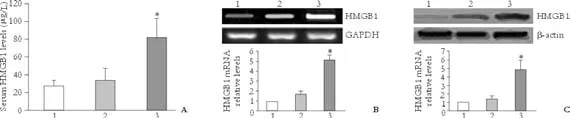
Fig. 1. HMGB1 levels in liver failure patients with chronic HBV infection. A: Serum HMGB1 levels detected by ELISA in liver failure patients with chronic HBV infection, CHB patients, and healthy controls. B: HMGB1 mRNA expression levels in PBMCs analyzed by real-time RT-PCR (graph) in enrolled patients and healthy controls, and a representative RT-PCR specific for HMGB1 and GAPDH (internal control) are shown in the upper panel. C: HMGB1 protein expression levels in liver tissue analyzed by Western blotting in enrolled patients and healthy controls. 1: healthy controls; 2: CHB patients; 3: liver failure patients with chronic HBV infection. *: P<0.01, compared with CHB patients.
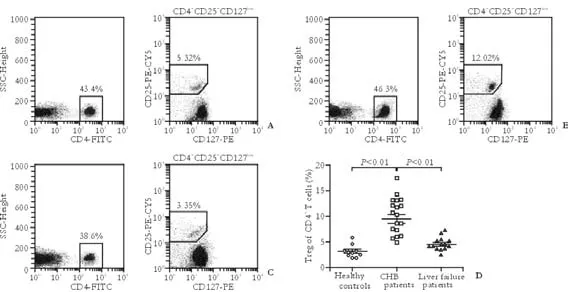
Fig. 2. Percentage of CD4+CD25+CD127lowTreg cells within the CD4+cell fraction in liver failure patients with chronic HBV infection. A-C: Typical dot plots obtained by flow cytometry using antibodies against CD4, CD25, CD127 from a representative liver failure patient, a representative CHB patient, and a representative healthy control respectively. CD4+T cells were gated in PBMCs, and then CD25+CD127lowT lymphocytes, a phenotype of Treg cells, were gated in CD4+T cells to analyze the proportion of Treg cells relative to CD4+T cells. D: CHB patients showed a higher percentage of Treg cells within their population of CD4+T cells in PBMCs compared with healthy controls, while liver failure patients with chronic HBV infection showed a decreased proportion of Treg cells relative to CD4+T cells compared with CHB patients. Bar represents median percentage of Tregs.
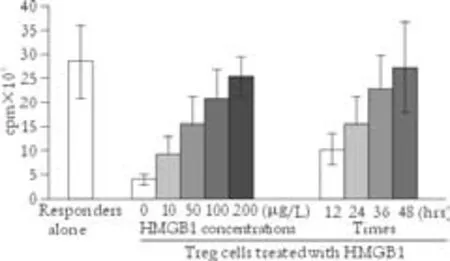
Fig. 3. Suppression assay of allogeneic MLR. 5×104CD4+CD25+CD127lowTreg cells treated with HMGB1 at different concentrations or at various intervals were combined with 1×105autologous PBMCs as responders, and 1×105allogeneic anti-CD3 depleted, irradiated third-party PBMCs as stimulators and incubated together for 7 days at 37 ℃ in 5%CO2. Sixteen hours before the end of incubation, 1 μCi3[H]-thymidine was added to each well. Plates were harvested and data was detected using a liquid scintillation counter. The results are presented as counts per minute (cpm), and showed that the ability of CD4+CD25+CD127lowTreg cells to suppress an allogeneic MLR was significantly weakened after treatment with HMGB1 compared with untreated Treg cells. Moreover, the reduced ability of Treg cells to suppress the MLR was related to HMGB1 treatment in a dose- and time-dependent manner.
Lower proportion of Treg cells in liver failure patients
Recently, a novel combined panel of biomarkers, CD4+CD25+CD127low, was confirmed by intracellular staining of Foxp3 antigen to be effective in identifying Treg cells.[36]To investigate the roles of Treg cells in the pathogenesis of liver failure caused by chronic HBV infection, the percentage of CD4+CD25+CD127lowTreg cells within the CD4+cell fraction among the PBMCs from liver failure patients with chronic HBV infection, CHB patients, and healthy controls was determined by this novel flow-cytometric approach. Representative dot plots obtained by flow cytometry from enrolled patients and healthy controls are shown in Fig. 2A-C. CHBpatients had a higher percentage of Treg cells within their population of CD4+T cells in PBMCs than healthy controls (9.52±3.89% vs. 3.21±1.21%,t=4.97,P<0.01; Fig. 2D). But in liver failure patients with chronic HBV infection, a decreased proportion of Treg cells relative to CD4+T cells was found compared with CHB patients (4.55±1.34% vs. 9.52±3.89%,t=4.73,P<0.01).
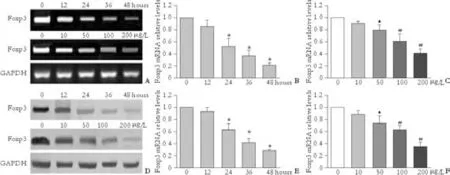
Fig. 4. Changes of Foxp3 expression after stimulating Treg cells with HMGB1. *: P<0.01, compared with 12 hours; ▲: P>0.05, #: P<0.01, compared with 10 μg/L. A: RT-PCR results for Foxp3 mRNA in Treg cells treated with HMGB1 at various intervals or at different concentrations. B: Detection of Foxp3 mRNA in Treg cells treated with 100 μg/L HMGB1 for 12, 24, 36 and 48 hours by real-time RTPCR. Untreated Treg cells highly expressed Foxp3 mRNA, while in Treg cells treated with HMGB1, this expression was significantly down-regulated at 12 hours to 48 hours, and the levels were lowest at 48 hours. C: Detection of Foxp3 mRNA in Treg cells treated with various concentrations of HMGB1 (10, 50, 100 and 200 μg/L) for 24 hours by real-time RT-PCR. Compared with untreated Treg cells, the Foxp3 mRNA expression were significantly down-regulated in Treg cells treated with various concentration of HMGB1, and the expression levels of Foxp3 mRNA were lowest at 200 μg/L. D-F: Assay of Foxp3 protein by Western blotting in Treg cells treated with HMGB1. Foxp3 protein levels were decreased in a dose- or time-dependent manner, when Treg cells were treated with HMGB1 in vitro.
HMGB1 inhibits Treg cells immune activity
To determine whether HMGB1 affects Treg cell activity, a suppression assay of allogeneic mixed lymphocyte response (MLR) was performed using CD4+CD25+CD127lowTreg cells isolated from the PBMCs of CHB patients and treated with HMGB1 at different concentrations or at various intervals to determine the changes of their ability to suppress T-cell proliferation. The ability of CD4+CD25+CD127lowTreg cells to suppress an allogeneic MLR was significantly weakened after treatment with HMGB1 compared with untreated Treg cells (Fig. 3). Moreover, reduction of the ability of Treg cells to suppress the MLR was related to HMGB1 treatment in a dose- and time-dependent manner. The results suggested that HMGB1 effectively inhibits the immune activity of Treg cells.
Down-regulation of Foxp3 expression in Treg cells treated with HMGB1
To explore whether HMGB1 affects Foxp3 expression in Treg cells and thereby weakens their immune activity, we assessed the changes of Foxp3 expression in Treg cells treated with HMGB1 at different concentrations or at various intervals by real-time RT-PCR and Western blotting. The results demonstrated that untreated Treg cells highly expressed Foxp3 mRNA and protein, while the expression levels were significantly down-regulated in Treg cells after treatment with 100 μg/L HMGB1 for 12 to 48 hours (0.86±0.10, 0.52±0.14, 0.37±0.09, and 0.21±0.04, respectively for mRNA; 0.92±0.08, 0.63± 0.11, 0.42±0.07, and 0.29±0.02, respectively for protein). The Foxp3 expression levels were the lowest at 48 hours. When Tregs were stimulated with various concentrations of HMGB1 (10, 50, 100, and 200 μg/L) for 24 hours, the Foxp3 expression was also significantly decreased (0.91± 0.04, 0.80±0.09, 0.61±0.13, and 0.42±0.06, respectively for mRNA; 0.89±0.06, 0.74±0.11, 0.62±0.08, and 0.35± 0.07, respectively for protein), and the expression levels were the lowest at 200 μg/L HMGB1 (Fig. 4).
Discussion
Liver failure caused by chronic HBV infection is a severe life-threatening condition, the pathogenesis of which is complex and still not entirely clear. Some believethat liver failure is related to "primary liver injury" induced directly or indirectly (i.e. immunopathological damage) by HBV and "secondary liver injury” induced by intestinal endotoxemia and the activation of Kupffer cells by endotoxin (LPS) with the progress of liver disease.[6]Intestinal endotoxemia results from overgrowth and translocation of gram-negative bacteria from the gut to the peritoneal cavity because of the increased permeability of the intestinal wall, which leads to spontaneous peritonitis and so plays a significant role in the progress of liver failure.[7,8]Recently, it has been shown that HMGB1 protein, a late inflammatory mediator, is involved in the processes of endotoxemia, and is a successful therapeutic target in diverse inflammatory and infectious disorders.[10,11]In the present study, we assessed the levels of HMGB1 in serum, PBMCs, and liver tissue from liver failure patients with chronic HBV infection, and found that serum HMGB1 levels were significantly greater in liver failure patients than in CHB patients (82.6±20.1 μg/L vs. 34.2±13.7 μg/L), and that there were no significant differences in serum HMGB1 levels between CHB patients and healthy controls. The results of RT-PCR and Western blotting further confirmed significantly higher expression of HMGB1 mRNA in PBMCs and HMGB1 protein in the liver tissues of liver failure patients than in those of CHB patients and healthy controls. It is suggested that HMGB1 is involved in the pathogenesis of liver failure caused by chronic HBV infection.
Previous studies have demonstrated that Treg cells play an essential role in maintaining immune tolerance and contributing to immunological hyporesponsiveness against HBV infection,[27-30]suggesting that Treg cells limit liver injury by controlling inflammation and reduce the incidence of liver failure simultaneously, and maintain persistent HBV infection by suppressing the immune response. However, the roles of Treg cells in the pathogenesis of liver failure in CHB patients are poorly known at present. For this reason, in our study, the percentage changes of CD4+CD25+CD127lowT cells, a novel combined panel of biomarkers for identifying Treg cells effectively,[36]were determined within the CD4+T cell fraction among the PBMCs from liver failure patients with chronic HBV infection, CHB patients, and healthy controls by a flow-cytometric approach. Currently, the most definitive marker for identification of Treg cells is the Foxp3 transcription factor.[37-39]However, identification of intracellular Foxp3 requires permeabilization of the cells, making it difficult to work with or culture the cells again once the staining is complete. Recently, a combination of monoclonal antibodies against CD4, CD25 (the α chain of the IL-2 receptor), and CD127 (the α chain of the IL-7 receptor) was used for flow cytometry-based delineation of a wellseparated CD4+CD25+CD127lowpopulation of putative Treg cells, which had the most highly suppressive ability in functional assays, a poor response to T-cell receptor signaling, and the highest levels of Foxp3 compared with other cell subsets.[35,36,40,41]We found that CHB patients had a significantly higher percentage of Treg cells within their population of CD4+T cells in PBMCs than healthy controls (9.52±3.89% vs. 3.21± 1.21%). However, liver failure patients with chronic HBV infection had a significantly decreased proportion of Treg cells within their population of CD4+T cells in PBMCs compared with CHB patients (4.55±1.34% vs. 9.52±3.89%). These results suggested that downregulation of Treg cells occurs in liver failure patients with chronic HBV infection, which can aggravate liver injury by enhancing the immunological responsiveness to HBV, thereby resulting in liver failure in chronic HBV infection. Therefore, down-regulation of Treg cells may play a significant role in the pathogenesis of liver failure in CHB patients.
Since we found that higher levels of HMGB1 and a lower percentage of Treg cells were both present in liver failure patients, whether HMGB1 affects Treg cell activity in liver failure patients is a question. To answer this, a suppression assay of allogeneic MLR was performed using CD4+CD25+CD127lowTreg cells isolated from the PBMCs of CHB patients and treated with HMGB1 at different concentrations or at various intervals to determine the changes of their ability to suppress T cell proliferation. We found that this ability was significantly weakened after treatment with HMGB1 compared with untreated Treg cells. Moreover, the reduced ability of Treg cells to suppress the MLR was related to HMGB1 treatment in a dose- and timedependent manner. The results suggested that HMGB1 effectively inhibits the immune activity of Treg cells in liver failure patients, which may be a reason for downregulation of Treg cells in such patients with high levels of HMGB1.
In the present study, to preliminarily explore the mechanism of the weakening of immune activity of Treg cells induced by HMGB1, the changes of Foxp3 expression were assessed in Treg cells treated with HMGB1 at different concentrations and at various intervals by real-time RT-PCR and Western blotting. The results demonstrated that untreated Treg cells highly expressed Foxp3, while the Foxp3 expression was significantly down-regulated after treatment with HMGB1 in a dose- and time-dependent manner. Foxp3 is a transcription factor specifically expressedby Treg cells, and the levels of Foxp3 gene expression directly reflect the phenotype and activity of Tregs.[39]Therefore, it is suggested that HMGB1 weakens the immune activity of Treg cells probably by reducing their expression of Foxp3.
Although advances in intensive care management and liver transplantation have improved the survival of liver failure patients, the prognosis of liver failure in CHB patients remains poor. Therefore, it is especially important to explore more effective interventions for the management of liver failure in these patients. In this study, the results showed that a high level of HMGB1, a late inflammatory mediator of endotoxemia, plays an important role in the pathogenesis of liver failure in CHB patients by inhibiting the immune activity of Treg cells. It is suggested that HMGB1 antagonists are a feasible way to treat liver failure by suppression of endotoxemia and have protective effects on liver damage as well as the activity of Treg cells. On the other hand, immuno-modulation therapy is also a potential intervention for liver failure in CHB patients. This hypothesis awaits further study.
Funding:This study was supported by a grant from the National Natural Science Foundation of China (No. 81071342).
Ethical approval:The study was approved by the Renmin Hospital of Wuhan University Medical Ethics Committee.
Contributors:WLW and GZJ proposed the study. WLW wrote the first draft. WLW and CH analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. GZJ is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Pappas G, Papadimitriou P, Falagas ME. World Wide Web hepatitis B virus resources. J Clin Virol 2007;38:161-164.
2 Zou L, Zhang W, Ruan S. Modeling the transmission dynamics and control of hepatitis B virus in China. J Theor Biol 2010;262:330-338.
3 Fang Y, Shang QL, Liu JY, Li D, Xu WZ, Teng X, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patients and healthy people in China. J Infect 2009;58:383-388.
4 Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver 2002;22:5-13.
5 Chen C, Li L, Wu Z, Chen H, Fu S. Effects of lactitol on intestinal microflora and plasma endotoxin in patients with chronic viral hepatitis. J Infect 2007;54:98-102.
6 Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol 2002;8:961-965.
7 Li L, Wu Z, Ma W, Yu Y, Chen Y. Changes in intestinal microflora in patients with chronic severe hepatitis. Chin Med J (Engl) 2001;114:869-872.
8 Sozinov AS. Systemic endotoxemia during chronic viral hepatitis. Bull Exp Biol Med 2002;133:153-155.
9 Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248-251.
10 Tang Y, Lv B, Wang H, Xiao X, Zuo X. PACAP inhibit the release and cytokine activity of HMGB1 and improve the survival during lethal endotoxemia. Int Immunopharmacol 2008;8:1646-1651.
11 Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta 2010;1799:149-156.
12 Ditsworth D, Zong WX, Thompson CB. Activation of poly(ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem 2007;282:17845-17854.
13 Lange SS, Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog 2009;48:571-580.
14 Ugrinova I, Pashev IG, Pasheva EA. Post-synthetic acetylation of HMGB1 protein modulates its interactions with supercoiled DNA. Mol Biol Rep 2009;36:1399-1404.
15 Assenberg R, Webb M, Connolly E, Stott K, Watson M, Hobbs J, et al. A critical role in structure-specific DNA binding for the acetylatable lysine residues in HMGB1. Biochem J 2008;411:553-561.
16 Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 2008;180:2531-2537.
17 Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J Leukoc Biol 2007;81:49-58.
18 Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 2005;61:1-9.
19 Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 2005;201:1135-1143.
20 Feng Y, Yang Q, Xu J, Qian G, Liu Y. Effects of HMGB1 on PMN apoptosis during LPS-induced acute lung injury. Exp Mol Pathol 2008;85:214-222.
21 Goldstein RS, Bruchfeld A, Yang L, Qureshi AR, Gallowitsch-Puerta M, Patel NB, et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med 2007;13:210-215.
22 Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock 2009;32: 348-357.
23 Livesey KM, Tang D, Zeh HJ, Lotze MT. Not just nuclear proteins: 'novel' autophagy cancer treatment targets - p53 and HMGB1. Curr Opin Investig Drugs 2008;9:1259-1263.
24 Sakaguchi S, Wing K, Miyara M. Regulatory T cells-a brief history and perspective. Eur J Immunol 2007;37:S116-123.
25 Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression-a diverse arsenal for a moving target. Immunology 2008;124:13-22.
26 Tang Q, Bluestone JA. The Foxp3+regulatory T cell: a jackof all trades, master of regulation. Nat Immunol 2008;9:239-244.
27 Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005;41:771-778.
28 Franzese O, Kennedy PT, Gehring AJ, Gotto J, Williams R, Maini MK, et al. Modulation of the CD8+-T-cell response by CD4+CD25+regulatory T cells in patients with hepatitis B virus infection. J Virol 2005;79:3322-3328.
29 Stoop JN, van der Molen RG, Kuipers EJ, Kusters JG, Janssen HL. Inhibition of viral replication reduces regulatory T cells and enhances the antiviral immune response in chronic hepatitis B. Virology 2007;361:141-148.
30 Feng IC, Koay LB, Sheu MJ, Kuo HT, Sun CS, Lee C, et al. HBcAg-specific CD4+CD25+regulatory T cells modulate immune tolerance and acute exacerbation on the natural history of chronic hepatitis B virus infection. J Biomed Sci 2007;14:43-57.
31 Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Diagnostic and treatment guidelines for liver failure. Zhonghua Gan Zang Bing Za Zhi 2006;14:643-646.
32 Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-408.
33 Moniuszko M, Edghill-Smith Y, Venzon D, Stevceva L, Nacsa J, Tryniszewska E, et al. Decreased number of CD4+and CD8+T cells that express the interleukin-7 receptor in blood and tissues of SIV-infected macaques. Virology 2006;356:188-197.
34 Moniuszko M, Kowal K, Rusak M, Pietruczuk M, Dabrowska M, Bodzenta-Lukaszyk A. Monocyte CD163 and CD36 expression in human whole blood and isolated mononuclear cell samples: influence of different anticoagulants. Clin Vaccine Immunol 2006;13:704-707.
35 Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006;203:1693-1700.
36 Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+T reg cells. J Exp Med 2006;203:1701-1711.
37 Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol 2005;6:331-337.
38 Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005;6:345-352.
39 Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005;22:329-341.
40 Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods 2007;319:41-52.
41 Moniuszko M, Kowal K, Zukowski S, Dabrowska M, Bodzenta-Lukaszyk A. Frequencies of circulating CD4+CD25+CD127low cells in atopics are altered by bronchial allergen challenge. Eur J Clin Invest 2008;38:201-204.
March 23, 2010
Accepted after revision May 5, 2010
Author Affiliations: Department of Infectious Diseases, Renmin Hospital of Wuhan University, Wuhan 430060, China (Wang LW, Chen H and Gong ZJ)
Zuo-Jiong Gong, MD, PhD, Department of Infectious Diseases, Renmin Hospital of Wuhan University, Wuhan 430060, China (Tel: 86-27-88041911ext88385; Fax: 86-27-88042292; Email: zjgong@163.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Advances in prognostic factors in acute pancreatitis: a mini-review
- Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma
- Simultaneous breast and ovarian metastasis from gallbladder carcinoma
- An eight-year journey of Hepatobiliary & Pancreatic Diseases International
- Interferon and lamivudine combination therapy versus lamivudine monotherapy for hepatitis B e antigen-negative hepatitis B treatment: a meta-analysis of randomized controlled trials
