Expression level of augmenter of liver regeneration in patients with hepatic failure and hepatocellular carcinoma
2010-06-29HaiYingYuDaiRongXiangHaiJunHuangJunLiandJiFangSheng
Hai-Ying Yu, Dai-Rong Xiang, Hai-Jun Huang, Jun Li and Ji-Fang Sheng
Hangzhou, China
Expression level of augmenter of liver regeneration in patients with hepatic failure and hepatocellular carcinoma
Hai-Ying Yu, Dai-Rong Xiang, Hai-Jun Huang, Jun Li and Ji-Fang Sheng
Hangzhou, China
BACKGROUND:Augmenter of liver regeneration (ALR) is an important polypeptide in the process of liver regeneration. This study aimed to determine the expression level of ALR in different liver diseases and its significance.
METHODS:We prepared murine polyclonal antibody against ALR protein from Balb/C mice and purified the IgG fraction, which specifically combined to ALR protein as shown by Western blotting. Serum ALR levels in patients with hepatocellular carcinoma (HCC), hepatic failure (HF), chronic hepatitis B, and healthy persons were compared by ELISA. ALR mRNA expression levels in liver tissues in some of these patients were also compared by real-time RT-PCR. Immunohistochemical analysis was carried out on HF and HCC liver tissues.
RESULTS:Different serum ALR levels foreshowed completely different prognoses in 18 HF patients. Higher ALR levels were noted in 6 improved patients (1613.5±369.6 pmol/ml) than in 12 deteriorating patients (462.3±235.8 pmol/ml). Similar levels were found in 20 HCC patients (917.9±332. 7 pmol/ml), 24 chronic hepatitis B patients (969.2±332.5 pmol/ml) and 10 healthy persons (806.9±240.8 pmol/ml). ALR mRNA levels in HCC liver tissues [10E6.24 (1.74×106) copies/μl] were much higher than in those of HF patients receiving orthotopic liver transplantation [10E3.45 (2.82×103)copies/μl] or in healthy liver tissues [10E4.31 (2.04×104) copies/μl]. In immunohistochemical analysis, positive immunostaining in HCC liver tissue was more intense than that in HF liver tissue.
CONCLUSION:Serum ALR level is helpful in estimating the survival time of patients with HF, and ALR may play an important role in hepatocarcinogenesis.
(Hepatobiliary Pancreat Dis Int 2010; 9: 492-498)
liver regeneration; expression level; hepatic failure; hepatocellular carcinoma; chronic hepatitis B
Introduction
Human liver diseases such as chronic hepatitis, hepatic failure (HF), and hepatocellular carcinoma (HCC) are all accompanied by hepatocyte injury and regeneration.[1]Liver regeneration is a complicated process involving many agents, and augmenter of liver regeneration (ALR) is an important polypeptide in this process. Early experiments postulated the presence of a heat-stable hepatic stimulatory substance (HSS) in the liver, since extracts from weanling or hepatectomized rat livers result in augmentation of hepatocyte proliferation. Later, the peptide purified from HSS was renamed ALR. Unlike complete mitogens such as hepatocyte growth factor (HGF) or transforming growth factor (TGF-α), ALR was defined as a co-mitogen or "secondary" mitogen.[2]It was found to be constitutively expressed in hepatocytes in an inactive form, and released from the liver after activation during regeneration. Animal research revealed changes in the level of ALR in serum at the early stage of liver regeneration, earlier than other growth factors:[3]
after 70% hepatectomy the level of ALR in the blood rises dramatically, peaking after 12 hours. Serum ALR level well reflects hepatocyte ALR expression level and the activation of liver regeneration. Recent experiments also showed an increase of ALR expression in human liver cirrhosis and carcinoma,[4]and the suppressedexpression of ALR subsequently inhibits the growth of HepG2 cells. Anti-hALR antibody was found to partially inhibit the autonomous growth of HepG2 cells.[5,6]All the above findings confirm the relationship between ALR and hepatocyte regeneration. The balance between hepatocyte injury and regeneration determines the turnover of many liver diseases.[7]In the present study, serum ALR levels and ALR mRNA expression levels were measured in different liver diseases to determine the relationship between ALR, hepatocyte regeneration, and liver disease, especially HF and HCC.
Methods
The study protocols and methods were approved by the Animal Care Ethics Committee and Clinical Investigation Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China).
Preparation of natural human ALR (nhALR) and anti-nhALR antibody
The recombinant expression vector of pET28a(+)/ hALR was constructed, and the recombinant human ALR (rhALR) protein of about 23 kDa was expressed and purified as reported previously.[8]To obtain nhALR, an Xa-arrest Kit (Novagen Germany) was used to excise the recombined peptide part of rhALR, and the Xa protease recognized as the continuous amino acids Ile-Glu-Gly-Arg. Thus we obtained a soluble nhALR protein of about 15 kDa. Amicon Ultra-4 (Millipore, USA) was used to desalt and concentrate the protein. The concentration of the nhALR protein was determined in a SmartSpecTMplus spectrophotometer (Bio-Rad, USA) by a Bradford protein assay kit and stored at -80 ℃ until use.
We obtained murine polyclonal antibodies against nhALR from Balb/C mice. Twenty-four female mice (8 weeks old, weighing 19-22 g) were used for immunization and they were divided into an immune group and a control group, each of 12 mice. Briefly, approximately 40 mg of nhALR protein emulsified with an equal volume of Freund's complete adjuvant (Sigma, USA) was injected at multiple intradermal sites to the immune group (nhALR was diluted to 80 mg/ml, and 0.5 ml nhALR was mixed with 0.5 ml Freund's complete adjuvant); physiologic saline was injected into the control group for negative control. The same dose of protein or physiologic saline emulsified in Freund's incomplete adjuvant was injected 2 and 4 weeks after the first injection. After 7 days, blood was collected from the eyeballs and clotted by overnight incubation at room temperature, then centrifuged at 3000 g for 15 minutes. The serum was collected and the IgG fraction was purified before use as antibodies. A Montage antibody purification kit (Millipore, USA) was used to isolate the IgG fraction from the murine antiserum. The serum was diluted with binding buffer A and loaded on a PROSEP®-A spin column to collect the IgG fraction. IgG collected from the control group was used as control antibody.
Identification of anti-nhALR antibody by Western blotting
nhALR protein was separated on 15% SDS-PAGE and transferred onto a 0.2 μm pore polyvinylidene fluoride membrane (Millipore USA) at 220 mA for 2 hours in a Bio-Rad Transblot electrophoretic transfer cell for Western blotting analysis. The non-specific sites were blocked with 5% nonfat milk in TBS, the membrane was washed in TBS and incubated with anti-nhALR antibody (1 μg/ml) in blocking buffer, followed by washing and incubation with horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulins (1∶1000, DakoCytomation, Denmark). The ALR-anti-ALR complex was treated with a mixture of 2× stable peroxide solution and 2× luminol/enhancer solution (Novagen, Germany), and chemoluminescence was recorded on Fuju X-ray film.
To determine whether anti-nhALR antibody combines with ALR in hepatocytes, we extracted total proteins from HepG2 cells and immortalized human hepatocytes[9]with Mammalian Protein Extraction Reagent (Pierce, America) and separated the protein on 15% denatured or natured SDS-PAGE; further detection was performed as described above.
Enzyme-linked immunosorbent assay (ELISA)
Serial dilutions of nhALR protein were prepared: 10 and 1 μg/ml, 100, 50, 10, 5, 2, and 1 ng/ml, and 500, 250, 125, and 62.5 pg/ml. Well plates (96 wells) were coated with anti-nhALR antibody (0.1 μg/well) in 100 μl coated buffer overnight at 4 ℃ and incubated with blocking solution (PBS containing 5% nonfat milk) at 37 ℃ for 2 hours. After washing with PBS-T, serial dilutions of nhALR protein were incubated at 37 ℃ for 1.5 hours, then anti-nhALR antibody (0.1 μg/well in 100 μl blocking solution) was added. IgG collected from the control group was used for negative control. After 2 hours incubation at room temperature, plates were rinsed three times with PBS-T, and 100 μl of 1∶2000-diluted horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulins (1∶1000, DakoCytomation Denmark) was added to each well for 1 hour incubation at room temperature. After washing five times, the enzyme activity was determined by adding 100 μl of 3, 3', 5,5'-tetramethyllbenzidine substrate solution. Optical density was read at dual absorbance of 450 nm and 630 nm with an ELISA plate reader (Bio-Rad) after incubating for 10 minutes.
Determination of ALR level in human serum by ELISA
Human serum was obtained from 72 patients classified into 4 groups (Table 1): 20 patients with HCC, of whom 6 accepted surgical resection; 18 with HF caused by hepatitis B virus, of whom 5 received orthotopic liver transplantation (OLT); 24 with chronic hepatitis B; and 10 healthy persons without liver disease. Of the 18 HF patients, 6 were improved through artificial liver support system (ALSS) therapy and rehabilitation and left hospital after 1-2 months, whereas the other 12 deteriorated in spite of ALSS therapy (7 died and 5 received OLT). When the patients met the diagnostic criteria of HF, their serum was collected on days 1, 2, and 3 before ALSS therapy. Blood samples collected from the 72 patients were stored at room temperature overnight and centrifuged at 3000 ×g for 10 minutes to isolate the serum. ELISA detection was performed as described above.
HCC was diagnosed pathologically and HF was diagnosed according to the clinical symptoms, laboratory results, or pathological findings. Diagnostic workup included physical examination, laboratory tests, and liver pathology according to the criteria described inLiver Diseases of the Chinese Medical Associationin 2000.
ALR mRNA expression in liver tissue
HCC tissues were obtained from the 6 patients with HCC who accepted surgical resection; necrotic or inflamed liver tissues from the 5 patients with HF who received OLT; and control liver tissues were from healthy donors for OLT. The operations were performed at our hospital. Informed consent to utilize the liver tissues for mRNA extraction was given by the patients or their relatives. HCC and HF were diagnosed pathologically.
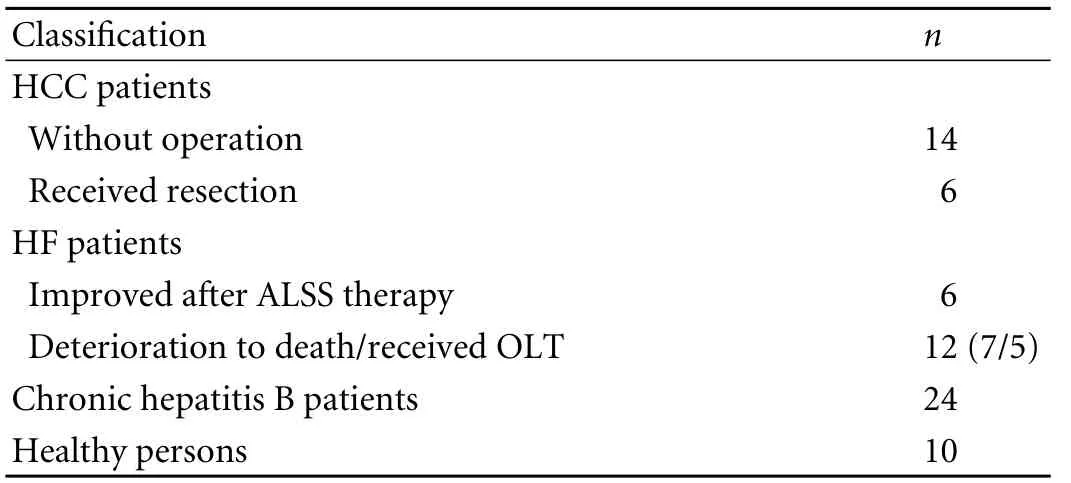
Table 1. Patient characteristics
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
ALR and β-actin were synthesized in our laboratory, and their concentrations were determined by absorbance at 260 nm (SmartSpecTMplus Spectrophotometer, Bio-Rad). Ten-fold serial dilutions from 1×100 to 1×1010copies/μl were prepared.
One hundred milligrams of each liver tissue sample was homogenized in a small earthenware basin with liquid nitrogen and total RNA was isolated using Trizol reagent as described by the manufacturer (Invitrogen. Grand Island, NY, USA). RNA concentration was determined by absorbance at 260 nm, transformed into cDNA, and stored at -80 ℃ until use.
The level of β-actin mRNA was measured to determine the efficiency of cDNA synthesis and reverse transcription of different batches of mRNA. The PCR primers specific for ALR cDNA were 5'-TAA GGA TCC ATG CGG ACG CAG CAG AAG-3' (sense) and 5'-TAA CTC GAG CTA GTC ACA GGA GCC ATC-3' (antisense), and for β-actin cDNA 5'-GGC ATC CTC ACC CTG AAG TA-3' (sense) and 5'-TCG GGT GTT GAA GGT CTC AA-3' (antisense).
Real-time PCR (20 μl) was performed using the SYBR Premix Ex TaqTMkit (Takara Biotechnology) in accordance with the manufacturer's protocol on an Option II real-time detection system (Bio-Rad Laboratories, Veenendaal, The Netherlands). Real-time RT-PCR data were analyzed by MJ Option MonitorTManalysis software version 3.1. The Ct value analysis, PCR efficiency, linearity, slopes of the standard curves, relative quantity of fluorescence, and dissociation curve analysis were calculated by the Strata gene software program built into the Option II apparatus.
Immunohistochemical analysis
The liver samples from HF and HCC patients were embedded in paraffin wax and 4-μm sections were cut. Standard pretreatment was done; anti-nhALR antibody diluted in 1∶100 in block solution was pipetted onto the sections and incubated overnight at 4 ℃, then the sections were washed three times with PBS and 1∶1000-diluted horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulins were added and incubated at 37 ℃ for 1 hour. Staining was completed by diaminobenzidine (DAB) and counterstaining was with hematoxylin. The following negative controls were performed: 1) IgG collected from the Balb/C mice of the control group was used to replace the anti-nhALR antibody, and 2) anti-nhALR antibody was blocked with nhALR for 1 hour at 37 ℃ before addition.
Statistical analysis
Statistical analysis was performed using SPSS (version 13.0) for Windows. Student'sttest between two groups and LSDttest between several groups were used. APvalue less than 0.05 was considered statistically significant.
Results
rhALR and nhALR
SDS-PAGE analysis showed the expected rhALR protein of about 23 kDa before the recombined peptide was excised, and the nhALR protein of about 15 kDa which was consistent with that reported previously (Fig. 1).
Western blotting analysis

Fig. 1. SDS-PAGE analysis of rhALR and nhALR. M: protein marker; lane 1: rhALR protein about 23 kDa; lane 2: nhALR protein about 15 kDa.

Fig. 2. Western blotting analysis of nhALR and ALR in HepG2 cells/immortalized human hepatocytes. M: nhALR; lane 1: total protein extracted from immortalized human hepatocytes; lane 2: total protein extracted from HepG2 cells. A: Western blotting under denatured condition (100 mmol/L dithiothreitol, DTT); B: Western blotting under natural condition (without DTT).
Anti-nhALR antibody immunoreacted with nhALR protein (Fig. 2). Anti-nhALR antibody-positive protein was present in the extracts prepared from both cell lines: immunoreactive protein strips of total protein extracted from HepG2 cells and immortalized human hepatocytes was about 23 kDa (denatured condition) and more than 40 kDa (dimeric structure under natural conditions) as reported previously. Thus the anti-nhALR antibody was specifically combined with ALR in HepG2 cells and ALR in human serum (Fig. 2).
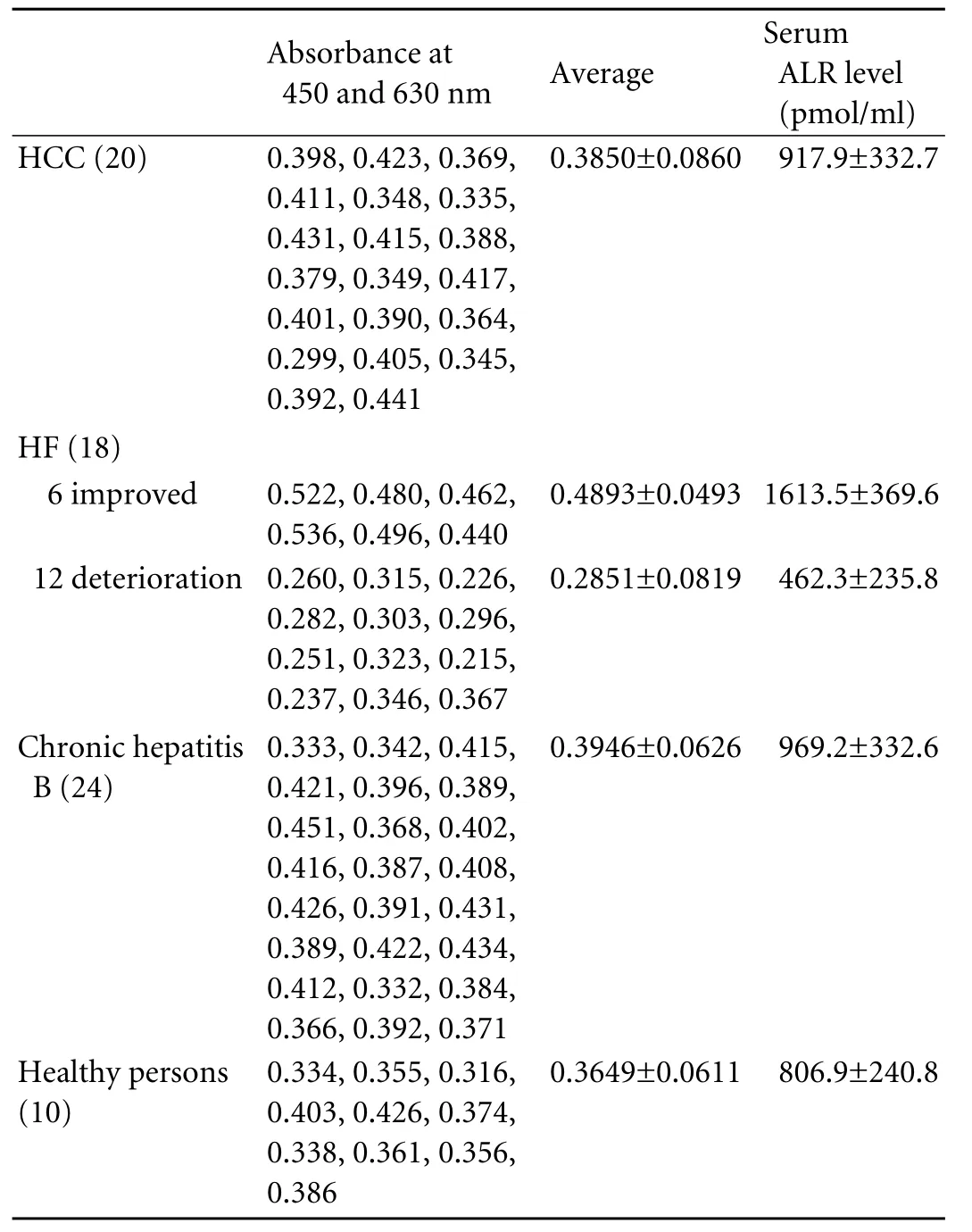
Table 2. Serum dual absorbance at 450 and 630 nm and serum ALR levels of 72 patients
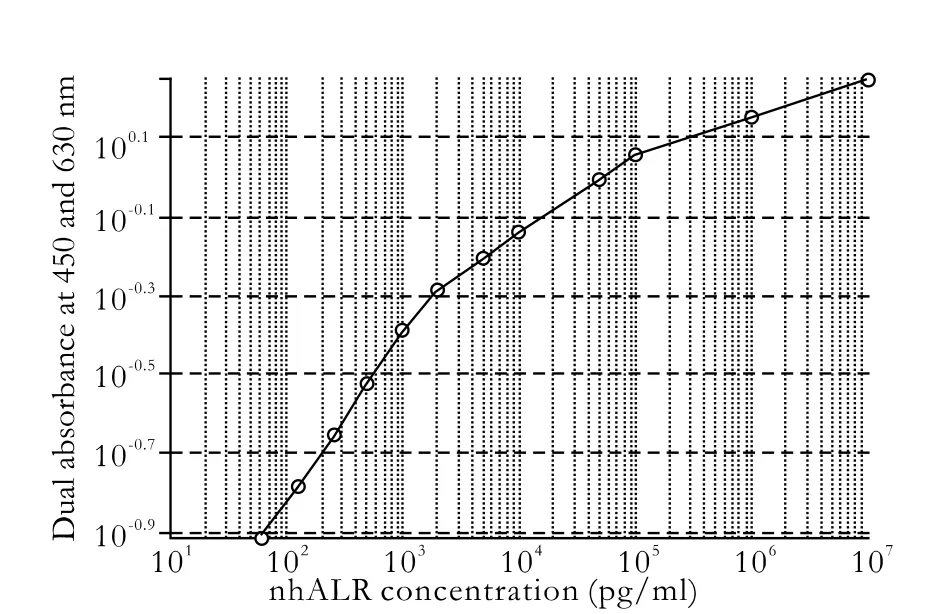
Fig. 3. Relationship between nhALR concentration and dual absorbance at 450 and 630 nm.

Table 3. Absolute ALR and β-actin mRNA levels of 11 patients and 2 controls
ELISA analysis
There was a close relationship between nhALR concentration dual absorbance at 450 and 630 nm. Besides a satisfactory linear relationship from 62.5 pg/ml to 2 ng/ml was found (Fig. 3), as a good reference to analysis of the ALR concentration in patient serum.
Determination of serum ALR levels
The serum ALR levels of the 72 patients (Table 2) were determined by ELISA. ALR levels were calculated by dual absorbance at 450 and 630 nm according to the linear relationship (Fig. 3). ALR levels were higher in the 6 improved patients (1613.5±369.6 pmol/ml) than in the 12 deteriorating patients (462.3±235.8 pmol/ml) or the other 54 patients and healthy persons (P<0.05). ALR levels in the 12 deteriorating patients were lower than those of the other 54 patients and healthy persons (P<0.05). ALR levels were similar, in the HCC patients (917.9±332.7 pmol/ml) and chronic hepatitis B patients (969.2±332.6 pmol/ml) and higher than in the healthy persons (806.9±240.8 pmol/ml) but these findings were not statistically different (P>0.05).
Real-time RT-PCR analysis
The absolute ALR and β-actin mRNA levels of the 11 patients and 2 controls are listed in Table 3. The ALR expression after normalization to β-actin was much higher in HCC liver tissues [10E6.24 (1.74×106) copies/μl] than in HF receiving OLT [10E3.45 (2.82×103) copies/μl] or healthy persons [10E4.31 (2.04×104) copies/μl].

Fig. 4. ALR protein expression in paraffin-embedded HCC and HF human liver tissue. A, B: Fields from HCC liver showing positive immunostaining of the majority of hepatocytes in cytoplasm but negative in nucleus, and rare in cholangiocytes and endothelial cells. C: Field from HF liver showing bile thrombus and less positive immunostaining than that in HCC liver sections. D, E: Negative controls (see Methods) showing lack of any positive immunostaining in hepatocytes, cholangiocytes, and endothelial cells.
Immunohistochemical analysis
Immunostaining was positive in the hepatocyte cytoplasm but negative in the nucleus, and rare in cholangiocytes and endothelial cells. Positive immunostaining was more intense in HCC liver tissue than in HF liver tissue. No positive immunostaining was found in negative controls (Fig. 4).
Discussion
ALR is a recently discovered hepatotrophic protein and unique cytokine which specifically stimulates cells of hepatic origin to grow regardless of genus.[10,11]ALR is absent from the serum of normal adult animals,[12]but a recent study[3]found that ALR is synthesized constantly in hepatocytes in an inactive form and is detected in normal adult mouse serum. Animal research found that the concentration of this factor in serum rises dramatically after 70% hepatectomy.[3]In our study, ALR was also detected in human serum, and the ALR levels in human liver diseases were significantly different. The fact that ALR is synthesized constantly and is activated during liver regeneration shows its importance in liver function and regeneration.
The liver has extraordinary regenerative power, and adequate regeneration usually occurs in patients with acute and chronic liver diseases, although hepatocyte necrosis is extensive. However, in some lethal or acute fulminant HF, the number of regenerative hepatocytesis clearly fewer than in surviving patients, and it appears that regeneration failure causes death in such patients.[13]In our study, different serum ALR levels were found in different HF patients, and they foreshowed completely different prognoses: patients with higher serum ALR levels had liver function improved enough through ALSS therapy and rehabilitation to leave hospital; conversely, patients with lower ALR levels deteriorated to the point of death or had to receive OLT. Decreased serum ALR levels may identify patients at risk of lethal liver failure and represent the trigger for maintaining intensive medical treatment or turning to salvage liver transplantation. High serum ALR levels represent a high level of hepatocyte regeneration. The survival rates of patients with different ALR levels were statistically significant, suggesting that ALR level is helpful in estimating the survival time of patients with HF.
HGF is another important polypeptide in the process of liver regeneration. A study found that the serum HGF level in HF patients is high,[14]and increasing HGF levels in HF patients result in deterioration, which seems opposite to our result. But we also found in surviving patients that the HGF was high at first and then decreased, while in the patients who got worse and died, the serum HGF level increased continuously. In our study, we only measured the early level of serum ALR (days 1, 2, and 3 before ALSS therapy), because all HF patients accepted ALSS therapy, and after this therapy the serum ALR level cannot reflect its real level. We found the early ALR level correlated with the prothrombin time and the prognosis. Unlike HGF, ALR is synthetized by hepatocytes, the early serum ALR level reflects the degree of regeneration of hepatocytes, and the regeneration level determines survival or death. ALR as a hepatotrophic factor takes a core place in the process of liver regeneration and may promote it by reducing liver-resident natural killer cell activity in human liver diseases.[15,16]
HCC is now the fifth most frequent malignant tumor in the world (564 000 cases annually) and the third cause for cancer death.[17,18]There is a growing understanding of the molecular mechanisms inducing hepatocarcinogenesis, which almost never occurs in healthy liver, but the cancer risk increases sharply in response to chronic liver injury at the cirrhosis stage.[19]A detailed understanding of epidemiologic factors and molecular mechanisms associated with HCC could ultimately improve our current concepts for screening and treatment of this disease.[20]
In our study, similar serum ALR levels were noted in HCC patients and chronic hepatitis B patients, and no significant difference was found between these patients and healthy persons. But the ALR mRNA expression levels in HCC liver tissues were higher than those in the liver tissue of healthy persons. All the above showed that ALR was expressed highly in HCC hepatocytes compared with healthy persons, but the serum ALR level in HCC patients was not high. The reason for the disparity between mRNA expression level and serum level of ALR in HCC is not yet understood. Immunohistochemical data showed that ALR existed predominantly in hepatocyte cytoplasm and positive immunostaining was more intense in HCC than in HF (which led to death). Recent research revealed that ALR stimulates the proliferation of HepG2 and QGY cells dose-dependentlyin vitro, but has no influence on the proliferation of primary rat hepatocytes. The expression of ALR is much higher in HCC liver tissues than in normal liver tissues, and it is not related to the differentiation and size of the carcinoma.[21]Another study also found that hALR is highly expressed by HepG2 cells and maintains the autonomous growth of hepatoma cellsin vitrothrough an autocrine mechanism, and anti-hALR antibody partly inhibits the autonomous growth of HepG2 cellsin vitro. The short interfering RNA targeting hALR gene specifically suppresses the expression of hALR, and subsequently inhibits the growth of HepG2 cells.[5,6]ALR might play an important role in the occurrence and development of HCC. The findings suggest an important role for ALR in hepatocarcinogenesis and therefore its potential value in clinical diagnosis and therapy of HCC.
Because of the core place of ALR in liver function and regeneration, and its close relationship with human liver diseases such as HF and HCC, there is an increasing necessity to extend research on mammalian ALR protein in different patients with different kinds of liver diseases or at different stages of hepatitis.
Funding:This work was supported by grants from the National Natural Science Foundation of China (30972592 and 30970747), and Zhejiang Provincial Natural Science Foundation (Y2090010).Ethical approval:Not needed.
Contributors:SJF and LJ proposed the study. YHY wrote the first draft. XDR analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. SJF is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Pawlowski R, Jura J. ALR and liver regeneration. Mol Cell Biochem 2006;288:159-169.
2 de Juan C, Benito M, Alvarez A, Fabregat I. Differential proliferative response of cultured fetal and regenerating hepatocytes to growth factors and hormones. Exp Cell Res 1992;202:495-500.
3 Gandhi CR, Kuddus R, Subbotin VM, Prelich J, Murase N, Rao AS, et al. A fresh look at augmenter of liver regeneration in rats. Hepatology 1999;29:1435-1445.
4 Thasler WE, Schlott T, Thelen P, Hellerbrand C, Bataille F, Lichtenauer M, et al. Expression of augmenter of liver regeneration (ALR) in human liver cirrhosis and carcinoma. Histopathology 2005;47:57-66.
5 Tang L, Sun H, Zhang L, Guo H, Zhang L, Liu Q. Inhibitory effect of blocking expression of human augmenter of liver regeneration (hALR) on proliferation of hepatocellular carcinoma cell line HepG2. Ai Zheng 2006;25:671-676.
6 Tang L, Sun H, Zhang L, Guo H, Chen XH, Deng JC, et al. Effects of anti-human augmenter of liver regeneration monoclonal antibody on proliferation of HepG2 cellsin vitro. J Chongqing Med Univ 2005;30:7-9, 17.
7 Elias E. Liver failure and liver disease. Hepatology 2006;43: S239-242.
8 Sheng J, Yu H, Li J, Sheng G, Zhou L, Lu Y. Cloning and expression of the human augmenter of liver regeneration at low temperature in Escherichia coli. J Biochem Biophys Methods 2007;70:465-470.
9 Li J, Li LJ, Cao HC, Sheng GP, Yu HY, Xu W, et al. Establishment of highly differentiated immortalized human hepatocyte line with simian virus 40 large tumor antigen for liver based cell therapy. ASAIO J 2005;51:262-268.
10 LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol 1975;248:273-284.
11 Hagiya M, Francavilla A, Polimeno L, Ihara I, Sakai H, Seki T, et al. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci U S A 1994;91:8142-8146.
12 Giorda R, Hagiya M, Seki T, Shimonishi M, Sakai H, Michaelson J, et al. Analysis of the structure and expression of the augmenter of liver regeneration (ALR) gene. Mol Med 1996;2:97-108.
13 Riordan SM, Williams R. Mechanisms of hepatocyte injury, multiorgan failure, and prognostic criteria in acute liver failure. Semin Liver Dis 2003;23:203-215.
14 Schirmacher P, Geerts A, Pietrangelo A, Dienes HP, Rogler CE. Hepatocyte growth factor/hepatopoietin A is expressed in fat-storing cells from rat liver but not myofibroblast-like cells derived from fat-storing cells. Hepatology 1992;15:5-11.
15 Vujanovic NL, Polimeno L, Azzarone A, Francavilla A, Chambers WH, Starzl TE, et al. Changes of liver-resident NK cells during liver regeneration in rats. J Immunol 1995;154: 6324-6238.
16 Tanigawa K, Sakaida I, Masuhara M, Hagiya M, Okita K. Augmenter of liver regeneration (ALR) may promote liver regeneration by reducing natural killer (NK) cell activity in human liver diseases. J Gastroenterol 2000;35:112-119.
17 Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002;35: 519-524.
18 Campbell JS, Johnson MM, Bauer RL, Hudkins KL, Gilbertson DG, Riehle KJ, et al. Targeting stromal cells for the treatment of platelet-derived growth factor C-induced hepatocellular carcinogenesis. Differentiation 2007;75:843-852.
19 Fausto N, Webber EM. Mechanisms of growth regulation in liver regeneration and hepatic carcinogenesis. Prog Liver Dis 1993;11:115-137.
20 El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-2576.
21 Sun H, Yu HF, Wu CX, Guan XQ, Liu Q. Expression of augmenter of liver regeneration in hepatic tumor cells and its clinical significance. Zhonghua Gan Zang Bing Za Zhi 2005; 13:205-208.
January 15, 2010
Accepted after revision April 27, 2010
Author Affiliations: State Key Laboratory of Infectious Disease and Department of Infectious Disease, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Yu HY, Xiang DR, Huang HJ, Li J and Sheng JF)
Ji-Fang Sheng, MD, PhD, Department of Infectious Disease, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236759; Fax: 86-571-87236755; Email: sania8@163.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- News
- Advances in prognostic factors in acute pancreatitis: a mini-review
- Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma
- Simultaneous breast and ovarian metastasis from gallbladder carcinoma
- An eight-year journey of Hepatobiliary & Pancreatic Diseases International
- Interferon and lamivudine combination therapy versus lamivudine monotherapy for hepatitis B e antigen-negative hepatitis B treatment: a meta-analysis of randomized controlled trials
