Diagnostic vaIue of the fluoroscopic triggering 3D LAVA technique for primary Iiver cancer
2010-06-29XiaoYongShenChunHuaChaiWenBoXiaoandQiDongWang
Xiao-Yong Shen, Chun-Hua Chai, Wen-Bo Xiao and Qi-Dong Wang
Hangzhou, China
Diagnostic vaIue of the fluoroscopic triggering 3D LAVA technique for primary Iiver cancer
Xiao-Yong Shen, Chun-Hua Chai, Wen-Bo Xiao and Qi-Dong Wang
Hangzhou, China
BACKGROUND:Primary liver cancer (PLC) is one of the common malignant tumors. Liver acquisition with acceleration volume acquisition (LAVA), which allows simultaneous dynamic enhancement of the hepatic parenchyma and vasculature imaging, is of great help in the diagnosis of PLC. This study aimed to evaluate application of the fluoroscopic triggering 3D LAVA technique in the imaging of PLC and liver vasculature.
METHODS:The clinical data and imaging findings of 38 adults with PLC (22 men and 16 women; average age 52 years), pathologically confirmed by surgical resection or biopsy, were collected and analyzed. All magnetic resonance images were obtained with a 1.5-T system (General Electrics Medical Systems) with an eight-element body array coil and application of the fluoroscopic triggering 3D LAVA technique. Overall image quality was assessed on a 5-point scale by two experienced radiologists. All the nodules and blood vessel were recorded and compared. The diagnostic accuracy and feasibility of LAVA were evaluated.
RESULTS:Thirty-eight patients gave high quality images of 72 nodules in the liver for diagnosis. The accuracy of LAVA was 97.2% (70/72), and the coincidence rate between the extent of tumor judged by dynamic enhancement and pathological examination was 87.5% (63/72). Displayed by the maximum intensity projection reconstruction, nearly all cases gave satisfactory images of branchesⅢandⅣof the hepatic artery. Furthermore, small early-stage enhancing hepatic lesions and the parallel portal vein were also well displayed.
CONCLUSIONS:Sequence of LAVA provides good multi-phase dynamic enhancement scanning of hepatic lesions. Combined with conventional scanning technology, LAVA effectively and safely displays focal hepatic lesions and the relationship between tumor and normal tissues, especially blood vessels.
(Hepatobiliary Pancreat Dis Int 2010; 9: 159-163)
liver neoplasm; diagnosis; imaging; LAVA sequence
Introduction
Primary liver cancer (PLC) is one of the common malignant tumors in China. It is important to detect the lesion early and define its identity, range, surrounding tissues, and vasculature.[1]Since the majority of PLCs develop characteristic changes in arterial phase imaging, a high resolution liver imaging technique is of great help for diagnosis and differential diagnosis.[2,3]This technique, known as liver acquisition with acceleration volume acquisition (LAVA), is a contrast-enhanced, multi-phase imaging method for the liver with high temporal and spatial resolution, large coverage, uniform fat suppression, and refined signal-to-noise ratio. After injection, when the contrast agents reach the pulmonary artery, the 3D LAVA scan is immediately started. In one breath-hold, LAVA acquires a stack of overlapping thin slices with high in-plane resolution. In this way, LAVA produces images of the arterial, portal and venous phases that not only precisely depict anatomy and contrast uptake, but also contain vascular information. With the ordinary magnetic resonance angiography protocol, the acquisition is repeated three or more times. But a single multi-phase LAVA acquisition, with one injection, provides more information than two traditional scans.
The diagnostic value of LAVA has aroused the interest of hepatobliliary pancreatic specialists and imaging specialists. This study aimed to evaluate the application value of the fluoroscopic triggering 3D LAVA technique in the imaging of PLCs and liver vasculature, and discuss its role in clinical diagnosis, treatment, and postoperative monitoring.
Methods
PatientsWe collected the information from 38 patients from October 2007 to February 2008. All patients were subjected to magnetic resonance imaging (MRI) and were confirmed pathologically by surgical resection or biopsy. Thirty-one patients had hepatocellular carcinoma and 7 had cholangiocellular carcinoma. There were 22 men and 16 women, aged from 24 to 69 years (average 52 years).
MRI methodology
The same 1.5-T MRI system (Superconducting, GE, USA) with an 8-channel torso phased array coil was used for all patients. Firstly, patients' breathing was strictly controlled. After the routine scan, a bolus of Gd-DTPA (0.1 mmol/kg, 3 ml/s) was injected by a double tube high pressure injector, followed by 20 ml saline injection at the same speed. Before the injection, fluoroscopy was used to make coronal scans to show the main pulmonary artery[4,5]on both sides and its major ramus. After the injection, we observed the contrast agents reaching the pulmonary artery, then the 3D LAVA scan was performed immediately. With the patient holding his/her breath, 3 arterial phase images were achieved in 23 seconds. From the beginning of the injection, 2 portal vein phase images in 60 seconds and 2 equilibrium phase images in 180 seconds were obtained. The scan delay time was prolonged 5-6 minutes after rejection if needed. As long as the patient could hold his/her breath, the phase for rest and relaxation was kept to a minimum. The time-intensity curve of the aorta, portal vein and hepatic lesions was drawn in an ADW4.2 workstation, which helped to reconstruct the hepatic artery and portal vein by 3D maximum intensity projection (MIP). Scanning parameters included coronal, TR/TE=4.0/1.9 ms, FA=15°, matrix=320×192, field of view=36-40 cm, thickness=4-6 mm, and NEX=0.75. LAVA was integrated with the array spatial sensitivity encoding technique (ASSET). The phase accelerating factor was 2.5 Ph. The zero-filling interpolation (ZIP×2) method was used in postprocessing. The whole liver volume scan took 17-23 seconds with breath holding.
Image evaluation
Two experienced radiologists evaluated the acquired images by reading, scoring, and recording separately. The criteria were set by Danias, classified into 5 levels:[6]1) Poor image cannot be used for diagnosis; 2) Blood vessel can be observed but with obvious artifacts not reliable for diagnosis; 3) Blood vessel is identifiable, but with artifacts, reliable for diagnosis; 4) Good image is structure distinctly visible, not out of focus and no artifacts; 5) High signal-to-noise ratio, even fat saturation, main arteries supplying the tumor, draining veins, and swollen lymph nodes are shown clearly. A lesion with specific features such as abnormal tumor vessels including density, twisting, and dilatation are important for diagnosis of PLC.
Results
According to the judgment of 2 investigators, 65.8% of the LAVA images were scored above 3, and 34.2% were scored at 3. All images reached the threshold for diagnosis of PLC.
Enhancement of lesions
In total, 38 patients had 72 nodules in the liver, the diameters of which ranged from 0.4 to 7.5 cm. The accuracy of LAVA was 97.2% (70/72). For judgment of the extent of invasion of the tumor, the coincidence rate between dynamic enhancement images in LAVA and pathological examination was 87.5% (63/72). Timeintensity curves of lesions rose rapidly and remained at peak levels, or rapidly rose and then dropped at once. The nodule signal intensity exceeded approximately 45% of the hepatic parenchyma in the first phase, 35% in the second phase, and peaked in the third phase. While the signal intensity began to decrease in the fourth phase, it was slightly higher than that in the hepatic parenchyma (Fig. 1). After embolization, the signal intensity of lesions was lower than that of the parenchyma, but there was no statistically significant difference. Swollen lymph nodes were clearly shown and the connection with parallel vessels was flawless.
Enhancement of the hepatic artery and portal vein
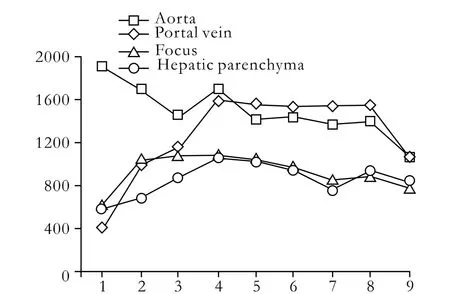
Fig. 1. 3D LAVA time-intensity curves of the aorta, portal vein, focus, and hepatic parenchyma.
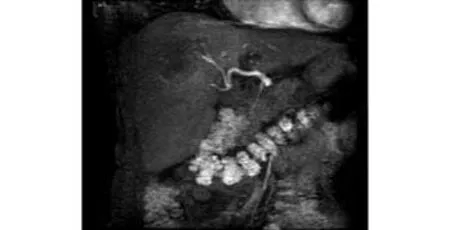
Fig. 2. Hepatic artery development in the second phase of 3D LAVA.
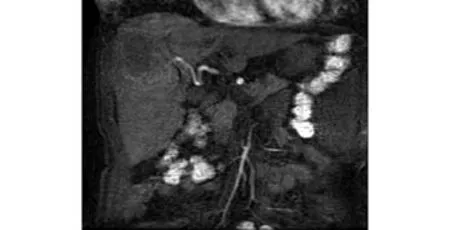
Fig. 3. Hepatic artery development in the first phase of 3D LAVA. Nearly all patients demonstrated satisfactory images of high signal-to-noise ratio, and the main arterial supply to the tumor was shown clearly.
Because the scanning covered the diaphragm to the renal hilus, it included the whole liver area, along with the abdominal aorta, celiac trunk, proper hepatic artery, portal vein, hepatic vein and embranchment in the liver. Abnormal tumor vessels including density, twisting, and dilatation are important for diagnosis. Since in this study the fluoroscopic triggering technique was used, all patients were diagnosed with high quality images. Hepatic artery enhancement developed in the second phase in 23 patients (Fig. 2), and in the first phase in 15 (Fig. 3). The aorta enhanced peak time in 27 patients was in the first phase, while in 10 it was in the second phase, and in 1 in the third phase. The peak time of enhanced portal vein appeared in the third (n=23), second (n=12), and fourth phases (n=3). In displays by MIP reconstruction, nearly all cases demonstrated satisfactory images of branches Ⅲ and Ⅳ of the hepatic artery. Small early-stage enhancing hepatic lesions and the parallel portal vein were also well depicted.
Discussion
PLC is the primary malignant tumor originating from hepatocytes and intrahepatic bile duct epithelial cells. In 2002, PLCs were found in 442 000 male patients, ranking the 5th most common tumor, and in 184 000 female patients, the 8th.[7-10]Diagnosis of hepatic lesions depends mainly on imaging. The key to clinical therapy and operation plan is accurate diagnosis and identification of the lesion and its area involved. Huang et al[11]suggested that the value of ultrasonography is to discover problems with certainty, but other examinations are needed for verification. MRI has excellent parenchyma resolution. LAVA provides higher quality multi-phase dynamic enhancement images of the liver in a shorter time, and shows the vascular anatomy.
As a 3D spoiled gradient echo technology on the basis of ASSET, LAVA takes segmented k-space techniques along the Z axis, which further shortens the scanning time. Compared with the previous multiphase dynamic enhancement scanning technology of the abdomen, the speed and the spatial resolution are improved about 25%. It improves the scanning range about 25%. Furthermore, a single breath-hold can produce multi-phase images of the artery. We can also reconstruct the original images of any other plane. So it can sensitively depict the anatomical structures of the liver and its surrounding area in detail. This provides the ability to depict direct signs, especially those of smaller lesions. Compared with the conventional dynamic enhancement scanning sequence, LAVA shows a significant advantage in the new fat suppression technique of lesions and the surrounding tissue, whose k-space fulfilling at slice selection adopts partial zerofulfilling methodology and uses small angle special inversion pulses to segment signal acquisition. This makes the best time for fat suppress inversion recovery at the center of k-space, and presents a symmetrical distribution. It greatly improves the fat suppression effect and provides a uniform fat suppression signal, and it decreases artifacts and the effect of edge enhancement. The improvement of LAVA time resolution, with the acquirement of the serial three-dimensional volumetric by thinner slices, increases the detection rate of small lesions. Furthermore, it clearly delineates how tiny vessels are arranged and the relationship of the blood vessels in normal tissues and lesions. This is important for surgery and chemoembolization.[12-16]
Only the arterial, portal vein, and equilibrium phases are delineated in conventional MR dynamic enhancement scanning. But the early arterial phase of lesions is not easy to handle. In this study, we obtained more accurate acquisition of the hepatic artery phase because of joint application of the fluoroscopic triggering technique. In order to collect information of the artery only but exclude enhanced information of tissue and vein, we put the fluoroscopic site on the main pulmonary artery. It may come into the arterial and portal vein phases, which are pollution-free virtually. Inthis study the enhancement time of the portal vein phase was not always 60 seconds after injection of contrast agents. In many patients (accounting for 31%), the peak time of the portal vein is enhanced in the second phase, as portal vein phase obtained by conventional scanning is likely to be equilibrium phase.[17]The fluoroscopic triggering 3D LAVA technique suffices the needs of diagnosis and differential diagnosis, and it is effective to find the problem of blood supply.[18-20]Accurate arterial phase and portal vein phase images are always obtained by the fluoroscopic triggering technique, which is not used to measure the circulation time or influenced by differences in circulation. In this study, the enhanced peak time of the portal vein in 3 patients with hepatic cirrhosis was shorter than that of the aorta. This phenomenon might be associated with the retardation of blood flow from the hepatic sinusoids because of cirrhotic collagen deposition.[21]Our results suggest that dynamic enhancement scanning of the liver reflects the homodynamic changes to some extent.
Since the degree and characteristics of the focus enhancement are delineated,[22]it is important to identify the nature of the disease. Most hepatocellular carcinomas demonstrate clear characteristics of enhancement,[19]which is important for differential diagnosis with image reconstruction of LAVA. Similarly it is also important to reveal the degree of tumor cell changes, to judge therapeutic effects, to guide subsequent therapy, and to determine the prognosis after treatment. Furthermore, monitoring of precancerous lesions can detect carcinoma, even liver cirrhosis with vascularization and morphological changes around the nodes,[23-27]all of which enhance the degree of enhancement.
LAVA sequence shows multi-phase dynamic enhancement of hepatic lesions. Combined with conventional scanning technology, LAVA can effectively and safely display focal hepatic lesions, the relationship between tumor and normal tissues, and the extent of aggressiveness, especially the highly valuable blood vessels. This technique may help to evaluate lesion activity as a routine means for follow-up of PLC patients and avoid the irradiation induced by repetitive X-rays and CT in young patients. This technique with a highspeed, as a high temporal resolution, multiple imaging modalities, and safety has become one of the most important diagnostic tools.
Funding:None.
Ethical approval:Not needed.
Contributors:SXY proposed the study and wrote the first draft. CCH analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. SXY is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Tsim NC, Frampton AE, Habib NA, Jiao LR. Surgical treatment for liver cancer. World J Gastroenterol 2010;16:927-933.
2 Voroney JP, Brock KK, Eccles C, Haider M, Dawson LA. Prospective comparison of computed tomography and magnetic resonance imaging for liver cancer delineation using deformable image registration. Int J Radiat Oncol Biol Phys 2006;66:780-791.
3 Zhou XP, Yang GS, Lu JH, Zhang HB, Li QG, Han LZ, et al. Prospective study of liver parenchyma volume in hepatectomy of primary liver cancer. Zhonghua Wai Ke Za Zhi 2005;43: 1370-1374.
4 Kirchner J, Kickuth R, Laufer U, Noack M, Liermann D. Optimized enhancement in helical CT: experiences with a realtime bolus tracking system in 628 patients. Clin Radiol 2000; 55:368-373.
5 Lee CH, Goo JM, Lee HJ, Kim KG, Im JG, Bae KT, et al. Determination of optimal timing window for pulmonary artery MDCT angiography. AJR Am J Roentgenol 2007;188:313-317.
6 Danias PG, McConnell MV, Khasgiwala VC, Chuang ML, Edelman RR, Manning WJ. Prospective navigator correction of image position for coronary MR angiography. Radiology 1997;203:733-736.
7 Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108.
8 Zhang M, Li B, Yan LN, Yin F, Wen TF, Zeng Y, et al. Development of a survival evaluation model for liver transplant recipients with hepatocellular carcinoma secondary to hepatitis B. World J Gastroenterol 2008;14:1280-1285.
9 Sun Z, Ming L, Zhu X, Lu J. Prevention and control of hepatitis B in China. J Med Virol 2002;67:447-450.
10 Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol 2009;9:31.
11 Huang ZQ. Advance and development of clinical diagnosis of obstructive. Chin J Pract Surg 2001;21:449.
12 Nonami T, Harada A, Kurokawa T, Nakao A, Takagi H. Advances in hepatic resection and results for hepatocellular carcinoma. Semin Surg Oncol 1996;12:183-188.
13 Burke D, Earlam S, Fordy C, Allen-Mersh TG. Effect of aberrant hepatic arterial anatomy on tumour response to hepatic artery infusion of floxuridine for colorectal liver metastases. Br J Surg 1995;82:1098-1100.
14 Yang GS, Wu ZQ, Wu MC. Comprehensive treatment for primary liver cancer. Hepatobiliary Pancreat Dis Int 2003;2:23-27.
15 Dugdale PE, Miles KA, Bunce I, Kelley BB, Leggett DA. CT measurement of perfusion and permeability within lymphoma masses and its ability to assess grade, activity, and chemotherapeutic response. J Comput Assist Tomogr 1999;23: 540-547.
16 Brix G, Bahner ML, Hoffmann U, Horvath A, Schreiber W. Regional blood flow, capillary permeability, and compartmentalvolumes: measurement with dynamic CT--initial experience. Radiology 1999;210:269-276.
17 Hawighorst H, Schoenberg SO, Knopp MV, Essig M, Miltner P, van Kaick G. Hepatic lesions: morphologic and functional characterization with multiphase breath-hold 3D gadoliniumenhanced MR angiography--initial results. Radiology 1999;210: 89-96.
18 Grazioli L, Morana G, Kirchin MA, Schneider G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine-enhanced MR imaging: prospective study. Radiology 2005;236:166-177.
19 Shimada K, Isoda H, Okada T, Kamae T, Arizono S, Hirokawa Y, et al. Unenhanced MR portography with a half-Fourier fast spin-echo sequence and time-space labeling inversion pulses: preliminary results. AJR Am J Roentgenol 2009;193:106-112.
20 Bian J, Sha L, Yang C, Sun CS. Three-dimensional dynamic contrast-enhanced MR angiography for evaluating recipient vessels in orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int 2008;7:476-480.
21 Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology 2005;234:661-673.
22 Tajima T, Honda H, Taguchi K, Asayama Y, Kuroiwa T, Yoshimitsu K, et al. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol 2002;178:885-897.
23 Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? Br J Radiol 2003;76:220-231.
24 Thorgeirsson SS, Lee JS, Grisham JW. Molecular prognostication of liver cancer: end of the beginning. J Hepatol 2006;44: 798-805.
25 Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer 2002;2:210-219.
26 Holland AE, Hecht EM, Hahn WY, Kim DC, Babb JS, Lee VS, et al. Importance of small (< or=20-mm) enhancing lesions seen only during the hepatic arterial phase at MR imaging of the cirrhotic liver: evaluation and comparison with whole explanted liver. Radiology 2005;237:938-944.
27 Efremidis SC, Hytiroglou P. The multistep process of hepatocarcinogenesis in cirrhosis with imaging correlation. Eur Radiol 2002;12:753-764.
March 13, 2009
Accepted after revision February 13, 2010
Author Affiliations: Department of Radiology, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Shen XY, Chai CH, Xiao WB and Wang QD)
Chun-Hua Chai, MD, Department of Radiology, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236506; Email: sxyzlj@163.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Endoscopic management of biliary disorders during pregnancy
- Efficacy of middIe hepatic vein reconstruction in aduIt right-Iobe Iiving donor Iiver transpIantation
- Recombinant adenovirus vector Ad-hIL-10 protects grafts from cold ischemia-reperfusion injury following orthotopic liver transplantation in rats
- CT and MRI diagnosis of hepatic epithelioid hemangioendothelioma
- HiIar inflammatory pseudotumor mimicking hiIar choIangiocarcinoma
- Xanthogranulomatous pancreatitis treated by duodenum-preserving pancreatic head resection
