STUDY OF pH VALUE EFFECT ON THE SOLUBILITY OF SLOW RELEASE POTASSIUM
2010-02-23HEZhenYINHairongWANGWeiLIUXinnianTIANPeng
HE Zhen, YIN Hai-rong, WANG Wei, LIU Xin-nian, TIAN Peng
(School of Materials Science and Engineering, Shaanxi University of Science & Technology, Xi′an 710021, China)
0 Introduction
N, P, K is three main elements required for plants; currently the most important feature of commercial fertilizers is the instant dissolution. Used of instant fertilizers, crops production had been growth. But for the released per unit time much higher than the number absorbed of nutrients of crops, it is resulted to resources wasted, environmental pollution and other issues. At the same time, a substantial part of the fertilizers getting into the groundwater, rivers, lakes, occurred to volatilization lost and losing[1-6], polluted the water environment. Moreover, the long-term used fertilizers may cause soil compaction, and it had serious implications in the increase of grain yields.
With the appeared of slow-releasing fertilizers and controlled-releasing fertilizers, it not only overcame some ills of instant fertilizers, but also increased the yields.Those fertilizers can reduce environmental pollution and the waste of resources. The nutrients can be fully absorbed by plants with slowing released of nutrients and controlling nutrients released based on actual,and that also reduced the strength of fertilizers[7-14].In this paper, slow-releasing potassium fertilizers based on K2O-RO-SiO2(R=Ca,Mg) glass system with raw materials of slag and K2CO3was prepared by the method of sintering, and the solubility of potassium, magnesium ions in different pH values were studied by atom assimilation, and the results analyzed.
1 Experimental
With slag and K2CO3as the raw materials, in the target composition of K2O · MgO·SiO2for ingredients, milled and mixed uniformly, at 900 ℃, 950 ℃, 1 000 ℃, 1 050 ℃, 1 150 ℃ respectively. The chemical composition of slag used in Tab.1.
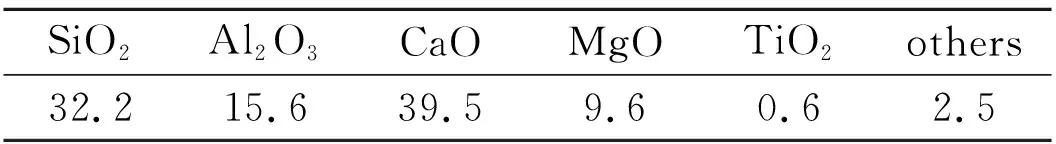
Tab.1 Slag chemical composition / wt%
To test the performance of fertilizers, we must accordingly do fertilizer experiment. In ideal situation, fertilizers directly applied to crops, then based on the growth of crops to determine the role of fertilizers. This process took a long time. In addition, plant roots ware able to release some of citric acid, which will be broken down the nutrients in the soil and turn to its own form for absorbing, in this view, fertilizers dissolved in citric acid can be detected its effects in some extent. The experiments were taken to pH=5,0.5 N hydrochloric acid solution, pH=2,2% citric acid and pH=4.5,2% of citric acid ammonium to make sure the soluble amounts of K2O, MgO, then determined the quality of fertilizers.We use the procedure below,first crush the samples, ball grind (through 80 sieve mesh),and then weigh 8 copies, each 1 g, dissolve four copies in 150 mL citric acid liquid and dissolve the others in 150 mL citric acid ammonium liquids. Mix 15,30,45,60 minutes, pump filter, dry the solid residue, weigh to determine the dissolution amount of atomic absorption spectrophotometer using 361MC.Recovery solution K+, Mg2+were detected, to determine whether K+, Mg2+and other ions dissolved.
2 Results and Discussion
2.1 Analysis weight loss of different dissolution media
In the process of dissolution, the solubility of a substance was connected with the property of the medium, different solvents had different solubility. Fig.1 was samples at pH=2, 2% of citric acid, pH=4.5, 2% of citric acid ammonium and 0.5 N hydrochloric acid dissolution weight loss curves.

Fig.1 Weightlessness curves of sample in different medium

Fig.2 K+ dissolved quantity of differentsamples in citric acidFig.3 K+ dissolved quantity of different samples in citric acid
We can see from Fig.1, samples dissolved in different solvents had very different weight loss, the dissolution capability of the samples in hydrochloric acid, citric acid, citric acid ammonium ware: hydrochloric acid>citric acid>citric acid ammonium. Such a result is mainly due to different pH values of the solutions, for the sample are alkaline substances. So the smaller pH values of the solvents, the greater the extent of the reaction. In this three solvents ,citric acid pH=2, citric acid ammonium pH=4.5, and 0.5 N hydrochloric acid was the acridest, so samples with hydrochloric acid reaction was most fully, thus, weight loss of sample′s dissolution was also biggest. The reaction of citric acid ammonium was most moderate; therefore the weight loss was smallest. In addition, in the course of the experiments, we also did groups of comparative tests with water as solvent. Stirred samples in 150 mL water with magnetic stirrer for 1 h, measured the weight loss solution only 4%,dissolved samples at room temperature in 8 days (no external mixing), only 13.9% weight loss solution,from this we can see the different solvent cause different weight loss solution. It is mainly due to K+, Mg2+, Ca2+and other metal ions can replacement H+in the solution, format soluble material, and then cause weight loss of the samples.
2.2 Analysis sample′s dissolution in different medium
To make sure the materials′ types and content, in the process of weight loss, dissolving about the samples with different sintered temperatures, we prepared the filtrate by atom assimilation, according to the data in the table to map out the stripping line of the dissolution about K+, Mg2+, show in Fig.2, Fig.3, Fig.4, and Fig.5.

Fig.4 Mg2+ dissolved quantity of different samples in ammonium citrateFig.5 Mg2+ dissolved quantity of different samples in ammonium citrate
As we can be see from the above chart that, K+, Mg2+, etc. dissolution change over the time, overall, the changes are relatively flat, indicated that each sample has a certain slow-releasing performance. In addition, as can be see part of the samples occurred in the process of dissolution peak (raised or sunken), focused on the dissolution time was about 45 min, this can be seen in the dissolution process of the specimen, there is a “solution-crystallization” of this dynamic equilibrium, and at the 45 min is exactly the turning point time of this dynamic equilibrium. Samples in different solution K+, Mg2+, etc. have different dissolution rates. Dissolution rate in citric acid is often greater than the percentage of citric acid ammonium, mainly because of the different pH values. Because the sample is alkaline substances, the smaller the pH values of solvents, the greater extent of the reaction. In this three solvents, citric acid pH=2, citric acid ammonium pH=4.5, while the hydrochloric acid solution of pH value was the smallest. In the same solution and dissolved time, the amount of dissolution percentage about K+was much greater than Mg2+. Under 900 ℃, K+dissolved amount was 80%~90%, while the Mg2+was 20 % to 30%.We can conclude that K+had a greater dissolution than Mg2+. According to the character of plant,at the begin of crop growth, citric acid quantity from base is little, dissolve of Potassium is little; latter of crop growth, citric acid quantity from base is more, dissolve of Potassium is more, satisfy the need pattern of Potassium in crop growth radically[4-10].
Fig.2, Fig.3 shows the specimens in the citric acid and citric acid ammonium, K+, Mg2+ions have a very good release. So by the above method, sample has a good solubility. Under 900 ℃, 950 ℃, 1 000 ℃ sintered,the samples have best performances of dissolution. In the citric acid, K+ion released rate was 70%, the highest value even reached more than 95% in some period of time, under 1 050 ℃sintered ,samples dissolution were 60% to 70%, and 1 150 ℃were 10% to 20%. K+ions dissolution rate in citric acid was often greater than the percentage of citric acid ammonium, mainly because of the different pH values. Because the sample was alkaline substances, the smaller pH values of solvents, the greater extent of the reaction. In this three solvents, citric acid pH=2, citric acid ammonium pH = 4.5, while the hydrochloric acid solution of pH value was the smallest. From Fig.4, Fig.5, show that Mg2+ions also has a good solubility in the citric acid and citric acid ammonium.In the process of citric acid to slow release of Mg2+ions, 900 ℃ sintering samples dissolution were higher than 950 ℃. For larger samples, 950 ℃ after sintering, the sample dissolution in citric acid was greater than 30%, maximum up to 70%, while the 900 ℃ sintering samples only 10% to 20%, from Fig.5 can draw similar conclusions.
3 Conclusion
The slow-releasing potassium fertilizer with slow releasing speed was prepared by the method of sintering from the raw materials of slag and K2CO3in the glass systems of K2O-RO-SiO2. Fertilizer′s dissolution capability in the 0.5 N hydrochloric acid, pH=2 of citric acid and pH=4.5 of citric acid ammonium was that: hydrochloric acid>citric acid>citric acid ammonium, presented alkalescency. By atom assimilation of fertilizers, the results showed that the solubility of potassium ions in citric acid is 95%, in citric acid ammonium is 86%, and so as the magnesium ions were 70%, 40%.
[1] GolovlvYuI, Zheplinskibm, Botsulavs. Slow-release granulated fertilizer[P]. USP 1224299, 1986-04-15.
[2] Waxman Anita, Lxpinmichaels. Controlled-release fertilizer[P]. IL107837, 1998-02-08.
[3] Toshihiro Kasuga, Yoshihiro Abe. Novel calcium phosphate ceramics prepared by power sintering and crystallization of glasses in the pyrophosphate region[J]. Journal of Material Research, 1998, 13(12): 3 357-3 359.
[4] Nakamura Hiroshi. Controlled-release fertilizer scooted with thermo setting resins[P]. JP09208355, 1997-08-12.
[5] ANDN Seninosuke, Yoshiware Hideo. Controlled-release fertilizes rand fertilization using them[P]. JP11116372, 1999-04-27.
[6] R K Brow, D R Tallent. Spectroscopic studies of the structure of titan phosphate and calcium titan phosphate glasses[J]. Physics and Chemistry of Glasses, 1997, 38(6): 300-306.
[7] Hideo Hosono, Yoshihiro Abe. Porous glass-ceramics composed of a titanium phosphate crystal skeleton: A review[J]. Journal of Non-crystalline Solids, 1995, 190: 185-197.
[8] SONG MeeYoug S. The controlled release matrix-type fertilizer and the process of preparation the reform[P]. WO9618591, 1996-01-20.
[9] Ibayaugustoc, Tenneylinwoodp. Polymers from hydrocyanic sand poly carboxylic acid[P]. US5206341, 1993-04-27.
[10] Richard K Brow, David R Tallant, T Myers,etal. The short-range structure of zinc polyphosphate glass[J]. Journal of Non-crystalline Solids, 1995, 191: 45-55.
[11] Honma Shocchi, Nakanmurat Sutomol,Vermiculiteas. Controlled-release fertilizer carrier[P]. JP6108039, 1986-03-11.
[12] Hiro SEHarao, Kawamura Masao, Mashitat akumi. Controlled-release fertilizer[P]. JP 60210585, 1985-10-23.
[13] Mitsubishi Chemical Industries Co, Ltd. Controlled-release nitrogen-phosphorus fertilizers[P]. JP5920673, 1984-08-14.
[14] E E Stroganova, N Yu Mikhailenko, O A Moroz. Glass-based biomaterials: present and future (a review)[J]. Glass and Ceramics, 2003, 60(9-10): 315-319.
