Numerical Investigation of Fuel Dilution Effects on the Performance of the Conventional and the Highly Preheated and Diluted Air Combustion Furnaces*
2009-05-15KiomarsAbbasiKhazaeiAliAsgharHamidiandMasoudRahimi
Kiomars Abbasi Khazaei**, Ali Asghar Hamidi and Masoud Rahimi
Numerical Investigation of Fuel Dilution Effects on the Performance of the Conventional and the Highly Preheated and Diluted Air Combustion Furnaces*
Kiomars Abbasi Khazaei1,**, Ali Asghar Hamidi2and Masoud Rahimi3
1Graduate School ofthe Environment and Energy, Science and Research Branch, Islamic Azad University, Tehran, Iran2Department of Chemical Engineering, Faculty of Engineering, Tehran University, Tehran, Iran3Department of Chemical Engineering, CFDResearch Center, Razi University, Kermanshah, Iran
This numerical study investigates the effects of using a diluted fuel (50% natural gas and 50% N2) in an industrial furnace under several cases of conventional combustion (air with 21% O2at 300 and 1273 K) and the highly preheated and diluted air (1273 K with 10% O2and 90% N2) combustion (HPDAC) conditions using an in-house computer program. It was found that by applying a combined diluted fuel and oxidant instead of their uncombined and/or undiluted states, the best condition is obtained for the establishment of HPDAC’s main unique features. These features are low mean and maximum gas temperature and high radiation/total heat transfer to gas and tubes; as well as more uniformity of theirs distributions which results in decrease in NOpollutant formation and increase in furnace efficiency or energy saving. Moreover, a variety of chemical flame shape, the process fluid and tubes walls temperatures profiles, the required regenerator efficiency and finally the concentration and velocity patterns have been also qualitatively/quantitatively studied.
highly preheated and diluted air combustion furnace, numerical modeling, chemical flame, fuel dilution, NOformation, energy saving
1 INTRODUCTION
The highly preheated and diluted air combustion (HPDAC) and the moderate or intense low oxygen dilution (MILD) combustion applied to industrial furnaces offer the main unique features such as: high energy savings, more uniform and relatively moderate gas temperature profile resulting in the reduction of pollutant emissions, a larger flame resulting in the low maximum local heat release rate and the possibility of lower combustion noise, and processing a higher quality of the furnace product at increased production rate [1-3]. These characteristics make this combustion process quite attractive, as most other abatement techniques result in some sort of financial penalty due to reduced thermal efficiency and productivity. Heat and diluted air recirculation in furnaces is an innovative approach to create the above mentioned main unique features. It is now well established that a mixture of reactants diluted with combustion products, at a temperature above auto-ignition, can achieve the desired outcome of reduced pollutant emissions and enhanced thermal efficiency, in other words narrowing the gap between these two objectives. The heat and the exhaust/inert gas recirculation quantities needed for fuel stream are less than those for air stream. Although this technology has been developed more than 10 years ago and has been commercially applied in different types of furnaces [2], the basic chemical-physical phenomenon still requires better understanding.
In order to obtain adequate knowledge of the main features of HPDAC or MILD combustion and creating them, Katsuki and Hesegawa [4] reviewed the latest development of MILD combustion and its applicability to industrial furnaces. They focused on the effects of heat-recirculating combustion under highly preheated air conditions (1200-1600 K). They found that intense mixing of the combustion air with burnt gases in the furnace, produced by high momentum ejection of combustion air, lowers the flame temperature and yields distributed reactions. Dally. [5, 6]reported on the structure of hydrocarbon non-premixed laminar and turbulent flames stabilized on a jet in a heated and diluted coflow. They found that major changes in the flame structure occurs when the oxygen level is reduced and that at higher jet Reynolds number and low oxygen concentration. Also, the effects of fuel mixture on the establishment of MILD combustion in a recuperative furnace were investigated. It was found that dilution of fuel with CO2or N2reduced the NOemission and made the flame inside the furnace invisible. Medwell. [7] reported the influence of fuel type on turbulent non-premixed jet flames under MILD combustion conditions. It is observed that under MILD combustion conditions, the fuel type there does not seem to be a significant effect on the structure of the reaction zone. The flow structure of the turbulent jet appears to control the flame more so than the chemistry in the MILD combustion conditions of the jet in hot coflow (JHC) burner. The valuable studies have been performed by Park[8-11]. In their studies, the flame structure and NO formation were investigated through the effects of CO2addition to either fuel- or oxidizer-side in H2-O2counterflow diffusion flame, the effects of H2O or CO2addition to either fuel- or oxidizer-side in CH4-O2-N2counterflow diffusion flame and in gas combustion of low calorific heating value.
The above review shows that a promising future for HPDAC or MILD technology is expected and the need for better fundamental understanding of the establishment and detailed structure of this combustion regime is warranted.
In addition, despite the above valuable numerical investigation of fuel dilution effects on the HPDAC or MILD combustion, attentions have been mostly focused on assessing the flame and combustion characteristics. In the present work, we make more efforts on the heat transfer and thermal behaviors.
The numerical study of this work is focused on the HPDAC burning system with a single burner in Fig. 1, because the literature review of the subject suggested that the study of a single burner and fuel jet allowed the explanation and measurement of many unique features of the HPDAC. For example, related experimental studies have been performed by Yang and Blasiak [12], Lille. [13] and other researchers.
The main objective of the present work is the study of the net/gross effects of applying a combined diluted fuel (50% natural gas and 50% N2) and oxidant under the following combustion conditions to an industrial furnace using an in-house computer program.
Using the undiluted air (21% O2) at 300 and 1273 K, and using the diluted air (90% N2and 10% O2) at 1273 K as oxidant.
As a new proposed combustion condition investigated in this paper which is not studied by other authors, a combined diluted fuel and oxidant instead of their uncombined and/or undiluted state is the best option for achieving the above mentioned HPDAC’s main unique features. In summary, the energy saving, the gas temperature profiles and uniformity and thus the pollutant emissions, the flame size and thus the local heat release rate, the temperature profiles of tubes wall and process fluids and thus the thermal performance of regenerator and furnace have been investigated through: (1) the net effects of the fuel/oxidant injection momentum flux ratio (by maintaining the temperature and oxygen concentration of oxidant) which is less than one, and (2) the net effects of the temperature and oxygen concentration [14] for the above mentioned oxidants (by maintaining the momentum flux ratio and fuel to oxidant velocity ratio which their values are less than one)varying the fuel and oxidant inlets sizes. As it will be seen later, by adjusting the operating fuel and thus the oxidant flow rates along with varying the size of their inlets, it is possible to optimize the performance of an existing furnace which is a candidate for revamping from a conventional combustion to an HPDAC or MILD combustion process.
2 MATHEMATICAL MODELING
2.1 Governing differential equations
The geometry of the furnace arrangement as shown in Fig. 1 is encountered in flows with substantial regions of recirculation. The equation used to represent conservation of the flow properties were, therefore, elliptic in form and were expressed in cylindrical coordinates. The general form of the equation is [15]:
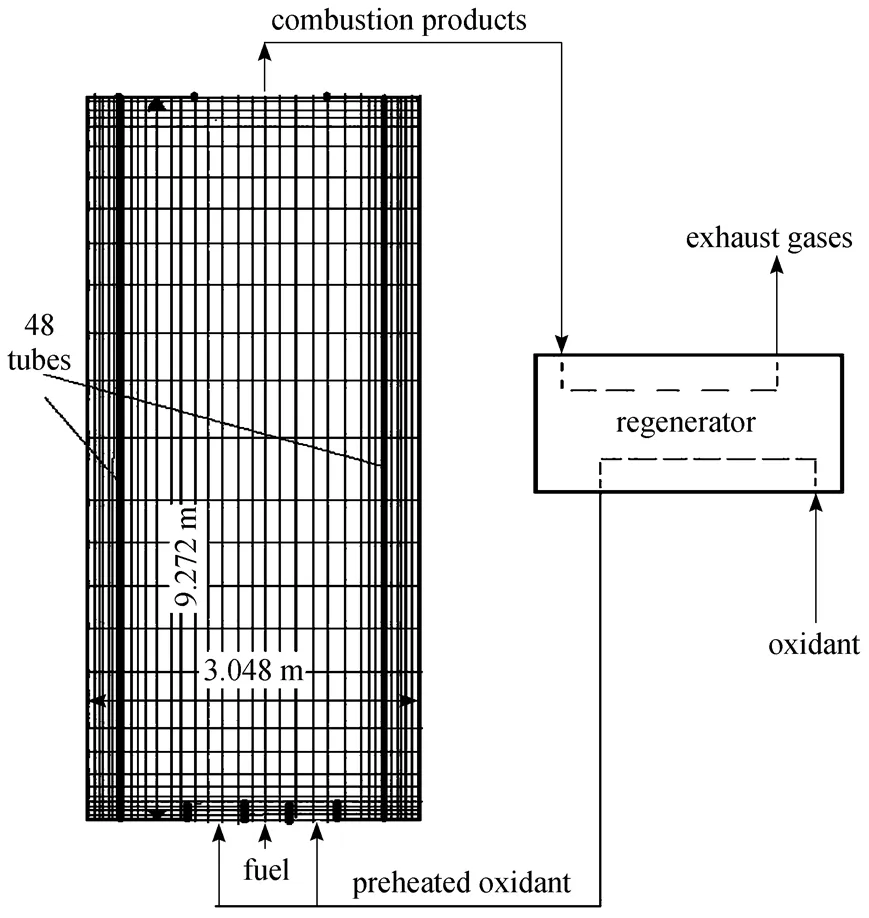
Figure 1 Furnace configuration and computational domain
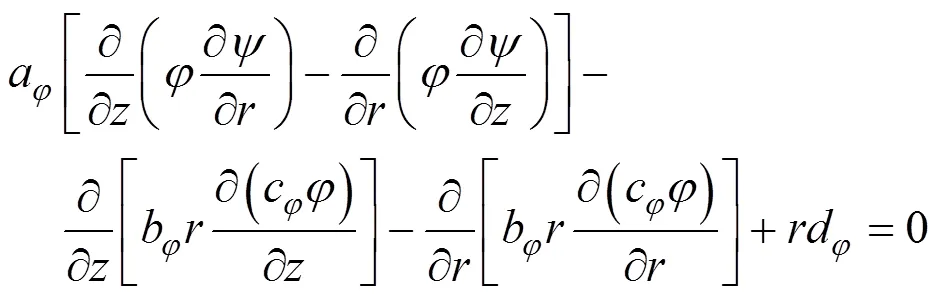

The general Eq. (1) is derived from the conservation of mass, momentum, energy and chemical species. The constitutive equations for the physical process such as turbulent transport and radiation are also added to the systems of equations.
It is assumed that (1) no external body forces act on the system, (2) species diffusion follows Flick’s law, (3) Lewis number for each chemical species is unity, (4) the kinetic heating terms in the energy equation are negligible, and (5) the gas follows the ideal gas equation of state. With these assumptions, the equations for conservation of mass, momentum, energy and chemical species may be written as illustrated by Eq. (1) and Table 1.
The instantaneous equations are transformed to yield equations for the time-averaged variables using a procedure known as Reynolds decomposition [16]. The pressure term is removed from the equations by application of the vorticity and stream function as the main hydrodynamic variables instead of the velocity components.
2.2 Turbulence models
In the momentum, chemical species and energy conservation equations, turbulent transport of momentum,mass and energy may be modeled using the Boussinesq approximation and the transport eddy diffusivities will be achieved by using a “two equations” or “-” model [17]. Here, for the sake of economy and the lack of sufficient boundary conditions and also the weakness of this model for prediction of the spreading rate for axisymmetric jets [18], another formula [19] is used:
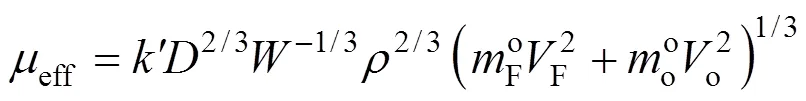
Further details of the mathematical models may be found in Refs. [15-17].
2.3 Turbulent combustion models
Despite the increase in computer power, there is interest in simple chemistry models that reproduce the main features of the combustion process accurately without excessive computing cost. Therefore, the lower computational cost of a simpler chemistry description is preferred. For instance, a simple one-step chemistry model has been adopted in recent works [20-22]. The equations for the species concentration and the species concentration fluctuation represent the combustion processes and will be referred to the combustion model. There are three main combustion models [23]. But for the sake of accuracy and the form of turbulence equation [Eq. (2)], only one of them is considered here. In this model, the fuel inlet is surrounded by the oxidant inlet as an annular orifice. The reaction is one step with fuel and oxidant unable to coexist at the same location and with the assumption of infinitely fast chemistry (physically controlled), according to the following reaction equation:

Table 1 The diffusion coefficients and source terms in Eq. (1)
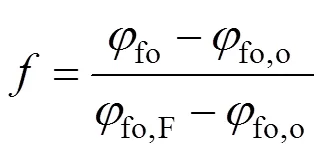
with
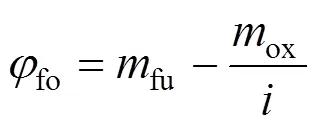
2.4 Radiation models
Thermal radiation enters as a source term in the enthalpy equation. For the evaluation of this term, radiation models are introduced. A simpler method is to replace the integro-differential equation by a system of differential equations using a flux approximation method [15]. The advantages which this transformation brings are twofold. First, the differential equations can be solved by standard finite difference techniques. Secondly, the finite difference equations are of the space “sparse matrix” kind, so that the zone temperature is linked only to its immediate neighbors. In the present research, equations of model are written for the rate of change of positive radiation fluxesandin the positive and negative coordinate directions.
In the absence of scattering, they are, for the axial direction [15]:
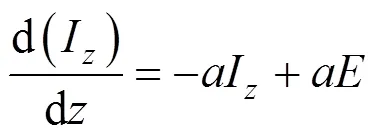
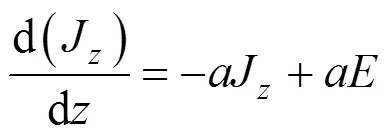
and for the radial direction:
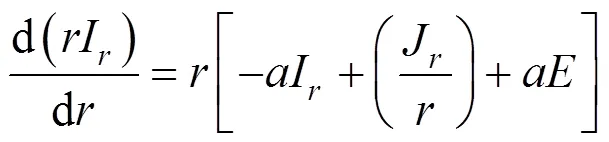
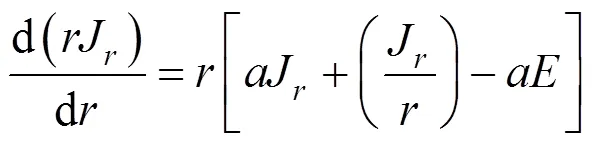
The working equations may be resulted from the combination of each pair of first order flux equations to yield a single second order equation:


Finally, the source term in the enthalpy equation is
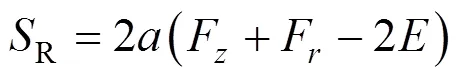
with [16]
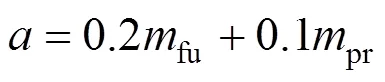
Further detail of this model can be found in Ref. [15]. These working equations are then cast into finite difference form; and the calculation of the radiation fluxes are embedded in the iteration cycle employed for the other variables.
2.5 Boundary conditions

3 NUMERICAL MODELING
The differential equations based on Eq. (1) were discretised in the finite difference form according to the control volume formulation as described by Patankar and Spalding [25]. To solve the resulted algebraic equation sets, the “point successive over relaxation” method [26] has been chosen. It is known that in certain circumstances this method is more rapid than the Gauss-Seidel method.

Table 2 Boundary conditions of dependent variables
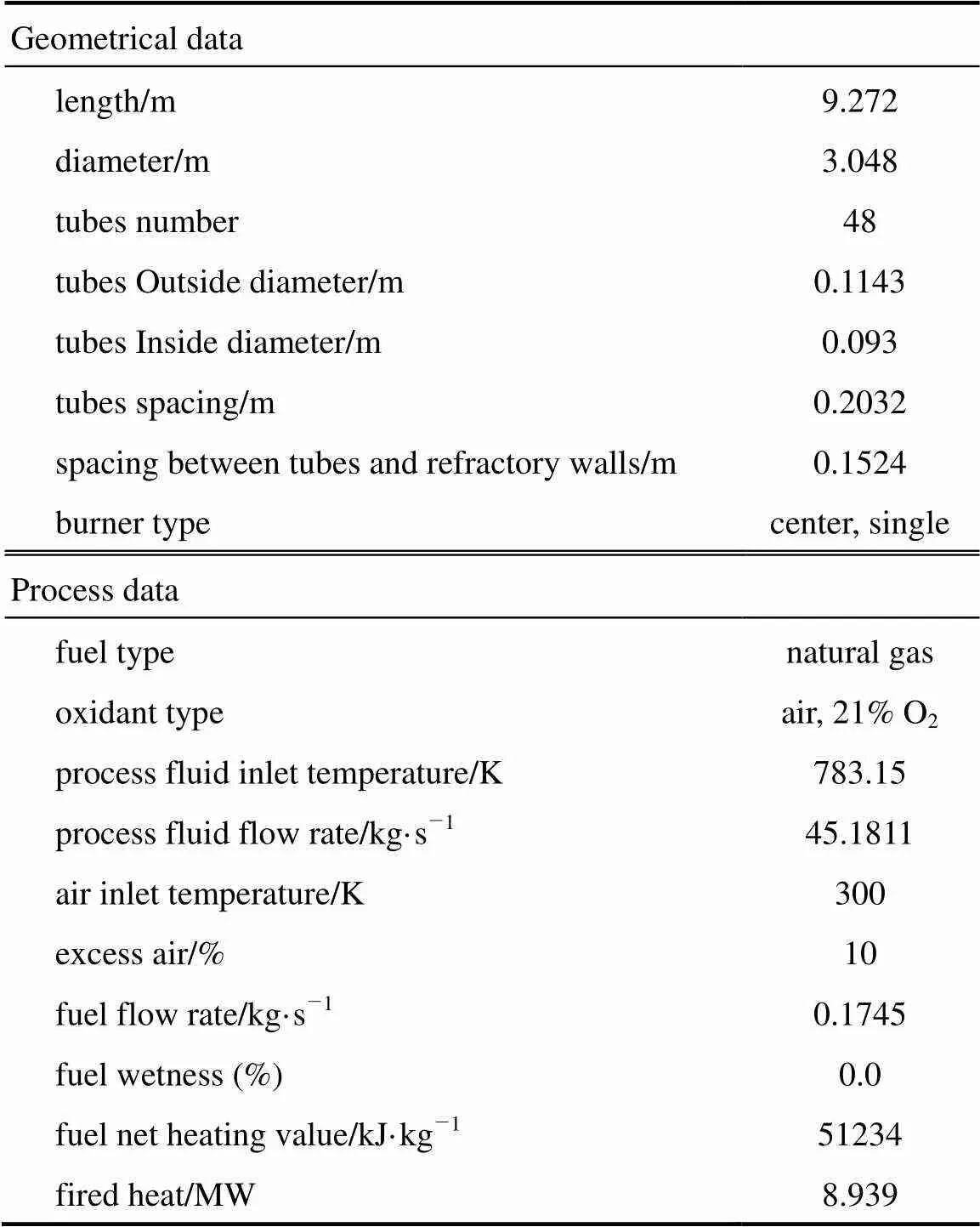
Table 3 Geometrical and process data of the existing industrial furnace
It should be noted that, for simulation of the HPDAC combustion, a steady state process was assumed in which the exhaust/firing cycles would not be reversed. A full description of the method is beyond the scope of this paper, so for the details of the numerical solution the reader is referred to Refs. [15, 23].
The accuracy which can be obtained with any finite-difference method of solution is closely tied up with the truncation error. By the reduction of the (tetrahedral) mesh size, this kind of error can be reduced. As can be seen from Table 4, the average difference between the overall results of case 4 (with 21×21 grid) and case 5 (with 71×71 grid), which are exactly the same, is about 2% relative to case 4 and between the overall results of case 1 (with 21×21 grid) and zone model case (with 80 surface and volume zones) [27] is about 10% relative to the zone model case. Moreover, the average relative discrepancies between the real [27] and the predicted bridge wall temperature are respectively about 0.7% for case 1 and 4.1% for zone model. These comparisons of results indicate the low truncation and round-off errors for the present model and as indicated by comparing between the real and predicted bridge wall temperature, its accuracy is higher than the zone model.
Furthermore to analyse the results of numerical simulation, authors proposed and used the new and/or modified parameters of chemical flame size, gas temperature uniformity ratio and flame peak temperature, as described in the following sections.
3.1 Chemical flame size
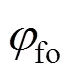
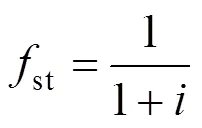
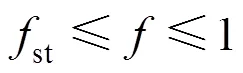
3.2 Gas temperature uniformity ratio
Numerous works in the literature state that the HPDAC combustion gives much more uniform temperature field than traditional combustion. Gas temperature uniformity ratio (GTU) is used to describe the gas temperature field uniformity inside the furnace as defined below:
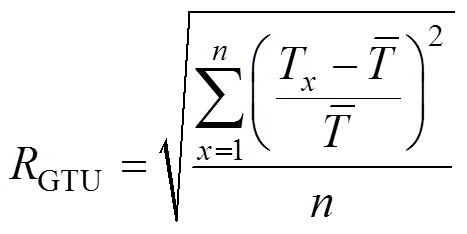
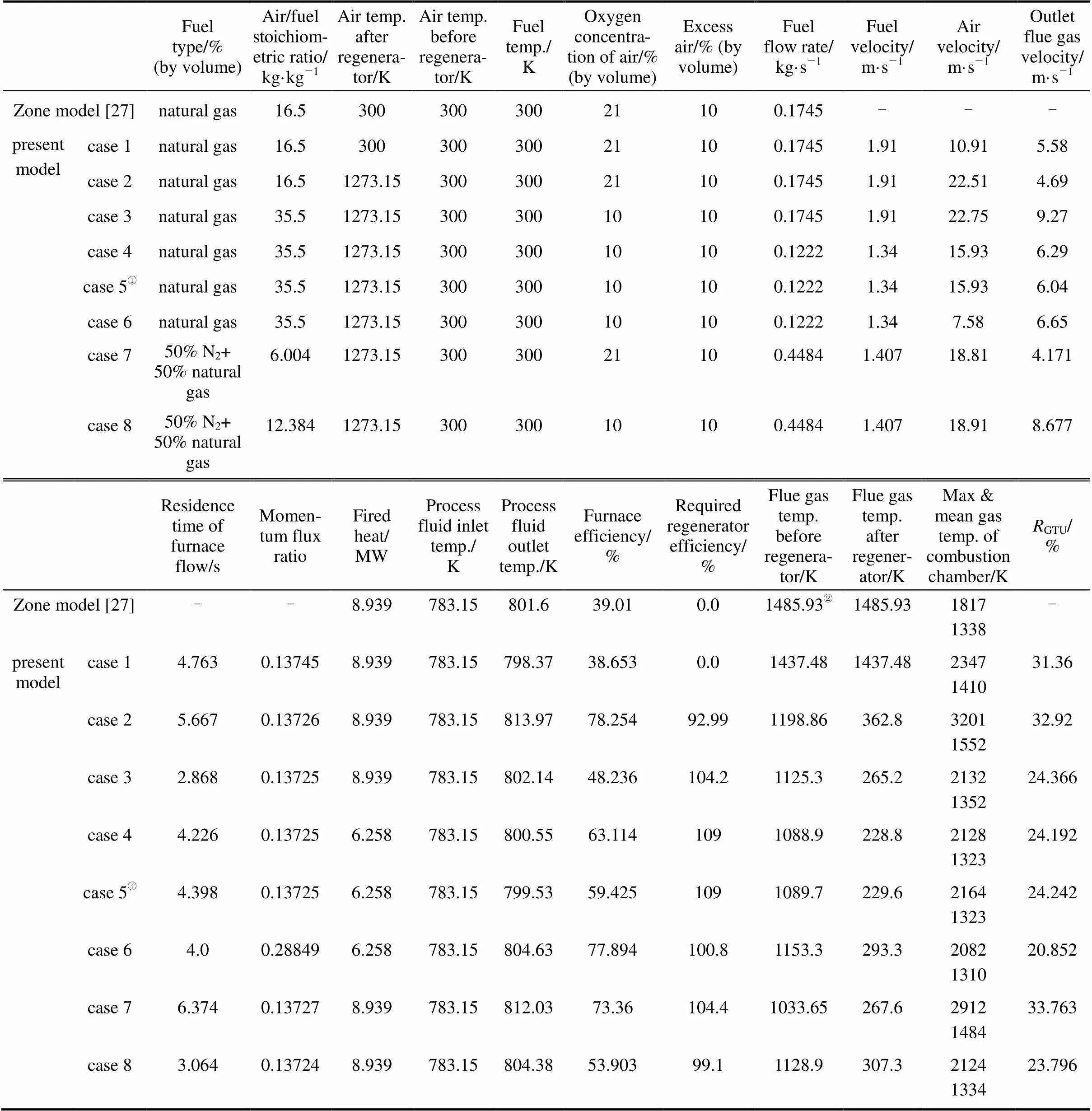
Table 4 The simulation results of the several cases studies
①Result according to 12 times increase of grid nodes number, respect to case 4.
3.3 Flame peak temperature
The higher flame temperature occurs somewhere in the flame envelope. At the flame peak temperature, it is assumed that the radiation fluxes can be neglected in comparison to the convection fluxes. So, the source term of energy equationRvanishes and from the similarity of the mixture fraction and energy equations and their boundary conditions it is then easy to deduce thatandmust be linearly related as follows:

Thus from Eqs. (13) and (15),
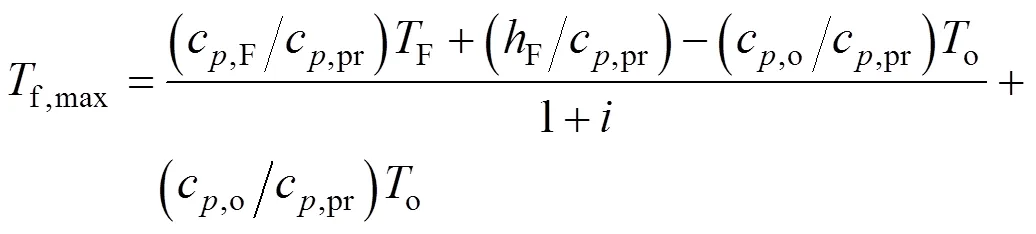
The simulation results, which have not been presented here, show that the average relative difference between the flame peak temperature calculated from the energy equation and the Eq. (16) is about 4%. This implies that, the above assumption is justified. From Eq. (16), it can be noted that for any oxidizer and fuel temperature, the flame peak temperature decreases with the reduction of oxygen concentration,.., the increase of, for oxidizer.
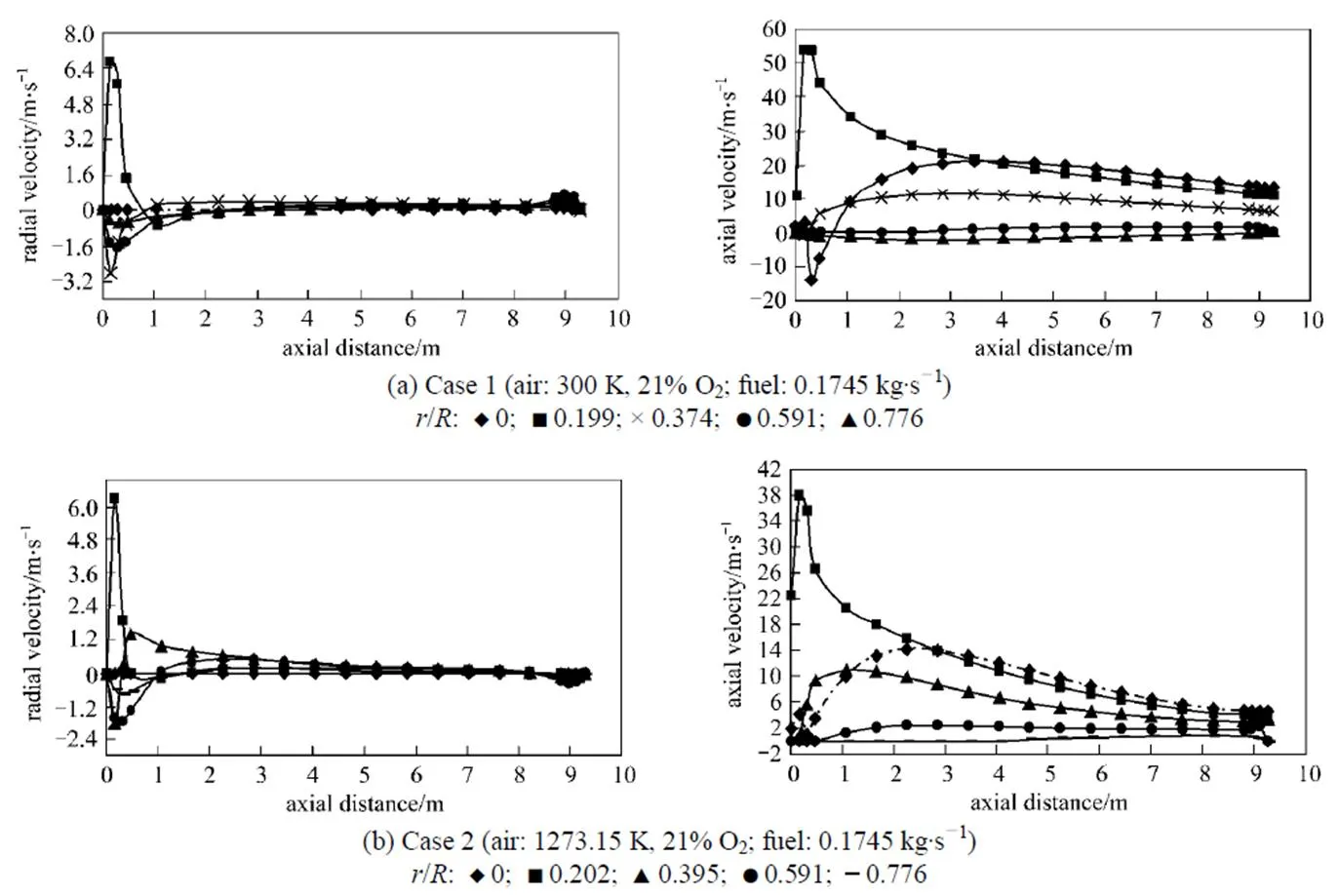
Figure 2 Axial and radial gas velocity profiles under the undiluted fuel and different conventional combustion conditions
(cases 1 and 2)
4 RESULTS AND DISCUSSION
In order to investigate the combustion phenomena and furnace performance, the simulation was carried out for the several cases of conventional combustion and HPDAC, as listed in Table 4.
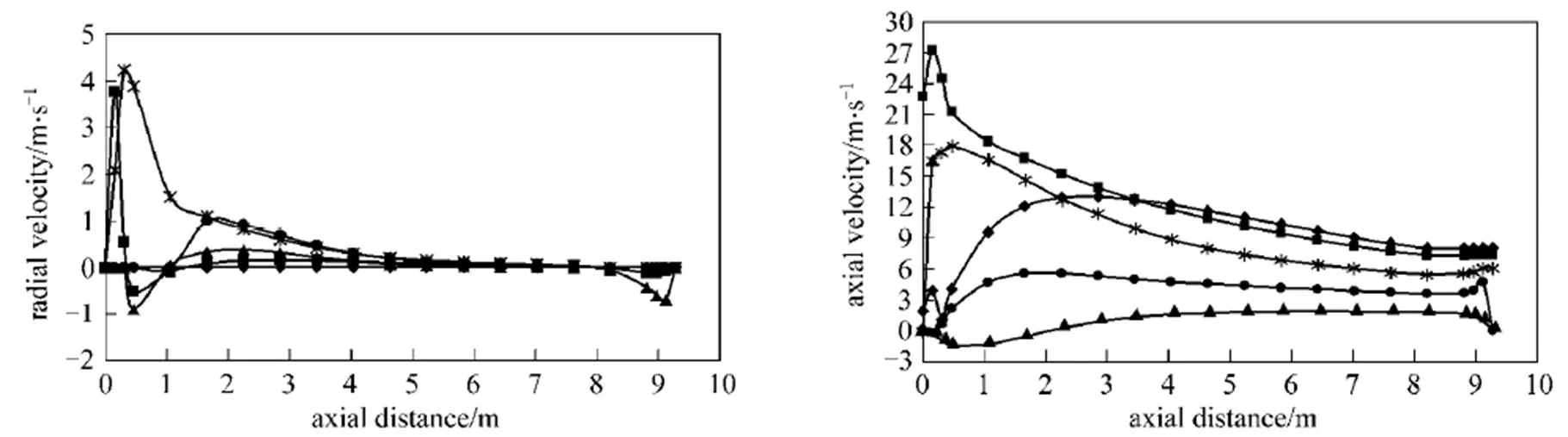
Figure 3 Axial and radial gas velocity profiles under the undiluted fuel and HPDAC condition [case 3: air (1273.15 K, 10% O2),fuel (0.1745 kg·s-1)]
/: ◆ 0; ■ 0.206; 0.41; ● 0.591; ▲ 0.776

Figure 4 Axial and radial gas velocity profiles under the diluted fuel and different conventional (case 7) and HPDAC (case 8) conditions
The momentum flux ratio [14] and velocity ratio [28] of the fuel gas jet to air flows are part of the conditions that was maintained constant for some of the studied cases. This provides a similarity in the mixing of fuel and air flows. Using this procedure, it is possible to identify the net effects of fuel dilution, combustion air preheat temperature and oxygen concentration. In other word, the simulated results can be related to the combustion process itself.
4.1 Velocity distribution
For the results illustrated in Figs. 2, 3 and 4, no experimental data are available for any comparison. However, it is should be noted that the trends of these results are in good agreements with those reported in the Refs. [14, 23, 29]. As can be seen from the above mentioned figures, the distributions of the mean axial and radial velocities are very complicated.
The corresponding fluctuations of the velocity close to the burner,.., the reaction zone, are comparatively large and tend to a uniform and lower value at downstream locations. As can be seen from Figs. 2, 3, 4, 9, 10 and 11, the maximum velocity corresponds to the flame peak temperature [14]. Recirculation zones which correspond to the negative values of velocities are related to the high temperature and correspondingly low values of density used in the solution of the momentum equation for the hotter regions of the flow. In the near wall regions, due to the lower values of the temperature and the correspondingly high value of density, and therefore more effects of the momentum changes, the flows circulate downward. Due to the higher heat capacity of diluted air and the lower heat capacity of diluted fuel with respect to the pure air and pure fuel, respectively [8], the reaction rate decreases and therefore, the jet expansion occurs over a larger volume, in both axial and radial directionsin the order of HPDAC>conventional combustion and/or as diluted fuel>undiluted fuel. Finally the lower velocities due to the lower flame temperature [6, 14]are obtained under the same values of the fired heat and the same momentum flux ratio/velocity ratio between the fuel gas jet and air flow. It can also be seen from the cases 3 and 8 of Table 4 or from the corresponding Figs. 3 and 4 that the change of the gas velocity with fuel dilution is very low, due to the lowest change of flame temperature [6]. These results are in a good agreement with the reported results in the Refs. [6, 8, 14, 28].
4.2 Fuel mass fraction distribution

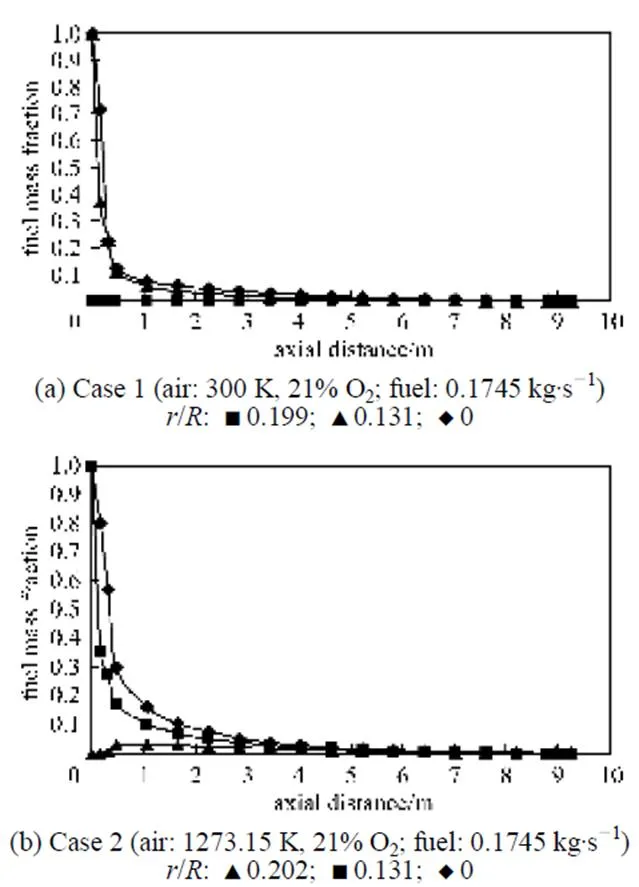
Figure 5 Fuel mass fraction distributions under the undiluted fuel and different conventional combustion conditions (cases 1 and 2)
Figure 6 Fuel mass fraction distributions under the undiluted fuel and HPDAC conditions [case 3: air (1273.15 K,10% O2); fuel (0.1745 kg·s-1)]
/: ■ 0.206; ▲ 0.131; ◆ 0
4.3 Temperature distribution
For the conventional combustion (low temperature air combustion), the results for tube wall, process fluid and gas temperature distributions of the zone model [27] and the present model are in good quantitative agreements [32]. From Fig. 8 and for the same values of the fired heat and momentum flux ratio/ velocity ratio between the fuel gas jet and air flow, it can be seen that the higher temperature uniformity and the lower maximum tube wall temperature vary as HPDAC-undiluted fuel>HPDAC-diluted fuel> conventional combustion (high temperature air)-undiluted fuel>conventional combustion (high temperature air)-diluted fuel. As illustrated in Table 4 and Figs. 9, 10 and 11, for any preheated oxidizer temperature, reduction of oxygen concentration or the dilution of fuel, results in a smaller region with high temperature [6] and lower value of flame peak temperature as calculated from Eq. (16). This means higher gas temperature uniformity. When the oxidizer temperature is increased, the zone of high temperatures tends to shrink; and therefore, a more intensive reaction takes place. As it was explained before, the thermodynamic limitations to the maximum temperature of the HPDAC combustion, the larger reaction volume and thus the low maximum local heat release rate, result in this uniform and relatively moderate temperature profile. Therefore formation of thermal-NO is suppressed in the HPDAC with respect to conventional combustion [12, 14, 31]. In addition, for the same values of the fired heat and momentum flux ratio/velocity ratio between the fuel gas jet and air flow, the lower flame peak temperature and hence the suppression of thermal-NO formation along with the gas temperature uniformity vary as HPDAC-diluted fuel>HPDAC-undiluted fuel>conventional combustion (low temperature air)-undiluted fuel>conventional combustion (high temperature air)-diluted fuel>conventional combustion (high temperature air)-undiluted fuel. From Table 4 and by comparing the cases 3, 7 and 8, it may be concluded that the change of furnace temperature with fuel dilution is very low [6] and that the flame peak temperature for fuel-side dilution is higher than that for oxidizer-side dilution [10].

Figure 7 Fuel mass fraction distributions under the diluted fuel and conventional (case 7) and HPDAC (case 8) conditions
It is interesting to note from Table 3 for cases 4 and 6, that a smaller ratio of fuel to oxidant injection momentum leads to a larger flame peak temperature and thus a larger NO production [14].
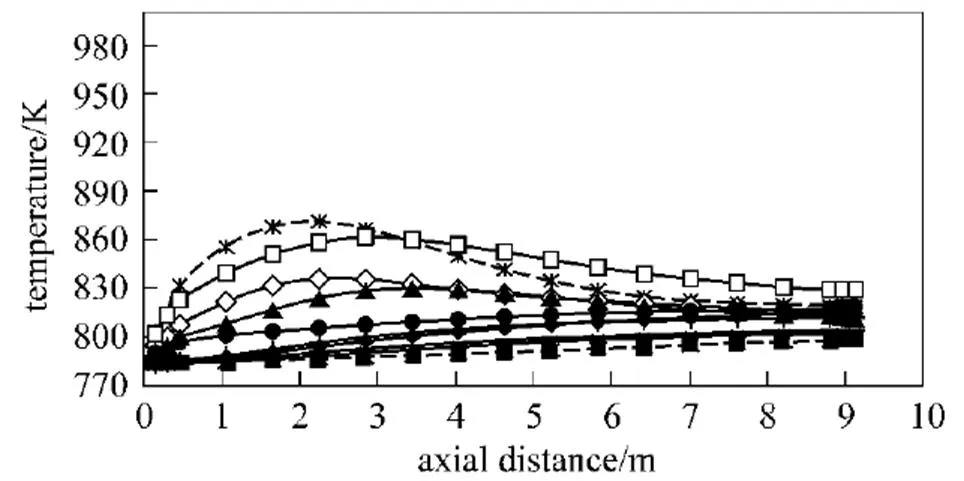
Figure 8 Tube wall and process fluid temperature distributions under the diluted and undiluted fuel and different conditions of conventional (cases 1, 2 and 7 with air of 21% O2) and HPDAC (cases 3 and 8 with air of 10% O2) for the present model
process fluid (air=1273.15 K): ◆ undiluted fuel, conv.; × undiluted fuel, HPDAC; + diluted fuel, conv.; —— diluted fuel, HPDAC;(air=300 K): ■ undiluted fuel, conv.
tube wall (air=1273.15 K): □ undiluted fuel, conv.; ▲ undiluted fuel, HPDAC; diluted fuel, conv.; ○ diluted fuel, HPDAC;(air=300 K): ● undiluted fuel, conv.
The trend of the results presented here, are in a good agreement with the results reported in the Refs. [6, 9, 10, 12, 14, 27, 30-33].
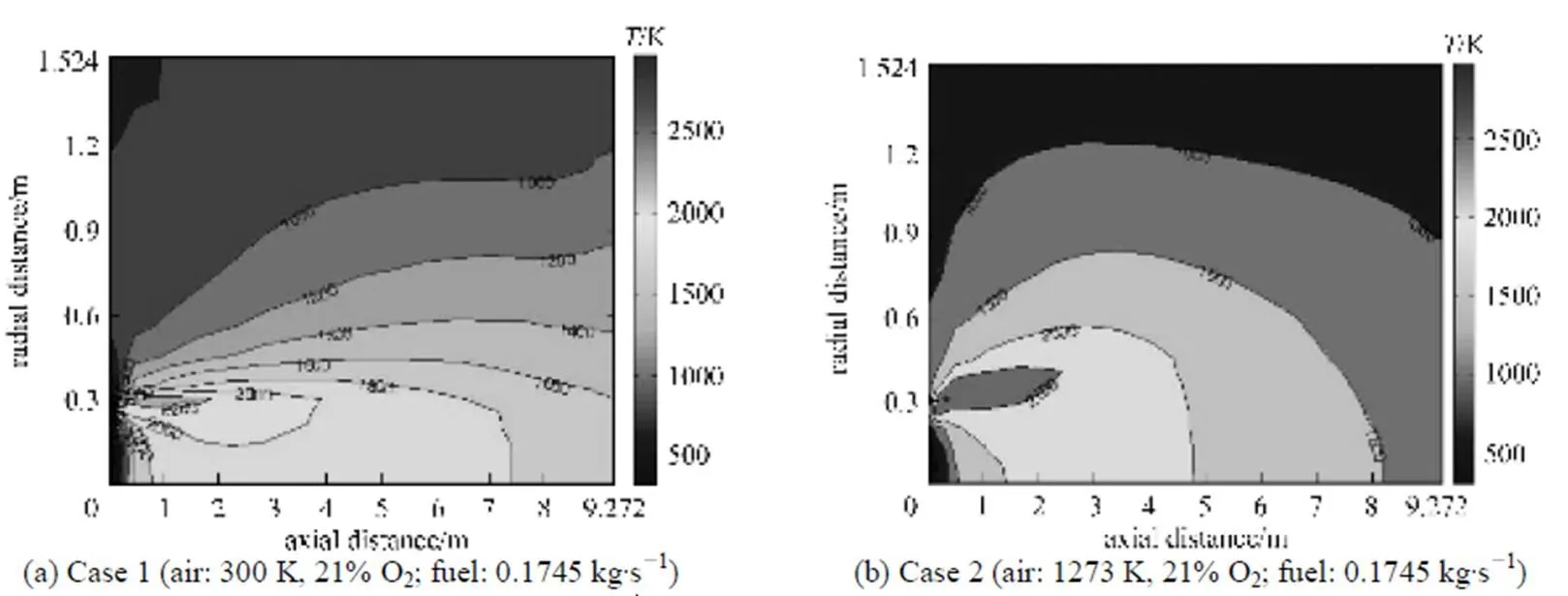
Figure 9 Gas temperature distributions under the undiluted fuel and different conventional combustion conditions (cases 1 and 2) for the present model
Figure 10 Gas temperature distributions under the undiluted fuel and different HPDAC conditions (cases 3 and 4) for the present model
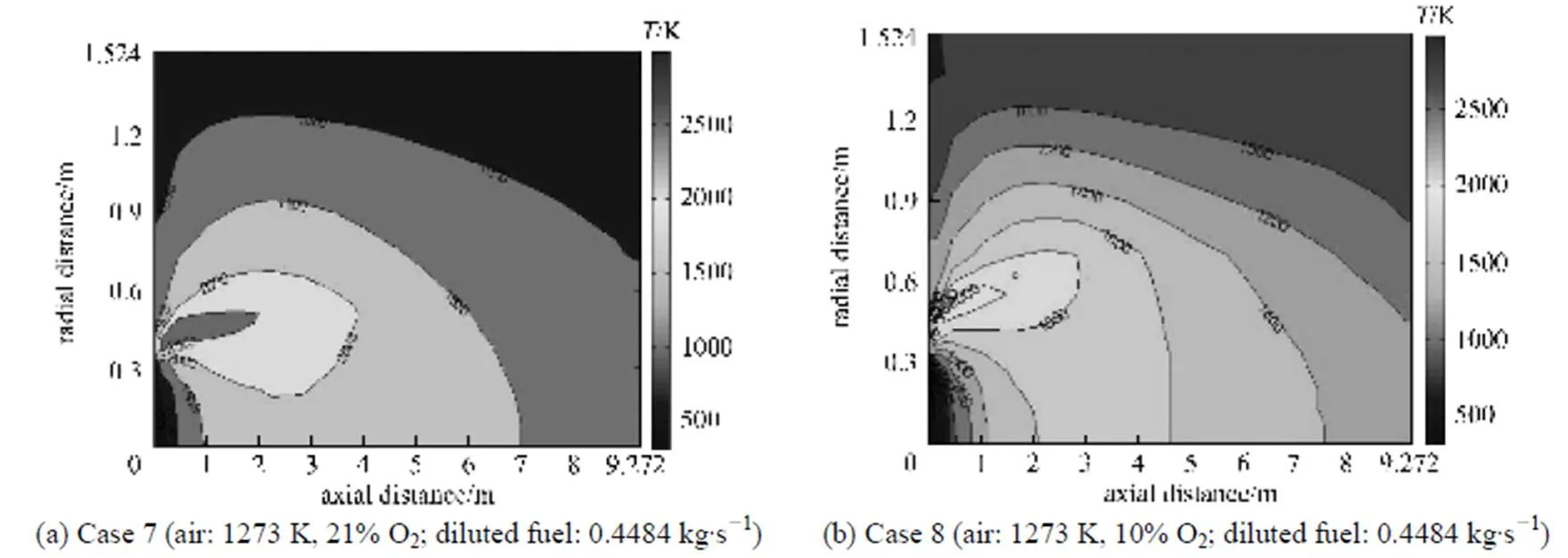
Figure 11 Gas temperature distributions under the diluted fuel and conventional (case 7) and HPDAC (case 8) conditions for the present model
4.4 Heat flux distribution
For the conventional combustion, it is interesting to note that, 96% of the total heat fluxes on the tubes are related to the radiation and only 4% are from convection heat transfer and also the results of tube wall total heat flux profiles for the present work and that of the zone model [27] are in a good quantitative agreement [32]. In spite of the variation of the lower flame peak temperature along with the gas temperature uniformity as discussed in the before section and according to the Fig. 12, the higher maximum tube wall total heat flux and the higher tube wall total heat flux non- uniformity vary as conventional combustion-diluted fuel>conventional combustion-undiluted fuel>HPDAC-diluted fuel>HPDAC-undiluted fuel. But and according to the Figs. 13, 14 and 15, the higher thermal radiation/total heat transfer for the gas field vary as conventional combustion (high temperature air)-diluted fuel>conventional combustion (high temperature air)-undiluted fuel>HPDAC-undiluted fuel>HPDAC- diluted fuel>conventional combustion (low temperature air)-undiluted fuel. These trends of lower flame peak temperature result in uniform heating of the furnace feed to be heated and therefore reduced energy requirements. This in turn means reduction of CO2and thermal-NO formations. It should be noted that, the negative values of radiation net heat flux are related to the radiation heat transfer in the negative coordinate directions. The trends of these latter results for undiluted fuel cases are in good agreements when compared with the results reported in the Refs. [14-16, 34, 35].
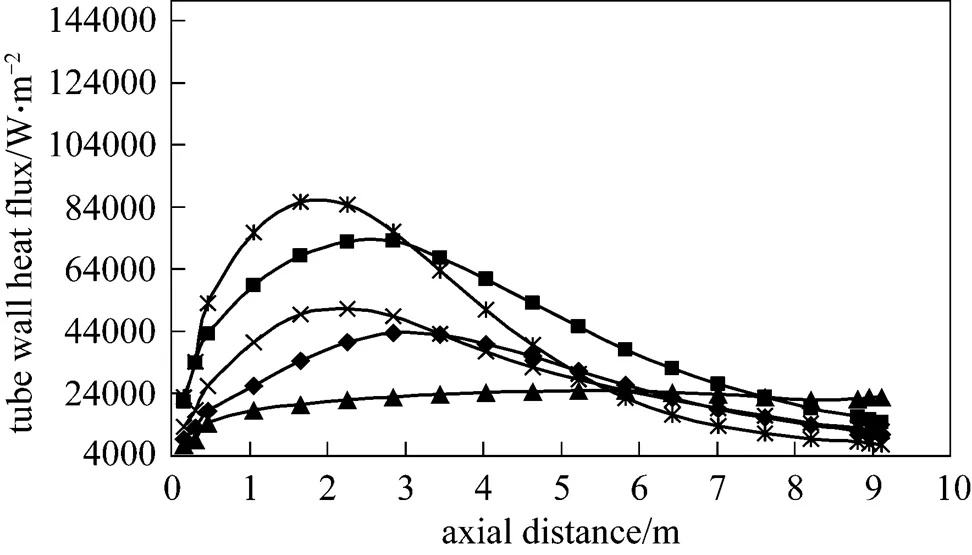
Figure 12 Tube wall total heat flux distributions under the undiluted and diluted fuel and different conventional (cases 1, 2 and 7 with air of 21% O2) and HPDAC (cases 3 and 8 with air of 10% O2) combustion conditions
undiluted fuel: ■ conv. withairof 1273.15 K; ◆ HPDAC withairof 1273.15 K; ▲ conv. withairof 300 K
4.5 Chemical flame volume
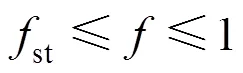
The flame zone is almost limited to the volume of the hypothetical cylinder created by the air jets due their strong injection momentum, and results in a rather long flame. The predicted results are found to agree well with respect to the shape of flame zone in the Refs. [14, 30, 35].
As can be clearly seen from Table 4 and the Figs. 5, 16 and 18, for the same values of the fuel to air momentum flux ratio/velocity ratio and the same value of the fired heat, when the preheat temperature of an oxidizer containing 21% oxygen is increased, the value of the fuel/diluted fuel to air density ratio increases and mixing becomes slower [28]. Finally a bigger chemical flame volume is resulted.
In the case of diluted fuel (Fig. 18), the stoichiometric mixture fraction shifts toward the rich side or centerline which has the highest scalar of dissipation and ensures the mixture of fuel and air is diluted before it can react [6]. Of course as the velocity ratio for cases 7 and 8 are higher than those of the cases 1 and 2, a shorter chemical flame lengths can be noted [28]. Also with a high preheat temperature, when the fuel is diluted or the oxygen concentration is reduced (Figs. 6, 7, 17 and 18), the reaction is less intense. Therefore an increased reaction,.., the chemical flame, volume in comparison to the conventional combustion is obtained. It is interesting to see from Fig. 17, that a maximum flame diameter occurs at the end of the flame [30] and that flame envelope terminates on the wall of furnace. This latter phenomenon implies that fuel would escape from the furnace without burning, an event which is undesirable. This can be controlled by adjusting the fuel to air flow rate ratio as illustrated in Fig. 17 and Table 4 (change from case 3 to case 6) or by diluting of the fuel as illustrated in Fig. 18.
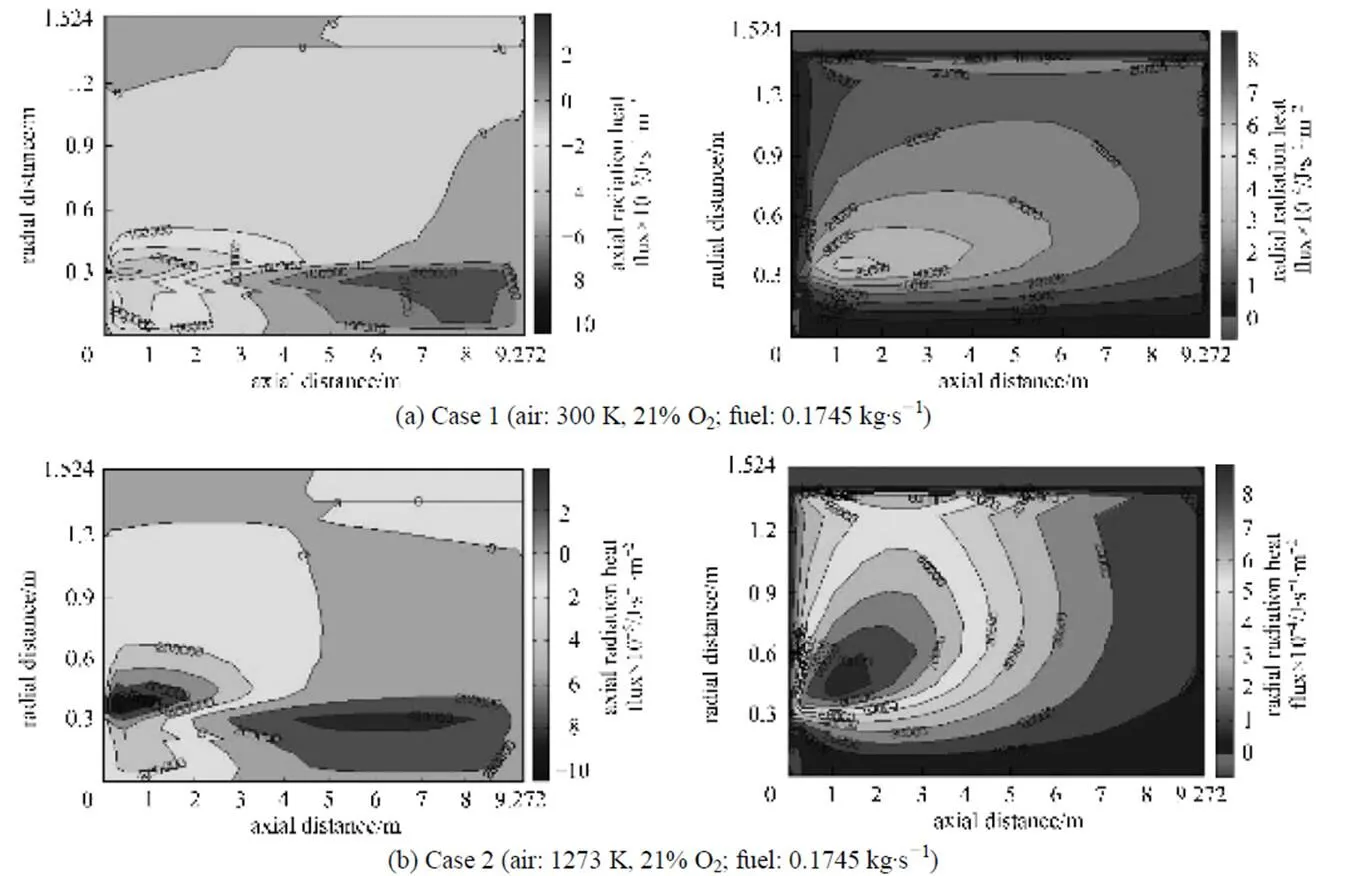
Figure 13 Axial and radial gas radiation net heat flux distributions under the undiluted fuel and different conventional combustion conditions (cases 1 and 2)
Figure 14 Axial and radial gas radiation net heat flux distributions under the undiluted fuel and different HPDAC conditions (cases 3 and 4)

Figure 15 Axial and radial gas radiation net heat flux distributions under the diluted fuel and conventional (case 7) and HPDAC (case 8) conditions
Figure 16 Chemical flame shapes under the undiluted fuel and different conventional combustion conditions (cases 1 and 2)
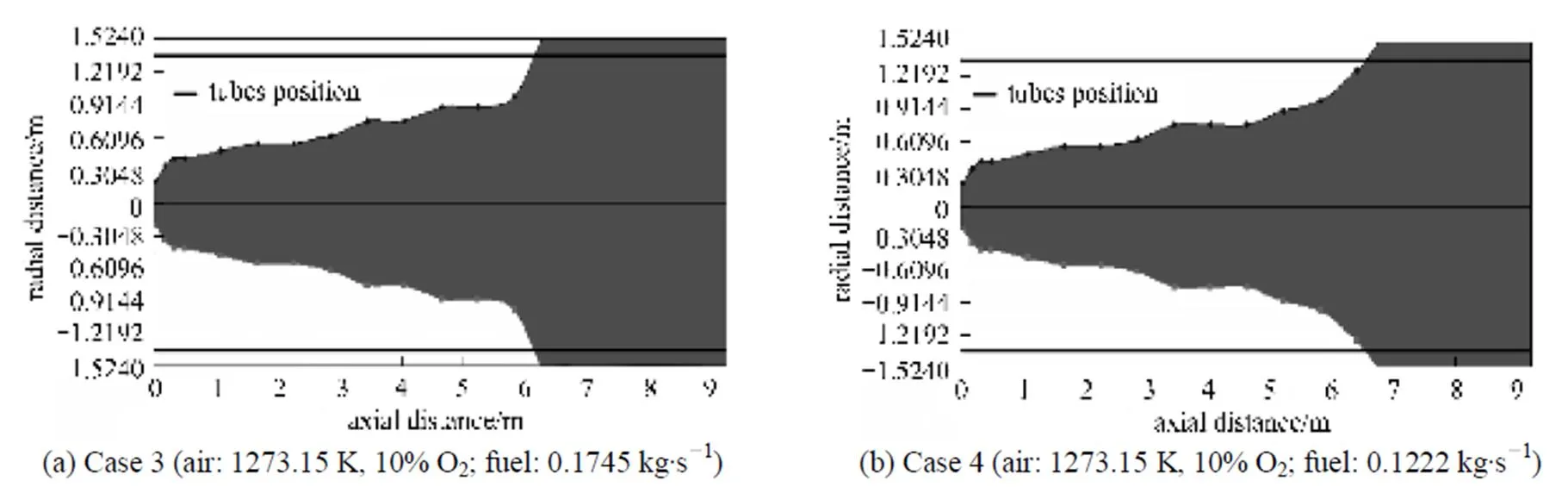
Figure 17 Chemical flame shapes under the undiluted fuel and different HPDAC conditions (cases 3 and 4)
Figure 18 Chemical flame shapes under the diluted fuel and conventional (case 7) and HPDAC (case 8) conditions
A maximum flame diameter occurring at the middle part of the flame, is another interesting result that may be concluded from this figure, that may be translated in to a burner cycle or twine flame HPDAC [35]. The trends of these results, specially, having a long flame for conventional combustion [27] and a maximum flame diameter occurring at the end of the flame (because of the only half a burner cycle or single flame calculation) [30], are also validated by comparing with the reported results [6, 27, 28, 30, 35].
4.6 Furnace and regenerator efficiency
As can be seen from Table 4, in spite of the high furnace efficiency for high temperature conventional combustion in the cases of using the diluted or undiluted fuel (cases 2 and 7), these cases are not desirable in comparison to the ambient temperature conventional one (case 1). This is due to the higher non-uniformity and thermal-NO formation resulted from the higher flame peak temperatures by about 36% and 24% for cases 2 and 7, respectively.
In addition, in the view of the world’s energy- environmental requirements, it appears that the HPDAC with diluted and then undiluted fuel combustion (cases 8 and then 3) are more suitable than the conventional combustion (cases 1 and 2). This is due to the lower flame peak temperatures,.., the suppression of the thermal-NO formation for cases 8 and 3 by about 9.5% and 9% in comparison to case 1, and by about 33.65% and 33% in comparison to case 2. Cases 8 and then 3, result in about 6% increase in furnace efficiency in comparison to each other and by about 16% and 10% in comparison to case 1. Their required regenerator efficiency is decreased by about 5% in comparison to each other and in comparison to that of case 2 also decreased by about 6% for case 8 and 11% for case 3. Finally using an appropriate fuel flow rate (for example, case 6 instead of case 3) would result in about 30% lower fuel or energy consumption and about 29%-39% higher furnace efficiency in comparison to cases 1 and 3. The regenerator would also have higher efficiency.
5 CONCLUSIONS
(1) For an industrial furnace under the conventional/HPDAC combustion conditions, using the diluted fuel instead of the undiluted one, lower radial and axial gas velocities, higher maximum tube wall temperature as well as their lower uniformity are experienced. Moreover, a fuel mass fraction distribution corresponding to a reaction zone with the higher width and length, the higher maximum radial and axial total heat fluxes for gas field and tubes wall are also noted. Having a variety of chemical flame shape, the lower flame peak temperature resulting in the lower thermal-NO formations along with the higher gas field temperature uniformity as well as the higher furnace and required regenerator efficiencies resulting in the lower CO2emission, are the other fuel dilution effects.
(2) Application of combined diluted fuel and oxidant instead of their uncombined and/or undiluted states is the best option for the establishment of HPDAC’s main unique features. This means the lower mean and maximum temperature of gas and the higher radiation/total heat transfer to it and to tube walls along with more uniformity of theirs distributions and thus a decrease and an increase in respectively NOpollutantformation and furnace efficiency or energy saving.
(3) From the qualitative/quantitative justification of the simulation results with respectively the results of other simulations reported in the literature and those of the zone model results, the present written computer program is found to be able to represent and simulate the conventional and HPDAC combustion processes along with the diluted/undiluted fuel usage for the furnace described in Ref. [27].
NOMENCLATURE
1,2tube wall side area, m2
flux-model absorption coefficient, m-1
1,2constants in turbulence model
cspecific heat capacity, J·kg-1·K-1
furnace diameter, m
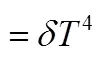

mixture fraction
square of the fluctuation of concentration
stagnation enthalpy, J·kg-1
cconvection heat transfer coefficient, W·m-2· K-1
Ffuel calorific value, J·kg-1
positive radiation flux in the positive co-ordinate direction, W·m-2
air to fuel stoichiometric ratio
positive radiation flux in the negative co-ordinate direction, W·m-2
kinetic energy of turbulence per unit mass, m2·s-2
mass fraction
omass flow rate, kg·s-1
number of grid nodes
furate of chemical reaction
radial distance from axis of symmetry, m
Kcorrelation related to-equation
Rsource or sink of energy per unit volume and time associated with radiation
absolute temperature, K
velocity, m·s-1
1,2fluid mean velocity in the axial and radial direction respectively, m·s-1
3normal tangential velocity, m·s-1
furnace length, m
axial co-ordinate along the furnace, m

stefan-boltzman constant, W·m-2· K-4
dissipation rate of turbulent kinetic energy, m2·s-3
effeffective viscosity, W·m-1·s-1
density, kg·m-3
vorticity
Subscripts
eff effective (related to combined laminar and turbulent effects)
F inlet fuel stream
f flame
fo “fuel-oxidant” in any points of flow inside the combustion chamber
fu fuel
o inlet oxidant stream
ox oxidant
p process fluid
pr product
radial direction
st stoichiometric
w tube wall
grid nodes number indicator
axial direction
1,2 two neighboring tube wall nodes
1 Hasegawa, T., Tanaka, R., Niioka, T., “Combustion with high temperature low oxygen air in regenerative burners”, In: The first Asia-Pacific Conference on Combustion, Osaka, Japan, 290-293 (1997).
2 Yasuda, T., Ueno, C., “Dissemination project of industrial furnace revamped with HTAC”, In: The Second International Seminar on High Temperature Combustion in Industrial Furnace, Stockholm, Sweden, 1-7 (2000).
3 Yang, W., Blasiak, W., “Combustion performance and numerical simulation of a high temperature air-LPG flame on a regenerative burner”,..., 33 (2), 113-120 (2004).
4 Katsuki, M., Hasegawa, T., “The science and technology of combustion in highly preheated air”,..., 27 (2), 3135-3146 (1998).
5 Dally, B.B., Karpetis, A.N., Barlow, R.S., “Structure of turbulent non-premixed jet flames in a diluted hot co flow”,..., 29 (1), 1147-1154 (2002).
6 Dally, B.B., Riesmeier, E., Peters, N., “Effect of fuel mixture on moderate and intense low oxygen dilution combustion”,., 137 (4), 418-431 (2004).
7 Medwell, P.R., Kalt, P.A.M., Dally, B.B., “Influence of fuel type on turbulent non-premixed jet flames under MILD combustion conditions”, In: The 16th Australasian Fluid Mechanics Conference, Gold Coast, Australia, 1350-1355 (2007).
8 Park, J., Choi, J.G., Keel, S.I., Kirn, T.K., “Flame structure and NO emission in gas combustion of low calorific heating value”,..., 27 (15), 1339-1361 (2003).
9 Park, J., Hwang, D.J., Chung, J.O., Keel, S.I., Shim, S.H., Lee, S.B., “Comparative study of flame structures and NOemission characteristics in fuel injection recirculation and fuel gas recirculation combustion system”,..., 28(10), 861-885 (2004).
10 Hwang, D.J., Park, J., Oh, C.B., Lee, K.H., Keel, S.I., “Numerical study on NO formation in CH4-O2-N2diffusion flame diluted with CO2”,..., 29 (2), 107-120 (2005).
11 Park, J., Hwang, D.J., Choi, J.G., Lee, K.M., Keel, S.I., Shim, S.H., “Chemical effects of CO2addition to oxidizer and fuel streams on flame structure in H2-O2counterflow diffusion flames”,..., 27 (13), 1205-1220 (2003).
12 Yang, W., Blasiak, W., “Numerical study of fuel temperature influence on single gas jet combustion in highly preheated and oxygen deficient air”,, 30 (2-4), 385-398 (2005).
13 Lille, S., Blasiak, W., Jewartowski, M., “Experimental study of the fuel jet combustion in high temperature and low oxygen content exhaust gases”,, 30 (2-4), 373-384 (2005).
14 Mortberg, M., “Study gas fuel jet burning in low oxygen content and high temperature oxidizer”, Ph.D. Thesis, Royal Institute of Technology, Sweden (2005).
15 Gosman, A.D., Lockwood, F.C., “Incorporation of flux model for radiation into a finite difference procedure for furnace calculation”, In: The Proceeding 14th International Combustion Symposium, Combustion Inst., USA, 661-671 (1972).
16 Truelove, J.S., Heat Exchanger Design Handbook, Hemisphere Publishing Corporation, New York (1983).
17 Launder, B.E., Spalding, D.B., Mathematical Models of Turbulence, Academic Press, New York (1972).
18 Fluent Inc., Fluent 5 Manual, New Hampshire (1998).
19 Pun, W.M., Spalding, D.B., “A procedure for predicting the velocity and temperature distributions in a confined, steady, turbulent, gaseous, diffusion flame”, In: Proceeding of International Astronautical Federation Meeting, Belgrade, Yugoslavia (1967).
20 Fernandez-Tarrazo, E., Sanchez, A.L., Linan, A., Williams, F.A., “A simple one-step chemistry model for partially premixed hydrocarbon combustion”,., 147 (1/2), 32-38 (2006).
21 Fernandez-Tarrazo, E., Vera, M., Linan, A., “Liftoff and blowoff of a diffusion flame between parallel streams of fuel and air”,.., 144 (1/2), 261-276 (2006).
22 Garrido-Lopez, D., Sarkar, S., “Effects of imperfect premixing coupled with hydrodynamic instability on flame propagation”,..., 30 (1), 621-628 (2005).
23 Khalil, E.E., Spalding, D.B., Whitlaw, J.H., “The calculation of local flow properties in two dimensional furnaces”,.., 18, 775-791 (1975).
24 Gosman, A.D., Pun, W.M., Runchal, A.K., Spalding, D.B., Wolfshetein, M., Heat and Mass Transfer in Recirculating Flow, Academic Press, London (1969).
25 Patankar, S.V., Spalding, D.B., “A calculation procedure for heat, mass and momentum transfer in three-dimensional parabolic flows”,.., 15, 1787 (1972).
26 Varga, R.S., Matrix Iterative Analysis, Prentice-Hall International, London (1962).
27 Nogay, R., Prasad, A., “Better design method for fired heaters”,., 91-95 (1985).
28 Ghia, K.N., Lavan, Z., Torda, T.P., “Turbulent mixing in the initial region of heterogeneous axisymmetric coaxial confined jets”, In: Report No. NASA CR-1615, Illinois Institute of Technology, Chicago (1970).
29 Hutchinson, P., Khalil, E.E., “The calculation of furnace flow properties and their experimental verification”,, 276-283 (1976).
30 Yang, W., Blasiak, W., “Numerical simulation of properties of a LPG flame with high temperature air”,...., 44 (10), 973-985 (2005).
31 Blasiak, W., Yang, W.H., Rafidi, N., “Physical properties of a LPG flame with high temperature air on a regenerative burner”,., 136 (4), 567-569 (2004).
32 Abbasi Khazaei, K., Hamidi, A.A., Rahimi, M., “Numerical modeling and simulation of highly preheated and diluted air combustion furnaces”,..., 22 (2), 107-118 (2009).
33 Yuan, J., Naruse, I., “Effects of air dilution on highly preheated air combustion in a regenerative furnace”,, 13 (1), 99-104 (1998).
34 Kobayashi, H., Yoshikawa, K., “Thermal performance and numerical simulation of high temperature air combustion boiler”, In: The Proceeding International Joint Power Generation Conference (IJPGC2000-15083), Miami Beach, Florida (2000).
35 Rafidi, N., “Thermodynamic aspects and heat transfer characteristics of HiTAC furnaces with regenerators”, Ph.D. Thesis, Royal Institute of Technology, Sweden (2005).
2008-11-12,
2009-09-03.
the National Iranian Oil Company (NIOC).
** To whom correspondence should be addressed. E-mail: kns_abbasi@yahoo.com
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
- Prediction of Critical Endpoints Based on the PR Equation of State*
- Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
- Calculation of Transport Properties of CF4+Noble Gas Mixtures
- Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
- A New Study on Combustion Behavior of Pine Sawdust Characterized by the Weibull Distribution*
