Kinetics of 2-Methyl-6-acetyl-naphthalene Liquid Phase Catalytic Oxidation
2009-05-14TIANWenyu田文玉XUEWeilan薛为岚ZENGZuoxiang曾作祥andSHAOJi邵记
TIAN Wenyu (田文玉), XUE Weilan (薛为岚), ZENG Zuoxiang (曾作祥),* and SHAO Ji (邵记)
Kinetics of 2-Methyl-6-acetyl-naphthalene Liquid Phase Catalytic Oxidation
TIAN Wenyu (田文玉)1, XUE Weilan (薛为岚)2, ZENG Zuoxiang (曾作祥)2,* and SHAO Ji (邵记)2
1Department of Chemical Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, China2Institute of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
In this paper, a kinetics model for the liquid-phase oxidation of 2-methyl-6-acetyl-naphthalene to 2,6-naphthalene dicarboxylic acid catalyzed by cobalt-manganese-bromide is proposed. The effects of the reaction temperature, catalyst concentration and ratio of catalyst on the time evolution of the experimental concentration for the constituents including raw material, intermediates and product are investigated. The model parameters are determined in a nonlinear optimization, minimizing the difference between the simulated and experimental time evolution of the product composition obtained in a semi-batch oxidation reactor where the gas and liquid phase were well mixed. The kinetics data demonstrate that the model is suitable to the liquid-phase oxidation of 2-methyl-6-acetyl-naphthalene to 2,6-naphthalene dicarboxylic acid.
2-methyl-6-acetyl-naphthalene, 2,6-naphthalene dicarboxylic acid, liquid phase catalytic oxidation, kinetics
1 INTRODUCTION
2-Methyl-6-acetyl-naphthalene (2,6-MAN), white or pale yellow powdery crystal with the melting point of 332.15 K, is an important intermediate [1, 2] for producing 2,6-naphthalene dicarboxylic acid (2,6-NDA) [3], which has very extensive application in light industry, electronic industry and defense industry and so on. In particular, 2,6-NDA is an important monomer for liquid crystal polyester material (LCP) and polyethylene naphthalene-2,6-dicarboxylate (PEN). PEN is a new thermoplastic polyester with high performance. After replacing benzene ring with naphthalene double ring, the intensity, stability resistance, thermo stability, chemical environmental resistance, hydrolytic resistance and gas retardance are superior to polyethylene terephthalate (PET) and polybutylene terephthalate (PBT) [4, 5], so its application and development are attracting much attention in the recent years.
The process for producing 2,6-naphthalene dicarboxylic acid from 2-methyl-6-acetylnaphthalene is a liquid phase catalytic oxidation process [6-8]. 2,6-MAN was oxidized with molecular oxygen-containing gas in the presence of a catalyst containing cobalt, manganese and bromine in solvent acetic acid. The oxidation of 2,6-MAN to 2,6-NDA occurs through radical chain elementary reactions, which involve a very large number of radicals as well as molecular species, the complexity of this reacting system prevents the accurate evaluation of the individual values of the kinetic constants by direct fitting of model results against experiment data, mostly because of the inability of measuring the concentration of the radical species.
The common approach is to lump the detailed mechanism into a set of global reactions which involve only molecular species, whose concentration can be, in principle, easily monitored as a function of time [9]. By accounting for the most important intermediates and final products of the process, that is 2-methyl-6-naphthoic acid (2,6-MNA), 2-formacyl- 6-naphthoic acid (2,6-FNA) and 2,6-NDA, we propose a lumped kinetic model. Due to the research of reaction mechanism and kinetics is not sufficient, this work aims to improve the comprehension of liquid phase catalytic oxidation reaction and derive relevant kinetic equations, which may be of great benefit to the design of reactors for industry.
2 Experimental
2.1 Reagents
Reagent 2,6-MAN which was prepared in the lab has been recrystallized prior to use. Its purity, determined by gas chromatograph (GC), is better than 99.0%. Other reagents, including cobalt acetate tetrahydrate, manganese acetate tetrahydrate, sodium bromide and acetic acid, are all chemically pure reagent from Shanghai Chemical Reagent Co. (China).
2.2 Synthesis of 2,6-NDA
A 250 ml autoclave (CQF0.3, Dalian Jingyi Reactor Limited Co., China) made of Ti and equipped with reflux condenser, stirring apparatus, heating apparatus, an inlet for gas and an exit for exhaust gas was used, and the experiment was carried out batchwise. After metal acetates, sodium bromide, 2,6-MAN (0.1 mol), acetic acid (156 ml) and water (4 ml) were added to the autoclave, nitrogen gas was introduced from the gas inlet to 1.0 MPa, and the mixture was heated to 180-210°C and 2.4 MPa. Then nitrogen was replaced by compressed air introduced from the inlet. The mixture was violently stirred while bubbling with compressed air, and the general reaction was carried out for 75 min. The sample (0.5 ml), which was withdrawn from the reactor at each time interval, was put into test tubes. The contents of 2,6-MAN and intermediates were measured by GC and high performance liquid chromatograph (HPLC). The autoclave was cooled and opened, and the reaction product slurry was obtained. The crystals were separated from the liquid by filtration. The resulting crystals were washed with acetic acid and dried. The product was identified by mass spectrometry (MS) for molecular weight. The analytic results showed that the molecular weight of the sample was 184, which was consistent with the actual value of 2,6-NDA.
2.3 Analytic method
The concentrations of 2,6-MAN in liquid samples were determined by a gas chromatograph (GC-2000II, Shanghai Institute of Calculation Technology, China) equipped with a flame ionization detector (FID). The column used was 10% SE30, 80/100 supelcoport, and 6.0 m by 0.0032 m (Dalian Institute of Chemical Physics, Chinese Academy of Sciences, China). The optimal operation conditions of GC are: injector temperature, 230°C; column temperature, 220°C; FID temperature, 220°C; carrier gas, pure hydrogen with a flow rate of 15 ml·min-1; sample volume, 1.5 μl. The concentrations of 2,6-MNA, 2,6-FNA and 2,6-NDA in liquid samples were determined by a high performance liquid chromatograph (HPLC, Agilent1100, USA) with a DAD detector [10, 11]. The column used was Zorbax eclipse XDB C8stainless steel (150 mm×4.6 mm). The optimum operation conditions of HPLC are: column temperature, 40°C; flow phase, acetonitrile (A)-potassium dihydrogen phosphate buffer solution (0.68%) (B) (40︰60); flow rate, 0.85 ml·min-1; detection wavelength, 210 nm; sample volume, 5 μl.
2.4 Reaction mechanism
Kamiya [12] elucidated the reaction mechanism for the formation of 2,6-NDA from 2,6-diethynaphthalene (2,6-DEN).The oxidation of the ethyl groups to carboxyl groups proceeded mainlyacetyl groups, and the scheme of Co-Mn-Br catalyzed oxidation of 2,6-DEN is shown in Fig. 1. The mixtures of the oxidation reaction from 2,6-MAN to 2,6-NDA were analyzed by HPLC and two intermediates (2-methyl-6- naphthoic acid and 2-formyl-6-naphthoic acid) were detected. The results showed that there were very few side reactions when the reaction took place under the conditions mentioned in Section 2.2.
Based on Ref. [12] and according to the analytic data here, it is considered that the oxidation reaction consists of three-steps as shown in Fig. 2.
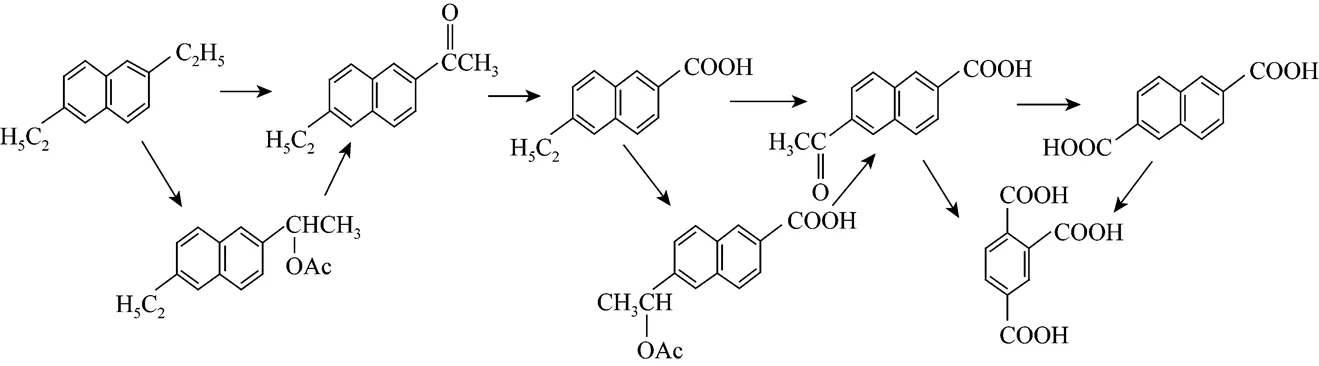
Figure 1 The scheme of Co-Mn-Br catalyzed oxidation of 2,6-DEN to 2,6-NDA [13]
Figure 2 The scheme of Co-Mn-Br catalyzed oxidation of 2,6-MAN to 2,6-NDA
3 Kinetics Rate Model
LetA,B,CandDrepresent the molar concentrations of A, B, C and D respectively. Supposed the concentration of O2be constant, thus we can assume the oxidation was zeroth order with respect to the oxygen partial pressure.




On the basis of the proposed mechanism, the following rate equations are obtained:
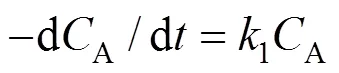


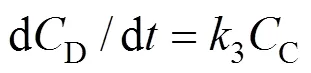

Table 1 The change of conversion with reaction time at 180°C



Substitution of Eq. (5) into Eq. (2) gives

and its solution is

The solution ofCandDare obtained in a similar manner:

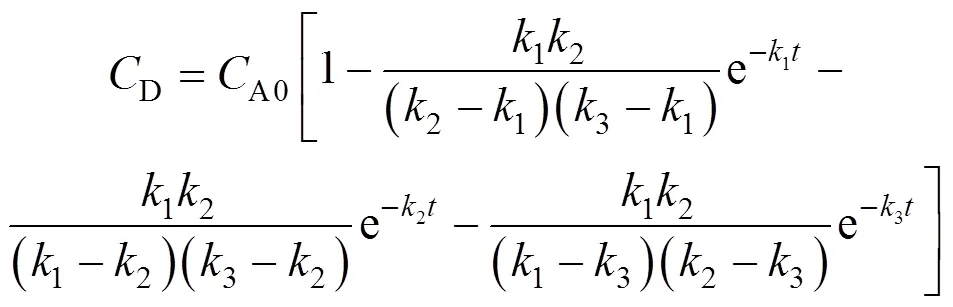
Eqs. (5), (7), (8) and (9) describe the variation of the concentration of 2,6-MAN, 2,6-MNA, 2,6-FNA and 2,6-NDA with time and temperature. Hereinafter we will validate its applicability by experimental data.
4 Results and Discussion
4.1 Effect of temperature

Table 2 Relationship between the reaction rate constant k and temperature T

Figure 4 Evolution of the experimental and simulated product concentrations (180°C)
■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model

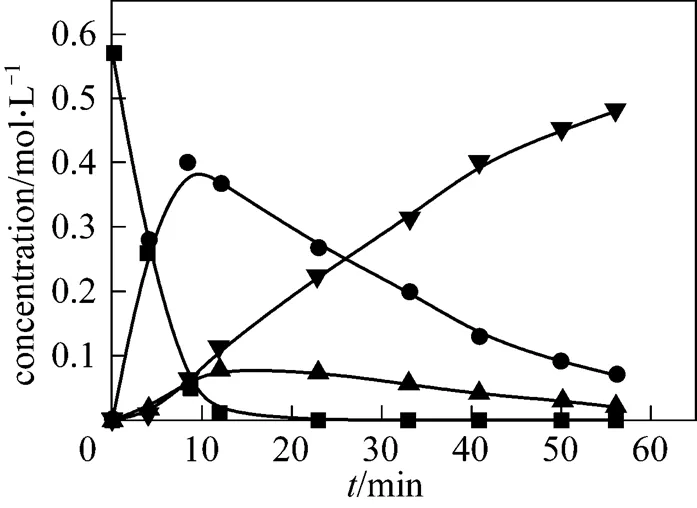
Figure 5 Evolution of the experimental and simulated product concentrations (190°C)
■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model

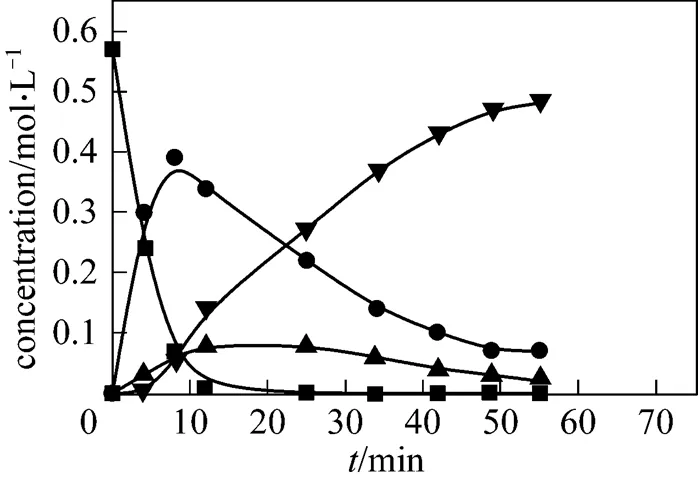
Figure 6 Evolution of the experimental and simulated product concentrations (200°C)
■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model
Based on Table 2, it is clear that the value of2is obviously less than that of1or3in the temperature range of 180-210°C, which indicate that the reaction rate for oxidating 2-methyl-6-naphthoic acid is the minimum one, and the second step is the rating-limiting reaction in the whole series reactions. In order to correlate the rate constant and temperature, plotting 1/against ln, the results show that the relationship between lnand 1/is linear, so ln1, ln2and ln3can be obtained by least square linear regression:


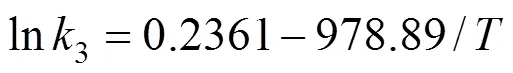
Based on the Arrhenius equation. the values ofdand0for the three reaction steps are listed in Table 3. From the value ofain Table 3, it is seen that the oxidation step of 2,6-MNA to 2,6-FNA is more sensitive to the temperature variation than other ones.

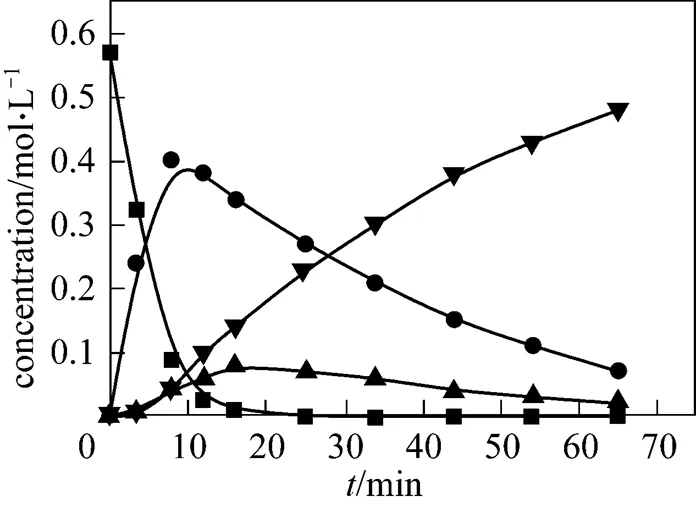
Figure 7 Evolution of the experimental and simulated product concentrations (210°C)
■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model
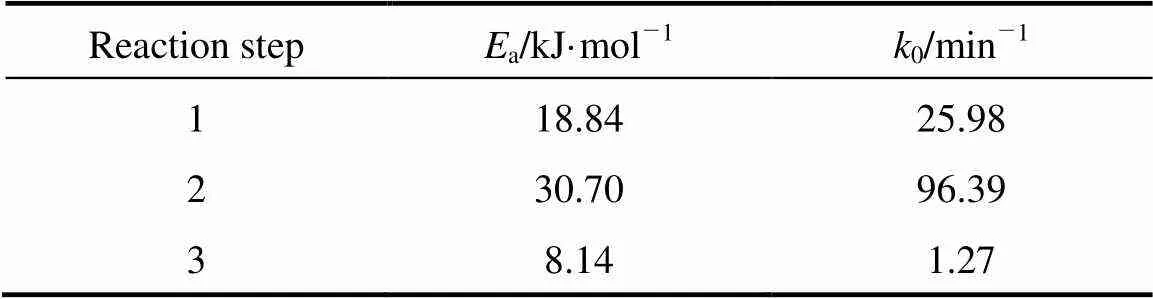
Table 3 Activation energy and preexponential factor
4.2 Effect of concentration of catalyst
4.3 Effect of ratio of catalyst
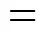


■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model


■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model

■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model

Table 4 Relationship between the reaction rate constant k and concentration of catalyst (200°C, n(Co)︰n(Mn)︰n(Br)2︰2︰1)

Table 5 Relationship between the reaction rate constant k and ratio of catalysts (200°C, 0.048 mol·L-1)


■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model

■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model
5 CONCLUSIONS
The mechanism and kinetics of oxidation reaction from 2,6-MAN to 2,6-NDA were investigated. A pathway including three reactions in series seemed reasonable, and a first order kinetic model was established to describe the relationship between the concentrations and the reaction time for the constituents including raw material, intermediates and product. In light of the dynamic data obtained here, the reaction rate constants of the three steps at 180-210°C were determined and discussed. It was concluded that the reaction rate increases with the increase of temperature and the second step reaction is more sensitive to the change of temperature.

■ 2,6-MAN; ● 2,6-MNA; ▲ 2,6-FNA; ▼ 2,6-NDA;
—— calculated by model

1 Hagen, G.P., Schmidt, G.E., Weis, J.M., Smith, T.G., “2-Acyl-6- methylnaphthalene preparation”, US Pat., 5138098 (1992).
2 Shao, J., Zeng, Z.X., Xue, W.L., Wu, C., “Experimental measurement and correlation of the solubility of methyl-acetyl-naphthalene in-heptane,-octane and-dodecane”,...., 14 (6), 780-783 (2006).
3 Xia, Q., Ma, P.S., “Measurement and correlation for solubility of dimethyl-2,6-naphthalene dicarboxylate in organic solvents”,...., 15 (2), 215-220 (2007).
4 He, Z.R., Zhu, L.H., “Preparation and application of 2,6-naphthalene dicarboxylic acid and dimethyl-2,6-naphthalene dicarboxylate”,-55 (2001). (in Chinese)
5 Hirose, I., “2,6-Naphthalenedicarboxylic acid”, Eng. Pat., 204119 (1986).
6 Tanaka, T., Inari, M., “Process for producing 2,6-napthalene dicarboxylic acid”, US Pat., 5110982 (1992).
7 Marcel, F., “Method for the preparation of 2,6-naphthalene dicarboxylic acid”, US Pat., 4764638 (1988).
8 Zhang, Y.K., Li, C.F., He, X.R., Chen, B.Z., “Simulation and optimization in the process of toluene liquid-phase catalytic oxidation”,...., 16 (1), 36-38 (2008).
9 Cavalieri d’Oro, P., Danoczy, E., Roffia, P., “On the low temperature oxidation of-xylene”,.., 1, 153-155 (1980).
10 Wu, Z.Q., Guo, Z.W., “High-performance liquid chromatograph determination of 2,6-naphthalene dicarboxylic acid”,...., 24 (1), 24-26 (2005).
11 Zheng, J.Z., Row, K.H., “Optimum of mobile phase condition for resolving isoflavones in RP-HPLC”,...., 15 (2), 291-295 (2007).
12 Kamiya, Y., “Formation of 2,6-naphthalenedicarboxylic acid by the Co-Mn-Br catalyzed autoxidation of 2,6-diethylnaphthalene in acetic acid”,..., 68 (1), 204-210 (1995).
13 Xia, Q., “The trend of the production of 2,6-naphthalene dicarboxylic acid by Co-Mn-Br catalytic oxidation of 2,6-dialkyl naphthalene”,.... (), 11 (4), 27-33 (1997). (in Chinese)
14 Cincotti, A., “Effect of catalyst concentration and simulation of precipitation processes on liquid-phase catalytic oxidation of-xylene to terephthalic acid”,..., 52, 4205-4213 (1997).
15 Cao, G., “Kinetics of-xylene liquid-phase catalytic oxidation”,., 40, 1156-1166 (1994).
16 Walt, P., Catalysis of Organic Reactions, Chapter 20, Marcel Dekker, New York (1990).
2008-04-11,
2008-10-03.
* To whom correspondence should be addressed. E-mail: zengzx@ecust.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Modeling and Optimization for Scheduling of Chemical Batch Processes*
- Simulation of Droplet-gas Flow in the Effervescent Atomization Spray with an Impinging Plate*
- Numerical Investigation of Constructal Distributors with Different Configurations*
- The Kinetics of the Esterification of Free Fatty Acids in Waste Cooking Oil Using Fe2(SO4)3/C Catalyst
- Multiple Model Soft Sensor Based on Affinity Propagation, Gaussian Process and Bayesian Committee Machine*
- Measurement and Correlation of Solid-Liquid Equilibria of Phenyl Salicylate with C4 Alcohols
