Steam Reforming of Dimethyl Ether over Coupled Catalysts of CuO-ZnO-Al2O3-ZrO2 and Solid-acid Catalyst*
2009-05-14FENGDongmei冯冬梅ZUOYizan左宜赞WANGDezheng王德峥andWANGJinfu王金福
FENG Dongmei (冯冬梅), ZUO Yizan (左宜赞), WANG Dezheng (王德峥) and WANG Jinfu (王金福)**
Steam Reforming of Dimethyl Ether over Coupled Catalysts of CuO-ZnO-Al2O3-ZrO2and Solid-acid Catalyst*
FENG Dongmei (冯冬梅), ZUO Yizan (左宜赞), WANG Dezheng (王德峥) and WANG Jinfu (王金福)**
Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
hydrogen production, dimethyl ether, steam reforming, hydrolysis, solid-acid catalyst,CuO-ZnO catalyst
1 INTRODUCTION
Interest in hydrogen production for fuel cell applications is steadily increasing due to environmental concerns [1]. Among the various hydrocarbon feed gases, dimethyl ether (DME) has the advantages of high energy density, non-toxicity, easy availability, safe handling/storage, and that the infrastructure in place for liquid petroleum gas (LPG) distribution can be readily adapted for DME [2]. These make it a promising source of hydrogen for fuel cell vehicles. The three major approaches for the conversion of dimethyl ether to hydrogen are steam reforming (SR), partial oxidation (PO), and autothermal reforming (ATR). Steam reforming of DME outperforms the others in giving higher hydrogen and lower CO concentrations. The production of hydrogen from DME SR has been studied by various groups [3-11]. The thermodynamics of DME SR processes has also been published [12, 13].
The overall DME steam reforming can be represented as follows:
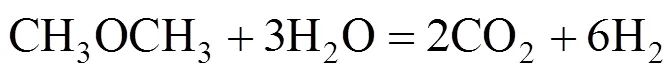
The reaction occurs as a combination of the hydrolysis of DME (reaction 2) and SR of methanol (reaction 3):
36.6 kJ·mol-1(2)
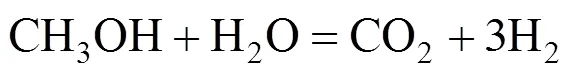
DME hydrolysis takes place on an acidic catalyst, while MeOH SR proceeds on metal catalysts,.. Cu- and Pt-based catalysts [14-19]. However, the hydrolysis of DME is limited by a low equilibrium value (the equilibrium conversion of DME at 200°C is 15%) when carried out without removal of the methanol produced, and it is considered the rate-limiting step of overall DME SR. Therefore, the enhancement of DME hydrolysis will give a high reforming conversion. When methanol form DME hydrolysis is rapidly eliminated by reaction (3), reaction (2) is accelerated and a high conversion of DME (reaction 1) can be obtained.

2 EXPERIMENTAL
2.1 Catalyst preparation

The resulting physical mixture was a bifunctional catalyst with the acid catalyst active for the conversion of DME to methanol, and the CuO-ZnO-Al2O3-ZrO2catalyst active for methanol SR. The mixture ratio of solid acid and CuO-ZnO-Al2O3-ZrO2catalyst was fixed at one-to-one by mass and all catalysts were ground and sieved to a particle diameter of 0.2-0.3 mm (60-80 mesh). The abbreviations used in the text for the catalysts are given in Table 1. The ZSM-5 catalyst is denoted as Z, and CuO-ZnO-Al2O3-ZrO2as CD. A quantity of 1g of this mixture was mixed with 2g of inert quartz of the same size and placed in the reactor as a fixed bed.
2.2 Catalyst characterization
The structure of the catalysts was characterized by scanning electron microscope (SEM), X-ray diffraction (XRD), temperature-programmed desorption (TPD) and N2BET adsorption. A scanning electron microscope (SEM) analysis of the catalysts before reaction was conducted on a HRSEM, JSM 7401F. Powder X-ray diffraction (XRD) patterns of the catalysts were obtained with a BRUKER D8 Advance type X-ray diffractometer using nickel-filtered Cu Kαradiation. The patterns were recorded for 10º<2<90º. The BET surface area was obtained using a high resolution BET equipment described in Wang[25, 26]. Temperature-programmed desorption (TPD) of NH3was carried out to estimate the amount of acid in the catalysts. Ammonia-TPD was carried out in the following manner; 100 mg of a powder sample was heated at a rate of 15°C∙min-1up to 500°C and kept for 1h in the He atmosphere to remove adsorbed molecules on the catalyst surface. The sample was cooled down to 100°C in the He atmosphere, then followed by adsorption of NH3in pure NH3flow for 1h. Consecutively, ammonia-TPD was initiated at a heating rate of 15°C∙min-1up to 650°C. The rate of NH3desorption was determined by using a TCD and recorded on an online personal computer. The amount of desorbed NH3was estimated.
2.3 Catalyst activity evaluation
Activity measurements were carried out using an isothermal fixed bed reactor (20 mm i.d.).The temperature ranged used was 200 to 400°C. Before the reaction, the catalyst was reduced with a 4% H2/96% N2mixture at atmospheric pressure by raising the temperature slowly to the reaction temperature over 10 h. A mixture of DME gas and deionized water fed to a custom-built vaporizer by means of a stratospheric piston pump was introduced into the reactor to start the reaction. The first sample of the effluent was taken 2 h after steady reaction conditions were established. Then samples were taken every 40 min. The final result shown is an average of five data points.
The compositions of the effluent gas were analyzed by an online gas chromatograph (GC) equipped with a TCD (VARIAN, GC-7890II). A Porapak T column was used for the separation of DME, CH3OH, and H2O, and a TDX-01 column was used for the separation of H2, CO, and CO2. DME conversion, hydrogen yieldand CO2selectivitywere defined as follows:
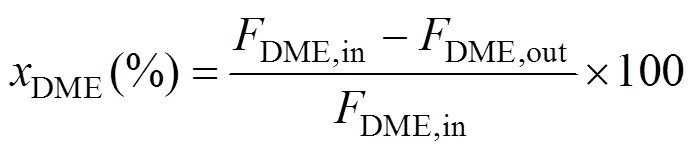

Table 1 Catalyst characterization: BET surface area, acidic property, crystalline phase and catalyst type
Note: n/c means “not detected ”.

Figure 1 SEM images of catalysts

The hydrogen yield, a parameter indexing the activity, was defined as the ratio of the molar amount of DME converted to hydrogen to the total molar amount of DME fed to the reactor and calculated by the following equation:

3 RESULTS AND DISCUSSION
3.1 Catalyst characterization
The specific surface area of the fresh catalysts, crystalline phases, and acid amounts on the catalysts estimated by NH3-TPD are summarized in Table 1. A higher surface area was found for ZSM-5, while the alumina had a low surface area. The BET surface area of γ-Al2O3was 162 m2∙g-1, which was greater than that of CuO-ZnO-Al2O3-ZrO2, 85.0 m2·g-1. Of the ZSM-5 catalysts, Z(38) had the highest surface area of 450.0 m2∙g-1.
Figure 1 shows SEM images of (a) CD, (b) γ-Al2O3, (c) Z(25), (d) Z (38), and (e) Z(50). The CD catalyst was mainly a copper spinel with a one-dimensional fibrous structure. The γ-Al2O3catalyst had an amorphous structure, and many particles were structured like the culmination of many whiskers that crisscrossed the surface of Al2O3. The appearances of the ZSM-5 and γ-Al2O3catalysts were quite different. Z(25) was large particles composed of fine grains, Z(38) was smooth-faced, Z(50) had both small grains and a film structure.
Figure 2 shows NH3desorption curves from NH3-TPD measurements. The desorption temperature indicated the acid strength of the catalyst: a high desorption temperature indicated a strong acid. The desorption characteristics of ZSM-5 were quite different from those of alumina. The three different types of ZSM-5 catalysts showed two distinct peaks appearing from 250°C to 300°C and 500°C to 600°C that are assignable to two types of acid sites. The low and high temperature peaks corresponded to weak and strong acid sites, respectively. A single major peak was observed for the alumina, signifying a single type of acid site. The majority of acid sites on alumina were weakly acidic. They are typically known as Lewis acid sites [3, 4]. ZSM-5 is known to possess mainly Brønsted acid sites with a small number of Lewis acid sites [6, 7]. The acid amounts corresponding to the amounts of adsorbed ammonia were estimated from the areas of the TPD curves. It was found that acid amount was inversely proportional to the Si-Al ratio (.., as the Si/Al ratio increased, the number of acid amount decreased). The acid amounts of Z(25), Z(38), Z(50) and alumina were 1.4537, 0.6303, 0.6151 and 0.2 mmol·g-1, respectively. Z(25) had the largest amount of desorbed NH3, indicating that Z(25) had the largest number of acid amount. In general, one can anticipate a higher activity for DME hydrolysis from a larger acid amount and stronger acidity.
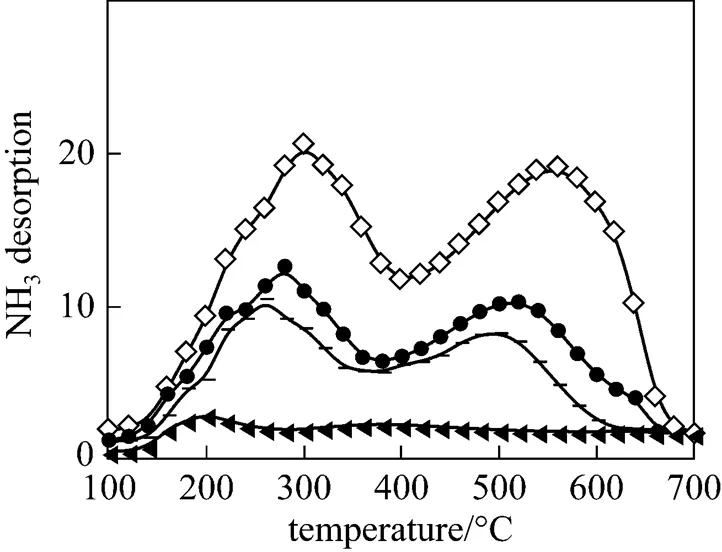


3.2 Hydrolysis of DME over solid acid catalysts
DME hydrolysis is reversible and thermodynamics- limited. The reaction equilibrium was calculated by using the Gibbs free energy minimization method and plotted in Fig. 4. The DME conversion obtained over the solid acid catalysts,.. Z(25), Z(38), Z(50), γ-Al2O3, are shown also in Fig. 4. Z(25), Z(38) and Z(50) showed comparable activities below the thermodynamics equilibrium. Z(25) exhibited the highest activity for DME hydrolysis. The high conversion over ZSM-5 in the low temperature range of 200 to 300°C is due to the strong acid strength, whereas no activity was observed at temperatures below 150°C. A temperature over 300°C is not recommended for DME hydrolysis over ZSM-5, since the acid sites can catalyze DME decomposition to hydrocarbon [27].

Figure 3 XRD patterns of the physical mixtures of CuO/ZnO/Al2O3/ZrO2 and solid acid catalysts
● Cu; ▽ CuO; □ ZnO; ◆ ZSM-5; Al2O3
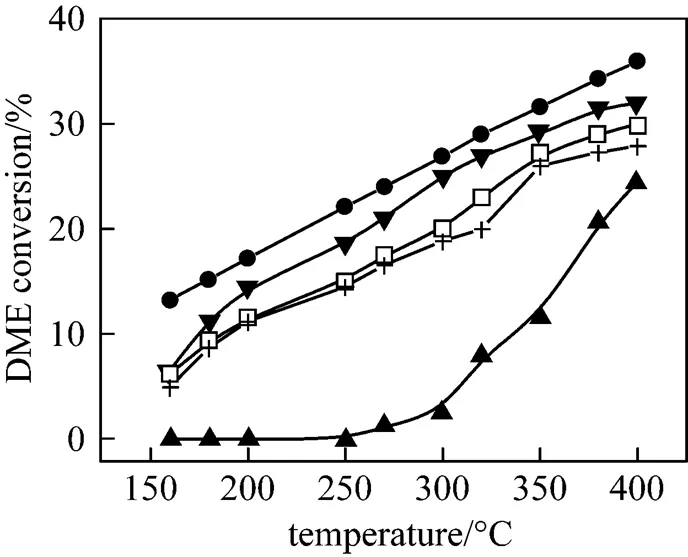
Figure 4 DME hydrolysis over solid acid catalysts (Catalyst, 0.75 g; n(DME)/n(H2O)=1/3.45; space velocity, 1660 L∙kg -1·h-1)● thermodynamic equilibrium; ▼ Z(25); □ Z(38); + Z(50); ▲ γ-Al2O3
Alumina showed only 1.1% DME conversion at 250°C. Howerer, the alumina catalysts became active at temperatures above 270°C. At temperatures above 350°C, small amounts of CH4and CO were detected indicating that some decomposition of DME (CH3OCH3→H2+CO+CH4) on alumina.
The activity for DME conversion depended on the acid amount and acid strength. DME hydrolysis needs relatively high acid strength at low temperature. The high acid strength may be responsible for the hydrolysis of DME and the Si/Al ratio greatly affects the conversion of DME hydrolysis. Therefore, the Z(25) catalyst having a larger number of Brønsted acid sites with moderate acid strength was the best catalyst component for DME hydrolysis and can be used at lower temperature. The observed trend of DME hydrolysis activity trend as a function of Si/Al ratio and catalyst type was:
Z(25)>Z(38)>Z(50)>γ-Al2O3.
3.3 Effect of solid acids on the steam reforming of DME
Since hydrogen produced from DME is from the methanol SR that follows DME hydrolysis, both reactions are equally important. Copper catalysts have a high low-temperature activity and good selectivity for methanol SR. A fibrous CuO-ZnO-Al2O3-ZrO2(CD) catalyst that is active for methanol production from CO2hydrogenation was developed by our group recently [23, 24]. This fibrous CuO-ZnO-Al2O3-ZrO2was used as one partner of the bifunctional catalyst in the work here. ZSM-5 or γ-Al2O3was the other partner of the bifunctional catalyst. Thus, physical mixtures containing a CD catalyst and a solid-acid catalyst were used for DME SR.
Figure 5 illustrates the effect of temperature on DME SR over the bifunctional catalysts expressed in terms of (a) DME conversion, (b) H2production and CO2selectivity. DME SR over CD/ ZSM-5 catalyst was carried out at 200 to 300°C and at 200 to 400°C for CD/γ-Al2O3catalyst. As depicted in Fig. 5, the conversion of DME over all bifunctional catalysts increased with increasing temperature. DME SR to a hydrogen rich gas proceeded more efficiently over the CD/ ZSM-5 catalysts than over CD/γ-Al2O3throughout the temperature studied. The CD/ ZSM-5 catalyst had a high activity even at 200°C with DME conversion of 10%-19%, while the CD/γ-Al2O3catalyst had no activity at temperatures below 250°C. The catalytic reforming activity strongly depended on the hydrolysis activity of the solid acids. A high hydrolysis activity over the solid acid led to a high SR activity over composites of CD and solid acid. There was not much activity difference among the CD/ZSM-5 catalysts. CD/Z(25) catalysts exhibited a little higher activity than CD/Z(38) and CD/Z(50). This suggested that CD/Z(25) has good potential as a bifunctional catalyst for DME SR.

Figure 5 Effect of temperature on DME SR over CD/Z(25) combined with CD501 catalysts (Catalyst 0.75g;(DME)/(H2O)=1/3.45; space velocity, 1660 L·kg-1·h-1)▼ CD+Z(25); □ CD+Z(38); + CD+Z(50); ▲ CD +γ-Al2O3
As discussed in the previous section, in addition to the acid amount, the catalyst type, acid type and acid strength play important roles in the hydrolysis activity. It is well known that alumina possesses mainly Lewis acid sites. ZSM-5 has strong Bronsted acid sites with some Lewis acid sites. Based on the result of DME hydrolysis and DME SR evaluation, we suggest that Bronsted acid sites are active for DME hydrolysis at low temperatures in the range of 200-300°C and Lewis acid sites need temperatures higher than 270°C to be active.
As shown in Fig. 5, the hydrolysis of DME conversion is very low only using solid catalyst, however, the conversions of DME SR with CD/Z(25) catalyst was much higher than that of DME hydrolysis. Although the hydrolysis of DME was the rate-limiting step of overall DME SR, if we add Cu base catalyst, the methanol product of DME hydrolysis is consumed and removed, and the DME hydrolysis is accelerated and a high conversion of DME can be obtained. The higher the methanol reforming reaction conversion over Cu base catalyst, the higher the DME reforming reaction conversion on Cu/ZSM bifunctional catalyst.experimental analysis, it was indicated that the synergy of the DME hydrolysis reaction-methanol steam reforming reaction is prominent and a higher one-way DME conversion can be obtained.
Figures 5 (b) and 5 (c) shows hydrogen yields and CO2selectivity from DME SR over the bifunctional catalysts. Similar to the DME conversion, the hydrogen yield increased with increasing temperature. As methanol SR proceeded, hydrogen and carbon dioxide increased while water decreased. Water was essentially consumed as a reactant, which shifted the water-gas shift (WGS) equilibrium to the right towards carbon monoxide and water.
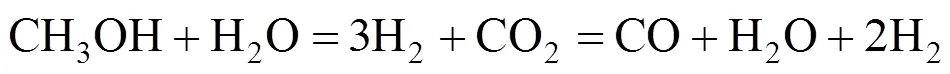
With increased temperature, CO2selectivity decreased and CO selectivity increased, indicating that the reverse WGS played an important role. The selectivity to methane also increased with increased temperature from 350 to 400°C.
The order of DME SR activity with respect to DME conversion in the temperature range of 200-400°C was a function of acidic property and acid-type as follows:
Z(25)+CD>Z(38)+CD>Z(50)+CD>γ-Al2O3+CD.
3.4 Effect of space velocity
The space velocity, which is a parameter that reflected reactor efficiency, was also tested with a steam/DME ratio of 3.5 under atmospheric pressure. Space velocities of 1179 to 9000 ml·(g cat)-1·h-1were used to test the catalytic behavior. Fig. 6 shows that with increased space velocity, DME conversion and hydrogen yield decreased and CO2selectivity increased. At the space velocity of 1179 ml·(g cat)-1·h-1, the conversion of DME was about 88% and CO2selectivity was 98% at 220 ºC. When it was increased to 9000 ml·(g cat)-1·h-1, DME conversion was decreased to 18% and CO2selectivity was increased to 100% because of the shorter residence time.

Figure 6 Effect of space velocity on DME SR over CD/Z(25) catalysts (Catalyst, 1 g;(DME)/(H2O)=1/3.5)temperature/°C: ● 200; ◇ 220; ■ 240; ☆ 270
DME can be completely converted when the space velocity was less than 2461 ml·(g cat)-1·h-1, and the H2yield was greater than 90%. At the same time, the CO content was low. When space velocity was decreased to 1179 ml·(g cat)-1·h-1, the conversion by the catalysts was greatly increased.
3.5 Effect of feed ratio
Figure 7 shows that DME conversion and hydrogen yield increased considerably when the steam/DME ratio was increased from 3 to 7. The CO2selectivity was increased to about 99 % at 240°C when the space velocity was 3935 ml·(g cat)-1·h-1. Hence, a higher steam/DME molar ratio is favorable for enhancing DME conversion and for reducing the CO concentration in the product. Taking into account the thermal load and energy supply, the optimum steam/DME ratio can be recommended as 3.5.

Figure 7 Effect of H2O/DME ratio on DME SR over CD/Z(25) catalysts (Catalyst, 1g; n(DME)/n(H2O) = 1/3.5)space velocity/ml·(g cat)-1·h-1: ● 9000 ; ▼ 6872; □ 4922;× 3935
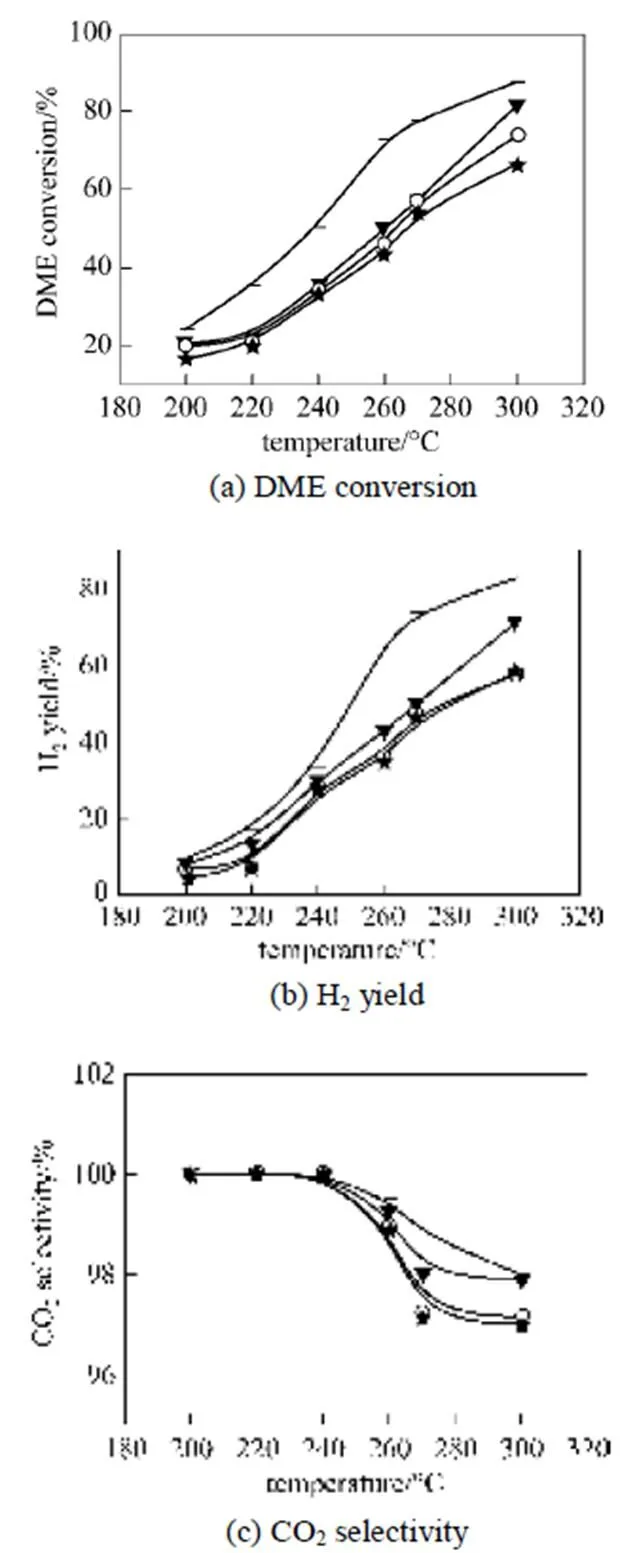
Figure 8 Effect of pressure ratio on DME SR over CD/Z(25)catalyst (Catalyst, 1 g;(DME)/(H2O)=1/3.5)
4 CONCLUSIONS
The hydrolysis of DME to methanol using various acidic catalysts: ZSM-5 and γ-Al2O3, has been investigated. It is found that stronger acidity gives higher activity for DME hydrolysis. ZSM-5 and γ-Al2O3catalysts are active for DME hydrolysis at different temperature ranges:>270°C for ZSM-5 and 200-300°C for γ-Al2O3. The hydrolysis of DME is the rate-determining step in the DME SR.
The bifunctional catalyst exhibited high performance at low temperature (200-300°C) for the DME SR. The performance of the bifunctional catalyst in DME SR depends on the DME hydrolysis activity of the solid acid: high hydrolysis activity corresponds to high SR activity. The observed DME SR activity trend as a function of Si/Al ratio and catalyst type was: CD+Z(25)>CD+Z(38)>CD+Z(50)>CD+γ-Al2O3. The reforming temperature, space velocity and steam/DME ratio played important roles in DME SR.
NOMENCLATURE
Fmolar flow rate ofcomponent, mol·s-1
Δheat of reaction, kJ·mol-1
gas constant (8.3145 J·mol-1·K-1)
Sselectivity ofcomponent
temperature, °C
xconversion ofcomponent
Yyield ofcomponent
1 Liu, Z.Q., Mao, Z.Q., Xu, J.M., “Operation conditions optimization of hydrogen production by propane autothermal reforming for PEMFC applications”,...., 14, 729-735 (2006).
2 Wang, Z.L., Diao, J., Wang, J.F., Jing, Y., “Study on synergy effect in dimethyl ether synthesis from syngas”,...., 9, 412-416 (2001).
3 Tanaka, Y., Kikuchi, R., Takeguchi, T., Equchi, K., “Steam reforming of dimethyl ether over composite catalysts of γ- Al2O3and Cu- based spinel”,..., 57, 211-222 (2005).
4 Faungnawakij, K., Tanaka, Y., Shimoda, N., Fukunaga, T., Kawashima, S., Kikuchi, R., Eguchi, K., “Influence of solid-acid catalysts on steam reforming and hydrolysis of dimethyl ether for hydrogen production”,..., 304, 40-48 (2006).
5 Semelsberger, T.A., Ott, K.C., Borup, R.L., Greene, H.L., “Generating hydrogen-rich fuel-cell feeds from dimethyl ether (DME) using Cu/Zn supported on various solid-acid substrates”,..., 309, 210-223 (2006).
6 Kawabata, T., Matsuoka, H., Shishido, T., Li, D., Tian, Y., Sano, T., Takehira, K., “Steam reforming of dimethyl ether over ZSM-5 coupled with Cu/ZnO/Al2O3catalyst prepared by homogeneous precipitation”,..., 308, 82-90 (2006).
7 Semelsberger, T.A., Ott, K.C., Borup, R.L., Greene, H.L., “Generating hydrogen-rich fuel-cell feeds from dimethyl ether (DME) using physical mixtures of a commercial Cu/Zn/Al2O3catalyst and several solid-acid catalysts”,..., 65, 291-300 (2006).
8 Faungnawakij, K., Tanaka, Y., Shimoda, N., Fukunaqa, T., Kikuchi, R., Equchi, K., “Hydrogen production from dimethyl ether steam reforming over composite catalysts of copper ferrite spinel and alumina”,..., 74, 144-151 (2007).
9 Laosiripojana, N., Assaburnrngrat, S., “Catalytic steam reforming of dimethyl ether (DME) over high surface area Ce-ZrO2at SOFC temperature: The possible use of DME in indirect internal reforming operation (IIR-SOFC)”,..., 320, 105-113 (2007).
10 Takeishi, K., Suzuki, H., “Steam reforming of dimethyl ether”,..., 260, 111-117 (2004).
11 Badmaev, S.D., Volkova, G.G., Belyaev, V.D., Sobyanin, V.A., “Steam reforming of dimethyl ether to hydrogen-rich gas”,...., 90, 205-211 (2007).
12 Semelsberger, T.A., Borup, R.L., “Thermodynamic equilibrium calculations of dimethyl ether steam reforming and dimethyl ether hydrolysis”,.., 152, 87-96 (2005).
13 Faungnawakij, K., Tanaka, Y., Shimoda, N., Fukunaqa, T., Kikuchi, R., Equchi, K., “Thermodynamic analysis of carbon formation boundary and reforming performance for steam reforming of dimethyl ether”,.., 164, 7-79 (2007).
14 Agell, J., Birgersson, H., Boutonnet, M., “Steam reforming of methanol over a Cu/ZnO/Al2O3catalyst: a kinetic analysis and strategies for suppression of CO formation”,.., 106, 249-257 (2002).
15 Peppley, B.A., Amphlett, J.C., Kearns, L.M., “Methanol-steam reforming on Cu/ZnO/Al2O3. Part 1: the reaction network”,..., 197, 21-29 (1999).
16 Peppley, B.A., Amphlett, J.C., Kearns, L.M., “Methanol-steam reforming on Cu/ZnO/Al2O3catalysts. Part 2. A comprehensive kinetic model”,..., 197, 31-49 (1999).
17 Jiang, C.J., Trimm, D.L., Wainwright, M.S., “Kinetic study of steam reforming of methanol over copper-based catalysts”,..., 97, 145-158(1993).
18 Mastalir, A., Frank, B., Szizybalski, A., Soerijanto, H., Deshpande, A., Niederbergere, M., Schomacker, R., Schlogl, R.,Ressler, T., “Steam reforming of methanol over Cu/ZrO2/CeO2catalysts: a kinetic study”,.., 230, 464-475 (2005).
19 Agrell, J., Birgersson, H., Boutonnet, M., “Steam reforming of methanol over a Cu/ZnO/Al2O3catalysts: a kinetic analysis and strategies for suppression of CO formation”,.., 106, 249-257 (2002).
20 Xie, F., Li, H.S., Zhao, X.L., “Adsorption and dehydration of methanol on Al2O3catalyst”,..., 25, 403-408 (2004). (in Chinese)
21 Wang, Z.L., Wang, J.F., Diao, J., Jin, Y., “The synergy effect of process coupling for dimethyl ether synthesis in slurry reactors”,..., 24, 507-511 (2001).
22 Ren, F., Li, H.S., Wang, J.F., Wang, D.Z., “Methanol synthesis from syngas in a slurry reactor”, In: 226th National Meeting of the American Chemical Society, 575 (2003).
23 An, X., F, Ren., Li, J.L., Wang, D.Z., Wang, J.F., “A highly active Cu/ZnO/Al2O3nanofiber catalyst for methanol synthesis through CO2and CO hydrogenation”,..., 26, 729-735 (2005). (in Chinese)
24 An, X., Li, J.L., Zuo, Y.Z., Zhang, Q., Wang, D.Z., Wang, J.F., “A CuO-ZnO-Al2O3-ZrO2fibrous catalyst that is an improved CO2hydrogenation to methanol catalyst”,.., 118, 264-269 (2007).
25 Chen, J.H., Li, W.Z., Wang, D.Z., “Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitors”,, 40, 1193-1197 (2002).
26 Wang, D.Z., Wei, F., Wang, J.F., “Method and apparatus to measure gas amounts adsorbed on a powder sample”, US Pat., 6981426 (2006)
27 Faungnawakij, K., Tanaka, Y., Shimoda, N., Fukunaqa, T., Kikuchi, R., Equchi, K., “A comparative study of solid acids in hydrolysis and steam reforming of dimethyl ether”,..., 333, 114-121 (2007).
2008-03-15,
2008-08-23.
the Ministry of Science and Technology (G1999022408) and the National Natural Science Foundation of China (20773075).
** To whom correspondence should be addressed. E-mail: wangjf@flotu.org
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Modeling and Optimization for Scheduling of Chemical Batch Processes*
- Simulation of Droplet-gas Flow in the Effervescent Atomization Spray with an Impinging Plate*
- Numerical Investigation of Constructal Distributors with Different Configurations*
- The Kinetics of the Esterification of Free Fatty Acids in Waste Cooking Oil Using Fe2(SO4)3/C Catalyst
- Multiple Model Soft Sensor Based on Affinity Propagation, Gaussian Process and Bayesian Committee Machine*
- Measurement and Correlation of Solid-Liquid Equilibria of Phenyl Salicylate with C4 Alcohols
