乙烯利处理菠萝不同成熟期果实中AcMYB103基因表达规律研究
2024-12-31韦巧云张秀芬韩骁赵静周彩霞蒋娟娟徐健
"
摘""要:菠萝属于凤梨科(Bromeliaceae)凤梨属(Ananas"Merr.)的一种重要热带水果,其花果发育理论与转录调控技术研究是菠萝产业的热点之一。乙烯是调控菠萝催花和果实发育的重要激素之一。菠萝基因组测序鉴定出94个菠萝R2R3-MYB转录因子,分为26亚族,其中S19亚族的MYB103与拟南芥等植物花药和叶片发育调控相关。为证明菠萝MYB103转录因子调控菠萝果实发育的潜在功能,从菠萝台农17号果实中克隆了1个MYB转录因子AcMYB103,全长1204"bp,编码区1038"bp,编码345个氨基酸。该基因编码蛋白含有2个典型的SANT结构域,定位在细胞核,无跨膜结构域和信号肽,是植物R2R3型MYB转录因子家族的一员。将菠萝和拟南芥MYB家族成员进行聚类分析发现,AcMYB103与拟南芥AtMYB103序列相似度最高,并与AtMYB80和AtMYB35聚类于S19亚族,据此命名为AcMYB103。进一步采用体积分数为0%、0.5%、1.0%和1.5%共4个乙烯利浓度处理台农17号、小目手撕和大目手撕3个品种小果期、膨大期和成熟期果实,并分析AcMYB103的表达量。结果表明,在小果期,随着乙烯利浓度的增加,台农17号AcMYB103表达规律呈下调模式,在小目手撕和大目手撕中呈上调模式。在膨大期,在台农17号和小目手撕品种中均呈现下调模式,在大目手撕果实中呈现上调模式。在成熟期果实中AcMYB103不表达,说明AcMYB103基因表达在乙烯利处理的品种和果实发育时期具有特异性,在果实发育早期起到差异化调控作用。本研究为深入研究AcMYB103基因在菠萝果实生长发育的调控机制奠定基础。
关键词:菠萝;AcMYB103;乙烯利;表达;成熟期中图分类号:S668.3""""""文献标志码:A
AcMYB103"Gene"Expression"Pattern"Treated"with"Ethephon"in"Different"Ripening"Stages"of"Ananas"comosus
WEI"Qiaoyun,"ZHANG"Xiufen,"HAN"Xiao,"ZHAO"Jing,"ZHOU"Caixia,"JIANG"Juanjuan,"XU"Jian*
Guangxi"South"Subtropical"Agricultural"Research"Institute,"Longzhou,"Guangxi"532415,"China
Abstract:"Pineapple,"an"important"tropical"fruit,"belongs"to"the"family"Bromeliaceae"(Ananas"Merr.),"and"its"flower"and"fruit"development"theory"and"transcriptional"regulation"technology"are"one"of"the"hot"spots"in"the"pineapple"industry."Ethylene"is"one"of"the"important"hormones"that"regulate"the"flowering"and"fruit"development"of"pineapple."In"the"study"of"watercore"disorder"in"Bali"pineapple"varieties,"it"was"found"that"two"MYB"transcription"factors,"AcMYB68"and"AcMYB109,"played"an"important"role"in"fruit"development."With"the"completion"of"pineapple"genome"sequencing,"94"pineapple"R2R3-MYB"transcription"factors"have"been"identified"in"the"pineapple"genome,"which"are"divided"into"26"subfamilies,"and"are"differentially"expressed"by"salt"and"other"stresses"and"ABA"hormones."In"this"study"we"proposed"a"scientific"hypothesis"that"ethylene"mediated"MYB"transcription"factors"to"regulate"the"development"of"pineapple"fruits."In"order"to"elucidate"the"molecular"regulatory"mechanism,"a"MYB"transcription"factor"AcMYB103"was"cloned"from"the"fruit"of"pineapple"Tainong"17,"with"a"total"length"of"1204"bp,"a"coding"region"of"1038"bp,"and"encoding"345"amino"acids."The"protein"contains"two"typical"SANT"domains,"localized"to"the"nucleus,"without"transmembrane"domain"and"signal"peptide,"and"was"a"member"of"the"plant"R2R3"MYB"transcription"factor"family."Cluster"analysis"of"pineapple"and"Arabidopsis"MYB"family"members"showed"that"AcMYB103"had"the"highest"similarity"with"Arabidopsis"AtMYB80,"AtMYB103"and"AtMYB35,"and"belonged"to"the"S19"subfamily."Furthermore,"four"ethephon"concentrations"(volume"fraction)"of"0%,"0.5%,"1.0%"and"1.5%"were"used"to"treat"the"fruits"at"the"small"fruit"stage,"expansion"stage"and"ripe"stage"of"the"three"cultivars"Tainong"17,"Xiaomushousi"and"Damushousi,"and"the"expression"level"of"AcMYB103"was"analyzed."The"results"showed"that"with"the"increase"of"ethephon"concentration"at"the"small"fruit"stage,"the"expression"of"AcMYB103"in"Tainong"17"showed"a"down-regulated"pattern,"and"upregulated"in"the"Xiaomushousi"and"Damushousi"mode."In"the"expansion"stage,"the"downward"adjustment"pattern"was"shown"in"the"Tainong"17"and"Xiaomushousi"varieties,"and"the"upward"pattern"was"shown"in"the"fruit"of"Damushousi."AcMYB103"was"not"expressed"in"fruits"at"the"ripening"stage,"indicating"that"the"expression"of"AcMYB103"gene"was"specific"in"ethephon-treated"varieties"and"fruit"development"periods,"and"played"a"differentiated"regulatory"role"in"the"early"stage"of"fruit"development."This"study"would"lay"a"foundation"for"in-depth"study"of"the"mechanism"of"AcMYB103"gene"in"the"regulation"of"growth"and"development"of"pineapple"fruit.
Keywords:"Ananas"comosus;"AcMYB103;"ethephon;"expression;"ripening"stages
DOI:"10.3969/j.issn.1000-2561.2024.11.005
菠萝(Ananas"comosus)属于凤梨科(Bro m eliaceae),其花果发育理论与技术研究是菠萝产业的热点之一[1]。植物激素在菠萝产业中具有重要作用。早在20世纪40年代就发现夜间低温、生产类激素2,4-D等具有促进菠萝花形成的作用。为了深入研究菠萝中植物激素转录调控的机制,生长素信号途径基因AcAUX和AcPIN等先后被克隆并证明其在抗逆中的作用[2]。除了生长素类激素之外,乙烯也是菠萝花发育影响激素之一。在乙烯信号转导途径中,乙烯响应因子AP2/ERF在花发育中具有重要作用[3]。然而,在菠萝果实发育分子机制研究较少。已经证明,植物MYB转录因子在多种植物果实发育过程中均有重要作用。例如,大麦HvGAMYB通过调控抽穗期进而调控产量[4]。在枣果实颜色的调控过程中,发现MYB基因家族成员是通过调控黄酮合成起到调控作用的[5]。鉴定出枣中的R2R3-MYB亚家族成员有118个,进一步筛选出了可能与果实膨大和色泽发育相关的候选基因ZjMYB59和ZjMYB104[6]。最新研究表明,矮牵牛花R2R3-MYB转录因子SG19既在花发育过程中的气味释放中具有特异性作用,又通过抑制乙烯的产生来抑制花蕾衰老[7]。其中,MYB103转录因子在菠萝、水稻、玉米等6种植物MYB基因家族中保守,其在植物花药发育、绒毛层形成和根毛形成中具有重要作用[8-9]。OsMYB103L的下调明显改变了β-1,4-葡聚糖聚合和纤维素微纤维的组装,从而增强了水稻生物质酶解糖化[10]。另一个在植物中功能保守的MYB80转录因子在绒毡层和花粉发育中具有重要作用[11]。芦笋的MYB35转录因子在雄花发育早期雄蕊中表达量高[12]。可见,MYB103的研究在拟南芥中主要是调控花药和根毛合成为主。在水稻中进一步发现MYB103具有调控叶片的机械强度和纤维强度的功能,但尚未发现其直接调控果实的研究报道。此外,乙烯作为重要的植物激素,其信号转导途径与MYB转录因子密切相关。例如,PlMYB308通过调控牡丹乙烯合成调控花的衰老。R2R3-MYB转录因子RhMYB108参与乙烯和茉莉酸诱导的玫瑰花瓣衰老过程。乙烯激活的PpERF105诱导抑制型R2R3-MYB基因PpMYB140的表达,抑制红梨花青素的生物合成。
在菠萝中,也初步开展了R2R3-MYB基因家族鉴定研究,发现菠萝顶端分生组织中多个MYB基因的表达受到乙烯利的诱导或抑制[13]。其中的S20亚族基因与逆境胁迫响应相关,且受到乙烯诱导,AcMYB108a在果实中的表达水平最高[14]。基于前期研究结果,本研究提出乙烯介导MYB转录因子调控菠萝果实发育的科学假设。揭示菠萝中MYB103基因的结构与功能将有助于解析其在乙烯利催花菠萝果实发育过程中的功能。据此,本研究拟从广西南亚热带农业科学研究所引进的台农17果实中克隆菠萝转录因子AcMYB103,采用生物信息学分析其编码蛋白结构并预测其功能;分析不同浓度梯度乙烯利处理菠萝品种果实后AcMYB103的表达规律,揭示AcMYB103在菠萝果实发育中的调控作用机制,为研发新型栽培调控技术打下坚实基础。
1""材料与方法
1.1""材料
1.1.1""植物材料""菠萝品种台农17号、小目手撕和大目手撕菠萝由广西南亚热带农业科学研究所(106°79¢85²E,22°34¢12²N)提供。选取3个品种在体积分数为0%、0.5%、1.0%和1.5%乙烯利处理后的小果期、膨大期和成熟期的果实用于AcMYB103基因的表达分析。
1.1.2""菌株和试剂""基因克隆与表达分析所需的大肠杆菌(E."coil)DH5-α感受态、反转录试剂盒,SYBR荧光染料等均购自南京诺唯赞公司[15]。
1.2""方法
1.2.1""提取总RNA和cDNA的合成""菠萝果实RNA的提取参考天根多糖多酚试剂盒(RNAprep"pure"Plant"Kit)说明书,反转录及浓度测定参考徐健等[15]的方法。提取RNA浓度与纯度采用超微量核酸蛋白分析仪测定。
1.2.2""菠萝AcMYB转录因子家族系统进化树分析""基于菠萝基因组数据库,使用HMMER"3.1对MYB"DNA结合域PF00249进行隐马尔可夫模型(Hidden"Markov"Model,"HHM)搜索。所有候选AcMYB采用NCBI在线分析和DNAMAN软件进行评估,去除冗余序列。将鉴定出的菠萝AcMYB与拟南芥AtMYB采用最大简约法(maximum"parsimony)、泊松校正模型和1000个重复的自举检验(bootstrap"test),利用MEGA"11软件进行系统发育分析。
1.2.3""克隆菠萝R2R3-MYB家族AcMYB103基因全长""利用Primer"primer6.0软件设计克隆引物AcMYB103-F:5¢-ACCCACCAACTTA GTTTC AT C TCA-3¢,AcMYB103-R:5¢-TGGTCCTC CT A CA GG T TGCT-3¢;设计荧光定量引物AcMY B 103-QF1:5’-TACCGCCGATCATCTCAACG-3¢,AcMYB103-QR1:5¢-TTGCGAAGGTGA GCAG A G A G-3¢。内参基因引物序列为β-actin-QF1:5¢-CT G GCCTACGTGGCACTTGACTT-3¢,β-actin-QR1:5¢-CACTTCTGGGCAGC GGAACCTTT-3¢。以反转录得到的菠萝品种健康果实cDNA为模板,按照高保真酶2×Phanta"Flash"Master"Mix试剂盒说明书进行PCR扩增,将AcMYB103目的条带按照Omega"Biotek(美国)胶回收试剂盒Gel"Extraction"Kit进行切胶回收,回收产物连接零背景pESI-"Blunt平末端载体,后通过热击法转化大肠杆菌DH5α感受态细胞中,经PCR检测后挑取阳性单克隆测序验证。在NCBI中利用Blast的功能进行比对后命名[15]。
1.2.4""AcMYB103生物信息学分析和表达分析""对AcMYB103进行生物信息学预测分析,利用DNAMAN多序列比对工具进行同源序列比对和相似性分析。表达分析以菠萝β-actin为内参基因,采用染料法荧光定量PCR分析AcMYB103基因的表达模式。每个处理均进行3次生物学重复和3次技术重复。
1.3""数据处理
AcMYB103基因在不同菠萝品种和果实发育时期的相对表达量用2‒ΔΔCt法进行分析计算,使用单因素方差分析ANVOA和图基检验分析差异显著性,使用OriginPro2018"(Origin"Lab"Corporation,"Massachusetts,"USA)软件制图[15]。
2""结果与分析
2.1""菠萝AcMYB103基因的克隆
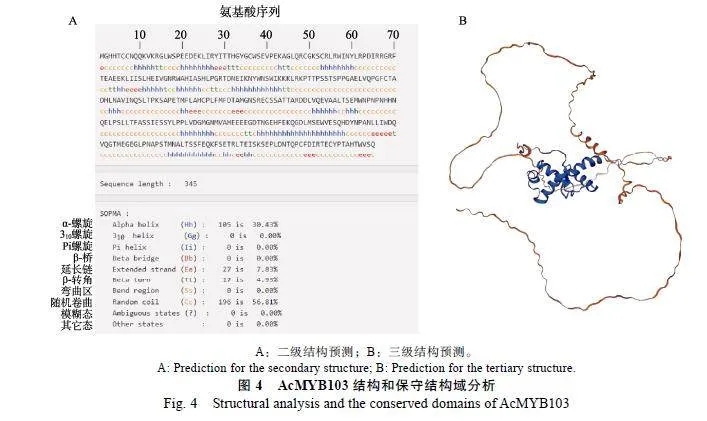
提取菠萝品种台农17号小果期健康果实的RNA反转录成的cDNA为模板,采用引物AcMY B103-F(5¢-ACCCACCAA CTTAGTTTCAT C TC A-3¢)和AcMYB103-R(5¢-TGGTCCTCCTACA G G T T GCT-3¢)扩增出AcMYB103基因。基因全长为1204"bp,开放阅读框(ORF)长度为1038"bp,编码345个氨基酸,其5¢端长度为120"bp,3¢端长度为46"bp。在第67~114位氨基酸位置有2个典型的SANT结构域,与刺子莞RpMYB和椰子CnMYB等相似,属于植物典型的R2R3-MYB转录因子(图1)。
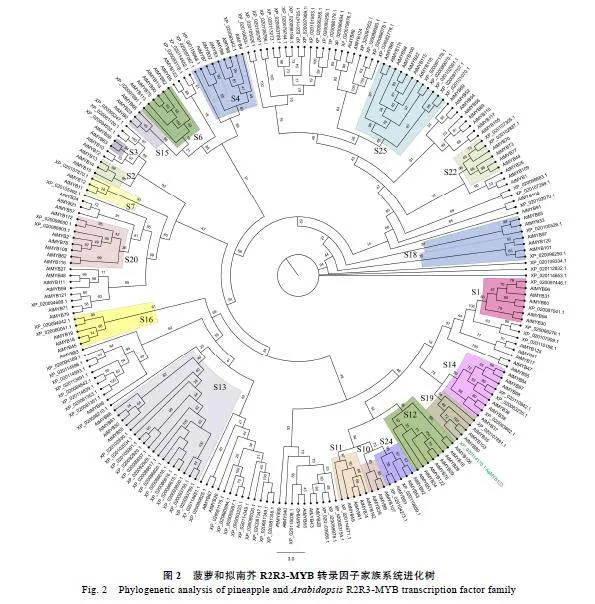
2.2""菠萝R2R3-MYB转录因子家族的系统进化分析
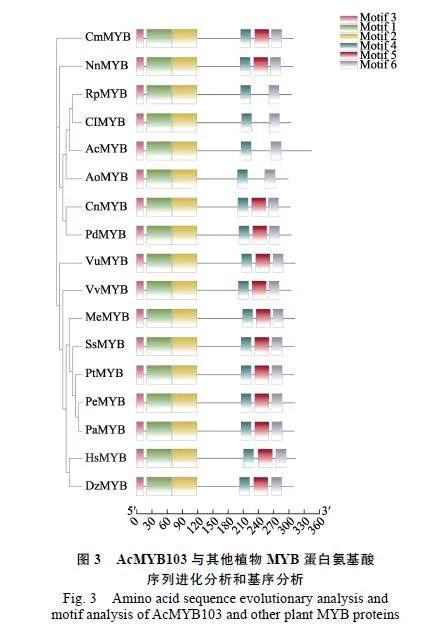
利用HMMER"3.1软件对菠萝基因组进行MYB结构域基因的搜索,经SMART和Pfam去除不具MYB转录因子保守结构域和冗余序列,在菠萝中共鉴定出103个菠萝R2R3-MYB家族基因。将所获得属于R2R3-MYB家族的103个菠萝AcMYB转录因子与已知的126个拟南芥AtMYB转录因子进行进化树分析。根据拟南芥MYB家族对菠萝MYB家族进行分类,结果显示,将103个AcMYB家族基因分为S1、S2、S3、S4、S6、S7、S10、S11、S12、S13、S14、S15、S16、S18、S19、S20、S22、S24、S2519个亚族(图2)。AcMYB103与拟南芥AtMYB103相似度最高,且与AtMYB80和AtMYB35共同聚为S19亚族。因CnMYB:椰子-KAG1359284.1;PdMYB:海枣-XP_008797178.1;RpMYB:刺子莞-KAJ4733963.1;AoMYB:芦笋-XP_020241891.1;SsMYB:簸箕柳-KAG5249367.1;PtMYB:毛果杨-XP_002304517.1;NnMYB:大紫玉-XP_010261333.1;VvMYB:葡萄-XP_002273004.1;DzMYB:榴莲-XP_022773261.1;PaMYB:银白杨-XP_034887886.1;PeMYB:胡杨-XP_011022623.1;CmMYB:沉水樟-RWR87806.1;MeMYB:木薯-XP_021622927.1;ClMYB:康藏嵩草-KAF3325127.1;HsMYB:木槿-XP_039068173.1;VuMYB:豇豆-QCD85070.1。黑色、粉色、蓝色区域分别表示氨基酸序列相似度100%、75%、50%。
2.3""AcMYB103蛋白的同源性分析
从图3可以看出,其他植物的MYB蛋白一般具有6个特异性基序,即基序1:EDEKL IR Y ITTHGYGCWSEVPEKAGLQRCGKSCRLRWINYLRPDIRRGRF,基序2:TPEEEKLIIS LHGVV GNR WAHIASHLPGRTDNEIKNYWNSWIKKKIRKPS,基序3:MGHHSCCNQQKVKRG,基序4:HHQQ AVL PPQATFSSGMDTNY,基序5:NMENMVPIE VQ S CS MBDEGEISLECLQRQ,基序6:WVES Q Q CSNFLFWDQVZGPLG。菠萝AcMYB103与刺子莞RpMYB、康藏嵩草ClMYB和芦笋AoMYB聚在一起,缺少基序5结构域,说明其是R2R3-MYB家族成员之一(图3)。
2.4""AcMYB103基因编码氨基酸生物信息学分析
利用ProtParam工具在线分析AcMYB103编码蛋白的理化性质:分子式C1702H2622N478O534S21,总原子数5357,分子量为38"997.68"Da,等电点为5.33,总平均亲水性为‒0.644,不稳定系数为52.35,推测AcMYB103蛋白是一个不稳定的疏水性蛋白。利用SOPMA分析AcMYB103蛋白的二级结构富含α-螺旋和随机卷曲结构(图4A)。利用PHYRE预测AcMYB103的三级结构与其二级结构相符合(图4B),属于典型的R2R3-MYB转录因子。
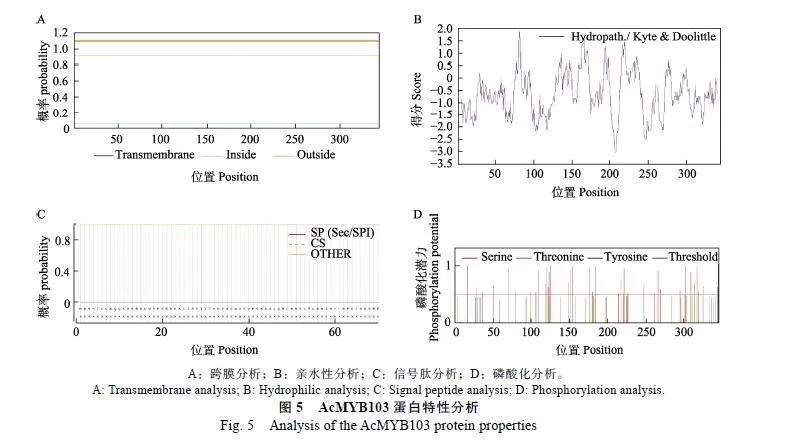
利用TMHMM-2.0在线工具分析预测表明AcMYB103蛋白无跨膜结构(图5A)。利用ProtScale工具证明它是疏水性蛋白(图5B)。利用SignalP-5.0"Server分析预测AcMYB103存在信号肽的概率是0.0009,无信号肽(图5C)。NetPhos"3.1磷酸化位点分析结果发现AcMYB103蛋白共有35个可能发生磷酸化的位点,表明AcMYB103蛋白在细胞信号转导过程中起重要作用(图5D)。DeepLoc-1.0预测AcMYB103蛋白定位于细胞核中,证明AcMYB103是定位在细胞核中的转录因子蛋白。
2.5""乙烯利处理菠萝果实中AcMYB103表达分析
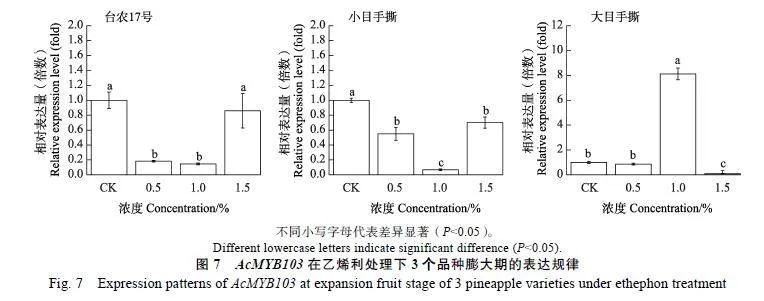
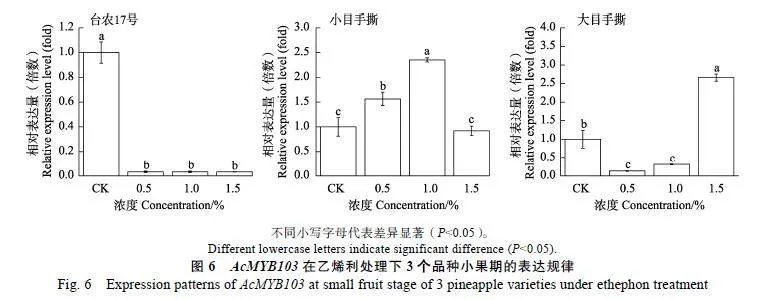
乙烯利是乙烯商用的释放剂,其在菠萝催花过程中具有明显的剂量效应。例如,台农17的夏季催花的最佳配方是1.0%~2.0%电石2次+0.6%乙烯利1次。台农16的乙烯利安全使用浓度为1.0%~1.5%。为了解析不同浓度梯度乙烯利处理广西3个栽培品种的3个时期的菠萝果实中AcMYB103基因表达规律,采用RT-qPCR技术进行表达模式分析。从图6中可以看出,在小果期随着乙烯利处理浓度提高,台农17号菠萝品种AcMYB103基因表达呈下调规律。小目手撕品种呈先上升后下降的规律,在1%"乙烯利浓度下表达量是CK的2.35倍(Plt;0.05)。大目手撕品种则是1.5%乙烯利浓度下表达最高,是CK的2.66倍(Plt;0.05)(图6)。而在膨大期随着乙烯利处理浓度的提高,台农17号菠萝品种AcMYB103基因表达呈下调规律。小目手撕品种中AcMYB103基因表达也呈现下降的规律。大目手撕品种则是1%乙烯利浓度下表达最高,是CK的8.13倍(Plt;0.05)(图7)。成熟期果实中,3个品种的AcMYB103基因均无表达。说明AcMYB103基因表达在乙烯利处理的品种和果实发育时期具有特异性。
3""讨论
菠萝水心病是近几年随着栽培技术和环境改变而产生的菠萝果实新病害,在苹果和梨中已有报道[16-17]。在菠萝中发现这个病害具有明显的品
种特异性。例如,台农17号菠萝果实水心病发病率明显高于其他品种,同时不同年份果实的发病程度也存在差异。研究表明,菠萝品种巴厘水心病果中内源激素玉米素(ZT)、茉莉酸(JA)和脱
落酸(ABA)含量均比对照显著下降,且随着发病程度的加深下降幅度增加[18]。目前,在梨和苹果中也尚处在初步研究阶段。在梨品种Akibae水心病果的转录组研究中,发现参与乙烯合成的1-氨基环丙烷-1-羧酸氧化酶(ACO)基因、1-氨基环丙烷-1-羧酸合成酶(ACS)和脱落酸合成的短链醇脱氢酶(SDR)和醛氧化酶(AAO)基因表达显著上调[19]。施用硼处理梨水心病感病品种,发现施用硼增加了梨果实淀粉降解、脂肪酸合成、IAA(吲哚-3-乙酸)降解和GA(赤霉素酸)合成基因的表达,抑制了乙烯合成基因的表达[20]。可见,果实内乙烯合成与水心病发病呈正相关。由于乙烯是菠萝有效的催花和座果剂[3,"21],因此有必要深入解析乙烯对菠萝果实发育的调控机制,阐明菠萝水心病发病机制。
R2R3-MYB转录因子在植物生长发育和转录调控中具有重要作用,并与乙烯信号转导机制紧密相关[22-23]。其中,AtMYB103/AtMYB80在植物生长发育过程中具有重要作用,且该基因功能多样。早期发现其调控绒毡层发育、胼胝质溶解和外壁形成[9]。随后,又证明它和MYB85、MYB52、MYB54诱导次生壁生物合成基因表达有关。其功能贯穿于花药发育过程中的细胞壁修饰、脂质代谢途径和信号转导途径,还可能与小孢子发生有关。除了拟南芥,相继在棉花[24]、烟草[25]和水稻[10]等植物中证明了其功能。本研究从菠萝果实中克隆的AcMYB103基因含有特征性的SANT结构域,与其他植物MYB家族成员高度同源,是典型的R2R3-MYB转录因子。前期研究表明,菠萝基因组含有103个R2R3-AcMYB转录因子,AcMYB10属于S22亚族[13]这与本研究结果不一致,因为本研究从基因组中克隆该基因,与多种植物基因进行对比,然后与拟南芥R2R3-MYB家族成员进行对比,最终重新命名为AcMYB103。该命名更加准确,便于进一步深入研究。在乙烯利处理的不同菠萝品种3个时期的表达规律表明,其在水心病敏感品种台农17中受乙烯利诱导下调表达,在小目手撕和大目手撕品种中上调表达,说明其在菠萝果实发育中具有重要作用。此外,AcMYB103基因在果实发育早期表达,而在成熟期完全不表达,说明其是早期调控果实发育的转录因子。这与前人在油菜中的研究结果相符,将油菜MYB80与EAR融合后,转基因油菜表现出绒毡层过早降解和花粉败育[26]。5个MYB80同源物都共享一个氨基酸序列,紧邻R2R3结构域,证明其在多种作物中结构和功能保守[11,"25]。本研究发现AcMYB103与拟南芥AtMYB80、AtMYB103和AtMYB35相似度最高,说明AcMYB103基因表达在乙烯利处理的品种和果实发育时期具有时空特异性,与其家族成员在植物发育调控中功能保守的结果一致[27-28]。本研究为深入探讨AcMYB103基因在菠萝果实生长发育调控机制中奠定基础,也为菠萝水心病的防控提供技术指导。
参考文献
<!--[if !supportLists]-->[1]"<!--[endif]-->YI"W,"LUAN"A,"LIU"C,"WU"J,"ZHANG"W,"ZHONG"Z,"WANG"Z,"YANG"M,"CHEN"C,"HE"Y."Genome-wide"identi f ication,"phylogeny,"and"expression"analysis"of"GRF"trans cription"factors"in"pineapple"(Ananas"comosus)[J]."Frontiers"in"Plant"Science,"2023,"14:"1159223.
<!--[if !supportLists]-->[2]"<!--[endif]-->ZHAO"H,"MAOKAI"Y,"CHENG"H,"GUO"M,"LIU"Y,"WANG"L,"CHAO"S,"ZHANG"M,"LAI"L,"QIN"Y."Charact eri z ation"of"auxin"transporter"AUX,"PIN"and"PILS"gene"families"in"pineapple"and"evaluation"of"expression"profiles"during"reproductive"development"and"under"abiotic"stresses[J]."PeerJ,"2021,"9:"e11410.
<!--[if !supportLists]-->[3]"<!--[endif]-->ZHANG"H,"PAN"X,"LIU"S,"LIN"W,"LI"Y,"ZHANG"X."Genome-wide"analysis"of"AP2/ERF"transcription"factors"in"pineapple"reveals"functional"divergence"during"flowering"induction"mediated"by"ethylene"and"floral"organ"development[J]."Genomics,"2021,"113(2):"474-489.
<!--[if !supportLists]-->[4]"<!--[endif]-->OGRODOWICZ"P,"KUCZYŃSKA"A,"KRAJEWSKI"P,"KE MPA"M."The"effects"of"heading"time"on"yield"performance"and"HvGAMYB"expression"in"spring"barley"subjected"to"drought[J]."Journal"of"Applied"Genetics,"2023,"64(2):"289-"302.
<!--[if !supportLists]-->[5]"<!--[endif]-->MUHAMMAD"N,"LUO"Z,"ZHAO"X,"YANG"M,"LIU"Z"G,"LIU"M"J."Transcriptome-wide"expression"analysis"of"MYB"gene"family"leads"to"functional"characterization"of"flavonoid"biosynthesis"in"fruit"coloration"of"Ziziphus"Mill[J]."Frontiers"in"Plant"Science,"2023,"14:"1171288.
<!--[if !supportLists]-->[6]"<!--[endif]-->李世佳,"吕紫敬,"赵锦."枣R2R3-MYB亚家族基因鉴定及其在果实发育中的表达分析[J]."中国农业科学,"2022,"55(6):"1199-1212.LI"S"J,"LYU"Z"J,"ZHAO"J."Identification"of"R2R3-MYB"subfamily"in"Chinese"jujube"and"their"expression"pattern"during"the"fruit"development[J]."Scientia"Agricultura"Sinica,"2022,"55(6):"1199-1212."(in"Chinese)
<!--[if !supportLists]-->[7]"<!--[endif]-->CHOPY"M,"BINAGHI"M,"CANNAROZZI"G,"HALITS CH KE"R,"BOACHON"B,"HEUTINK"R,"BOMZAN"D"P,"JAGGI"L,"VAN"GEEST"G,"VERDONK"J"C,"KUHLEMEIER"C."A"single"MYB"transcription"factor"with"multiple"functions"during"flower"development[J]."New"Phytologist,"2023,"239(5):"2007-2025.
<!--[if !supportLists]-->[8]"<!--[endif]-->LIU"C,"XIE"T,"CHEN"C,"LUAN"A,"LONG"J,"LI"C,"DING"Y,"HE"Y."Genome-wide"organization"and"expression"profiling"of"the"R2R3-MYB"transcription"factor"family"in"pineapple"(Ananas"comosus)[J]."BMC"Genomics,"2017,"18(1):"503.
<!--[if !supportLists]-->[9]"<!--[endif]-->ZHANG"Z"B,"ZHU"J,"GAO"J"F,"WANG"C,"LI"H,"LI"H,"ZHANG"H"Q,"ZHANG"S,"WANG"D"M,"WANG"Q"X,"HUANG"H,"XIA"H"J,"YANG"Z"N."Transcription"factor"AtMYB103"is"required"for"anther"development"by"regulating"tapetum"development,"callose"dissolution"and"exine"formation"in"Arabidopsis[J]."Plant"Journal,"2007,"52(3):"528-538.
<!--[if !supportLists]-->[10]"<!--[endif]-->WU"L,"ZHANG"M,"ZHANG"R,"YU"H,"WANG"H,"LI"J,"WANG"Y,"HU"Z,"WANG"Y,"LUO"Z,"LI"L,"WANG"L,"PENG"L,"XIA"T."Down-regulation"of"OsMYB103L"distinctively"alters"beta-1,4-glucan"polymerization"and"cellulose"microfibers"assembly"for"enhanced"biomass"enzymatic"saccharification"in"rice[J]."Biotechnology"for"Biofuels,"2021,"14(1):"245.
<!--[if !supportLists]-->[11]"<!--[endif]-->PHAN"H"A,"LI"S"F,"PARISH"R"W."MYB80,"a"regulator"of"tapetal"and"pollen"development,"is"functionally"conserved"in"crops[J]."Plant"Molecular"Biology,"2012,"78(1/2):"171-183.
<!--[if !supportLists]-->[12]"<!--[endif]-->TSUGAMA"D,"MATSUYAMA"K,"IDE"M,"HAYASHI"M,"FUJINO"K,"MASUDA"K."A"putative"MYB35"ortholog"is"a"candidate"for"the"sex-determining"genes"in"Asparagus"officinalis[J]."Scientific"Reports,"2017,"7(1):"41497.
<!--[if !supportLists]-->[13]"<!--[endif]-->陈哲,"胡福初,"阮城城,"范鸿雁,"郭利军,"张治礼."菠萝R2R3-MYB基因家族鉴定与表达分析[J]."热带作物学报,"2019,"40(10):"1958-1971.CHEN"Z,"HU"F"C,"RUAN"C"C,"FAN"H"Y,"GUO"L"J,"ZHANG"Z"L."Bioinformatics"and"gene"expression"analysis"of"pineapple"R2R3-MYB"gene"family[J]."Chinese"Journal"of"Tropical"Crops,"2019,"40(10):"1958-1971."(in"Chinese)
<!--[if !supportLists]-->[14]"<!--[endif]-->贺涵,"刘传和,"邵雪花,"赖多,"匡石滋,"肖维强."菠萝R2R3-MYB基因家族S20亚族的鉴定[J]."西北农林科技大学学报(自然科学版),"2022,"50(12):"117-127.HE"H,"LIU"C"H,"SHAO"X"H,"LAI"D,"KUANG"S"Z,"XIAO"W"Q."Identification"of"S20"subgroup"of"R2R3-MYB"sup erfa mily"of"Ananas"comosus"L."Merr[J]."Journal"of"Northwest"Aamp;F"University"(Natural"Science"Edition),"2022,"50(12):"117-127."(in"Chinese)
<!--[if !supportLists]-->[15]"<!--[endif]-->徐健,"韦巧云,"赵静,"樊松乐,"黄丽君,"罗晟昇,"蒋娟娟."菠萝AcMYB44基因的克隆及植物表达载体构建[J]."分子植物育种,"2022,"20(22):"7453-7460.XU"J,"WEI"Q"Y,"ZHAO"J,"FAN"S"L,"HUANG"L"J,"LUO"S"S,"JIANG"J"J."Cloning"and"expression"vector"construction"of"AcMYB44"gene"from"Ananas"comosus[J]."Molecular"Plant"Breeding,"2022,"20(22):"7453-7460."(in"Chinese)
<!--[if !supportLists]-->[16]"<!--[endif]-->WADA"H,"NAKATA"K,"NONAMI"H,"ERRA-BALSELLS"R,"TATSUKI"M,"HATAKEYAMA"Y,"TANAKA"F."Direct"evidence"for"dynamics"of"cell"heterogeneity"in"watercored"apples:"turgor-associated"metabolic"modifications"and"within-fruit"water"potential"gradient"unveiled"by"single-cell"analyses[J]."Horticulture"Research,"2021,"8(1):"187.
<!--[if !supportLists]-->[17]"<!--[endif]-->LIU"X,"LIU"D"H,"SHEN"Y,"LIU"J,"WEI"J,"WANG"C"L."Transcriptome"changes"associated"with"boron"applications"in"fruits"of"watercore-susceptible"pear"cultivar[J]."Molecular"Biology"Reports,"2022,"49(12):"12055-12061.
<!--[if !supportLists]-->[18]"<!--[endif]-->姚艳丽,"付琼,"周迪,"朱祝英,"杨玉梅,"张秀梅."水心病菠萝果实生理指标和内源激素含量变化[J]."热带作物学报,"2021,"42(9):"2587-2593.YAO"Y"L,"FU"Q,"ZHOU"D,"ZHU"Z"Y,"YANG"Y"M,"ZHANG"X"M."Changes"of"physiological"indexes"and"endogenous"hormone"content"of"watercore"pineapple"fruit[J]."Chinese"Journal"of"Tropical"Crops,"2021,"42(9):"2587-2593."(in"Chinese)
<!--[if !supportLists]-->[19]"<!--[endif]-->LIU"X,"FAN"H"M,"LIU"D"H,"LIU"J,"SHEN"Y,"ZHANG"J,"WEI"J,"WANG"C"L."Transcriptome"and"metabolome"analyses"provide"insights"into"the"watercore"disorder"on"“Akibae”"pear"fruit[J]."International"Journal"of"Molecular"Sciences,"2021,"22(9):"4911.
<!--[if !supportLists]-->[20]"<!--[endif]-->LIU"X,"LIU"D"H,"CHEN"T,"ZHANG"J,"WANG"C"L."Watercore"pear"fruit"respiration"changed"and"accumulated"γ-ami no butyric"acid"(GABA)"in"response"to"inner"hypoxia"stress[J]."Genes"(Basel),"2022,"13(6):"977.
<!--[if !supportLists]-->[21]"<!--[endif]-->RUAN"C"C,"CHEN"Z,"HU"F"C,"FAN"W,"WANG"X"H,"GUO"L"J,"FAN"H"Y,"LUO"Z"W,"ZHANG"Z"L."Genome-wide"characterization"and"expression"profiling"of"B3"superfamily"during"ethylene-induced"flowering"in"pineapple"(Ananas"comosus"L.)[J]."BMC"Genomics,"2021,"22(1):"561.
<!--[if !supportLists]-->[22]"<!--[endif]-->XING"G,"LI"J,"LI"W,"LAM"S"M,"YUAN"H,"SHUI"G,"YANG"J."AP2/ERF"and"R2R3-MYB"family"transcription"factors:"potential"associations"between"temperature"stress"and"lipid"metabolism"in"Auxenochlorella"protothecoides[J]."Biotechnology"for"Biofuels,"2021,"14(1):"22.
<!--[if !supportLists]-->[23]"<!--[endif]-->LIU"M"Y,"YANG"H,"FAN"S"L,"GUO"B"B,"DAI"L"J,"WANG"L"F,"WANG"M."Genome-wide"identification"and"expression"analysis"of"the"R2R3-MYB"gene"family"in"rubber"trees[J]."Forests,"2023,"14(4):"710.
<!--[if !supportLists]-->[24]"<!--[endif]-->MACMILLAN"C"P,"BIRKE"H,"CHUAH"A,"BRILL"E,"TSUJI"Y,"RALPH"J,"DENNIS"E"S,"LLEWELLYN"D,"PETTOLINO"F"A."Tissue"and"cell-specific"transcriptomes"in"cotton"reveal"the"subtleties"of"gene"regulation"underlying"the"diversity"of"plant"secondary"cell"walls[J]."BMC"Genomics,"2017,"18(1):"539.
<!--[if !supportLists]-->[25]"<!--[endif]-->VERMA"N,"BURMA"P"K."Regulation"of"tapetum-specific"A9"promoter"by"transcription"factors"AtMYB80,"AtMYB1"and"AtMYB4"in"Arabidopsis"thaliana"and"Nicotiana"tabacum[J]."Plant"Journal,"2017,"92(3):"481-494.
<!--[if !supportLists]-->[26]"<!--[endif]-->XU"Y,"IACUONE"S,"LI"S"F,"PARISH"R"W."MYB80"homologues"in"Arabidopsis,"cotton"and"Brassica:"regulation"and"functional"conservation"in"tapetal"and"pollen"development[J]."BMC"Plant"Biology,"2014,"14:"278.
<!--[if !supportLists]-->[27]"<!--[endif]-->HIGGINSON"T,"LI"S"F,"PARISH"R"W."AtMYB103"regulates"tapetum"and"trichome"development"in"Arabidopsis"thaliana[J]."Plant"Journal,"2003,"35(2):"177-192.
<!--[if !supportLists]-->[28]"<!--[endif]-->ZHU"J,"ZHANG"G,"CHANG"Y,"LI"X,"YANG"J,"HUANG"X,"YU"Q,"CHEN"H,"WU"T,"YANG"Z."AtMYB103"is"a"crucial"regulator"of"several"pathways"affecting"Arabidopsis"anther"development[J]."Science"China"Life"Sciences,"2010,"53(9):"1112-1122.
图5""ShTPT蛋白质三级结构的预测
Fig."5""Prediction"of"the"tertiary"structure"of"ShTPT"protein

A:ShTPT跨膜结构预测;B:ShTPT磷酸化位点预测。
A:"ShTPT"transmembrane"structure"prediction"diagram;"B:"Prediction"of"phosphorylation"sites.
图6""ShTPT蛋白理化性质分析
Fig."6""Analysis"of"physical"and"chemical"properties"of"ShTPT"Protein

图7""TPT蛋白系统进化树分析
Fig."7""Phylogenetic"tree"of"TPT"protein

图8""ShTPT亚细胞定位
Fig."8""Subcellular"localization"results"of"ShTPT

不同小写字母表示差异显著(Plt;0.05)。
Different"lowercase"indicate"significant"differencenbsp;(Plt;0.05).
图9""ShTPT基因在甘蔗不同组织的表达分析
Fig."9""Expression"analysis"of"ShTPT"gene"in"different"tissues"of"sugarcane

不同小写字母表示在0.05"水平差异显著。
Different"lowercases"indicate"significant"difference"at"the"level"0.05.
图10""ShTPT在干旱胁迫下的表达
Fig."10""Expressions"of"ShTPT"gene"under"drought"stress
