Study on the Mechanism of Action of Glyasperin A in the Treatment of Atherosclerosis Based on Network Pharmacology and Molecular Docking
2024-06-10NaLIXiangPUYihuiCHAIYuqiYANGLailaiLI
Na LI Xiang PU Yihui CHAI Yuqi YANG Lailai LI

Abstract [Objectives] This study was conducted to investigate the mechanism of action of glyasperin A in the treatment of atherosclerosis using a network pharmacology approach.
[Methods] Targets related to atherosclerosis were searched in GeneCards database. An active ingredient-disease-target network was constructed by Cytoscape 3.7.1. A target protein interaction network was constructed by String database. Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed on the DAVID database.
[Results] Glyasperin A acted on 36 atherosclerosis-related targets, and the biofunctional and pathway enrichment analyses showed that it was mainly involved in response to xenobiotic stimulus, drug transport across blood-brain barrier, lipid oxidation, barrier, and lipid oxidation, etc. The results showed that glyasperin A acted on 36 atherosclerosis-related targets. The biofunctional and pathway enrichment analyses showed that it was mainly involved in response to xenobiotic stimulus, drug transport across blood-brain barrier, lipid oxidation, positive regulation of protein localization to nucleus, and hepoxilin biosynthetic process, and it played an anti-fatigue role through signal pathways such as serotonergic synapse, efferocytosis, arachidonic acid metabolism, chemical carcinogenesis-receptor activation and platelet activation.
[Conclusions] Glyasperin A has multi-target and multi-pathway effects in the treatment of atherosclerosis. This study provides reference for further research on glyasperin A in the treatment of atherosclerosis.
Key words Glyasperin A; Atherosclerosis; Network pharmacology; Mechanism of action
DOI:10.19759/j.cnki.2164-4993.2024.02.014
Atherosclerosis is a chronic vascular inflammation, which involves a complex process of plaque formation by various cells, lipids and debris in the intima of blood vessels, which is influenced by many traditional and non-traditional risk factors[1]. Atherosclerosis is a chronic progressive disease, which mainly affects large and medium-sized arteries, such as coronary artery, cerebral artery, renal artery and lower extremity artery. Its basic pathological process is the deposition of blood components such as lipids in the intima of arteries, the proliferation of smooth muscle cells and the increase of collagen fibers, forming atherosclerotic plaques, which lead to thickening and hardening of the arterial wall and narrowing of the vascular lumen. If the disease continues to develop, it may cause calcification, fibrosis and other changes in the arterial wall, which will eventually cause the vascular wall to be stiff and lose its elasticity, thus affecting the blood supply of organs[2]. According to the national health and nutrition examination data of the United States, the overall population prevalence rate of high- and low-density lipoprotein cholesterol in the United States had reached 33.5% from 2005 to 2008[3]. Annual Report of Cardiovascular Health and Diseases in China (2019) pointed out that the prevalence of cardiovascular diseases in China is increasing year by year, with the number of patients reaching 330 million, and 2 out of every 5 deaths died of cardiovascular diseases on average[4]. Therefore, it is particularly important to carry out the treatment of atherosclerosis to prevent and treat the risks brought by cardiovascular diseases.
Data and Methods
Screening of drug and disease targets
The structure of glyasperin A could be found in PubChem database (https://pubchem.ncbi.nlm.nih.gov/), and then corresponding gene abbreviations were searched according to the structure of effective active ingredient of the drug through Swisstargetprediction database (http://www.swisstargetprediction.ch/). In the GeneCards database (https://www.genecards.org/), with "atherosclerosis" (AS) as the key word, reported genes related to atherosclerosis were searched, and duplicate genes were removed, and genes with correlation below 1 were screened out, so that target genes related to “atherosclerosis” were obtained. Finally, target genes of the ingredient were compared with target genes of the disease, and common targets were screened out. As a result, there were 36 targets for the treatment of atherosclerosis by glyasperin A.
Construction of "target-disease target" network
Targets related to the active ingredient of crude glyasperin A and atherosclerosis were screened, and duplicate targets were deleted. The data were imported into software Cytoscape Version 3.7.1, and a target network of crude glyasperin A-atherosclerosis was constructed. The core architecture of Cytoscape software is network, and each node is a gene, protein or molecule. Edges between nodes represent the interaction between these biomolecules, and the degree of nodes represents the number of nodes connected to each other in the network. A greater degree indicates that the target is more likely to become the key target of compounds.
Construction of protein interaction network
The protein targets of glyasperin A was imported into the String database (https://string-db.org/, Version 11.0). The credibility of protein interaction in the String database was divided into three grades, with an interaction score greater than 0.7 as high credibility, 0.4-0.7 as medium credibility and 0.15-0.40 as low credibility. In this study, a protein interaction network of crude glyasperin A in the treatment of atherosclerosis was constructed by selecting targets with interaction greater than 0.4 and hiding free nodes. In specific, files in PNG and TSV formats were downloaded. The obtained data were imported into Cytoscape3.7.1 software to draw the interaction network, which was then analyzed. The network analysis results were saved, and Network Analyzer tools in Cytoscape were used to set node size and color for the reflection of degree, so as to obtain the final protein interaction network.
Biological function and pathway enrichment analysis
The targets of glyasperin A were input into DAVID database (https://david.ncifcrf.gov/, Version 6.8), and the species was limited to human. The targets of glyasperin A were analyzed by gene ontology (GO) and KEGG pathway, and the results were saved.
Molecular docking
The 3D structures of targets and the molecular structure of the drug were obtained by PDB and PubChem platforms, respectively. Molecular docking was carried out by Autodockvina software, and the docking strength was analyzed using PyMOL and other software.
Results and Analysis
Active ingredient and its targets
Glyasperin A had 100 active targets. 1 894 targets related to atherosclerosis with correlation score greater than 1 were obtained by GeneCards search. A total of 36 potential targets were obtained by intersecting the active targets of glyasperin A with those related to atherosclerosis. The Wenn diagram is shown in Fig. 1.
Construction of "ingredient-disease target" network
The ingredient target-disease target network diagram was constructed with Cytoscape, as shown in Fig. 2, with 37 nodes and 36 edges. In the figure, "diamond" represents the active ingredient glyasperin A, and a "circle" represents a target.
Construction and analysis of protein interaction network
The target proteins of glyasperin A were introduced into the String database, and a protein interaction network was constructed by limiting the species to "human" and selecting the targets with interaction greater than 0.4. As shown in Fig. 3, nodes represent targets; edges represent the association between targets; and the degree of nodes represents the number of nodes connected to each other in the network. A greater degree indicates that the target is more likely to become the key target of the compound. AKT1, PPARG, ESR1, PTGS2, SRC, MPO, XDH and other targets are located at the core of the network and play a key regulatory role in the protein-protein interaction network.
Biological function and pathway enrichment analysis
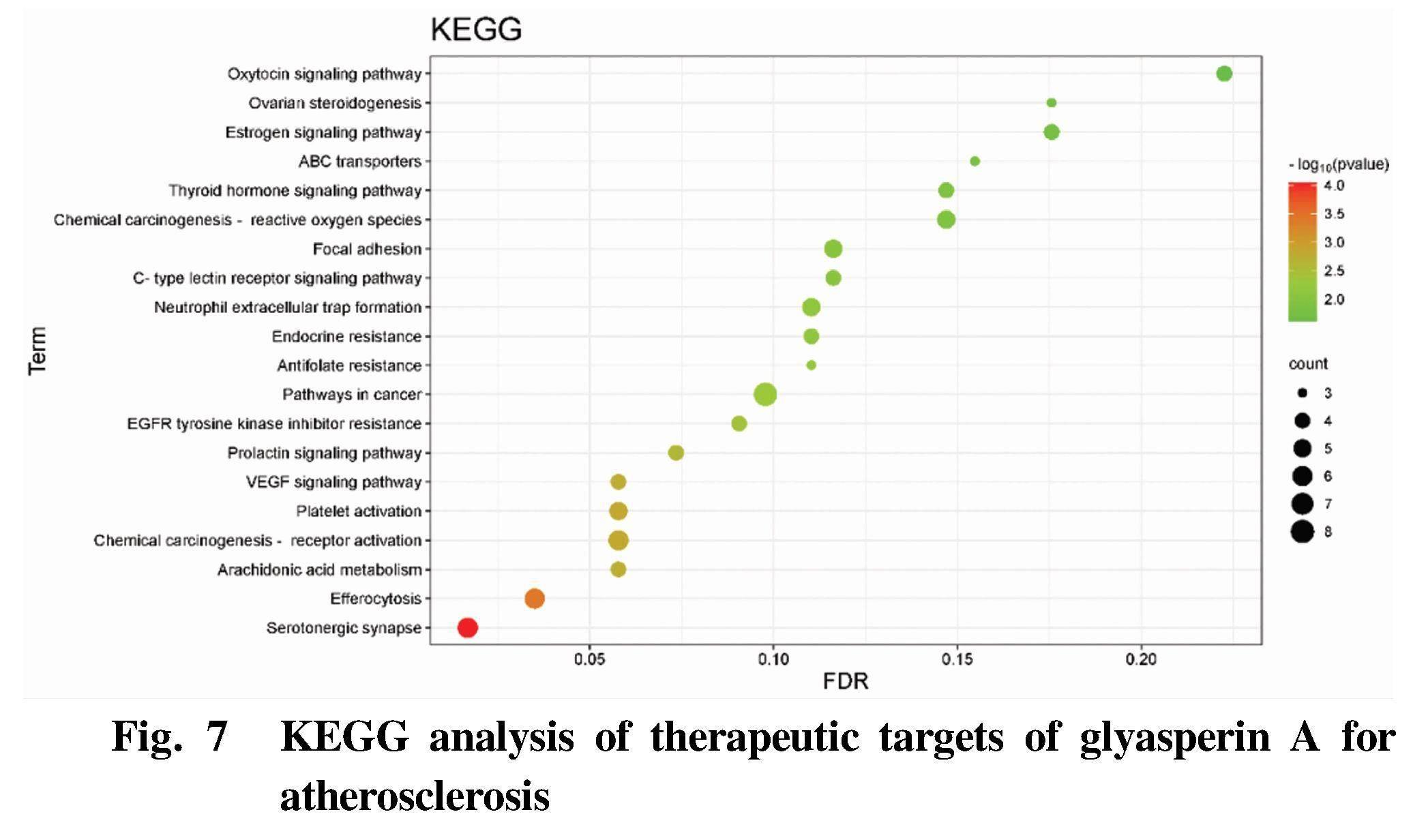
GO enrichment analysis and KEGG pathway analysis were carried out by using DAVID database. GO enrichment analysis includes three branches, namely biological process (BP), cellular component (CC) and molecular function (MF). The top biological processes or pathways were screened out to draw figures using Bioinformatics, as shown in Fig. 4-Fig. 7.
BP analysis is shown in Fig. 4. These targets were mainly involved in response to xenobiotic stimulus, drug transport across the blood-brain barrier, lipid oxidation, positive regulation of protein localization to nucleus, hepoxilin biosynthetic process and other biological processes.
CC analysis (Fig. 5) showed that the targets were mainly involved in cytosol, extrinsic component of cytoplasmic side of plasma membrane, macromolecular complex, azurophil granule lumen, cytoplasm and other cell components.
MF analysis (Fig. 6) showed that the targets were mainly involved in oxidoreductase activity, acting on single donors with incorporation of molecular oxygen, incorporation of two atoms of oxygen, enzyme binding, arachidonate12-lipoxygenaseactivity, iron ion binding, RNApolymerase II transcription factor activity, ligand-activated sequence-specific DNA binding and other molecular functions.
Na LI et al. Study on the Mechanism of Action of Glyasperin A in the Treatment of Atherosclerosis Based on Network Pharmacology and Molecular Docking
The results of KEGG path analysis (Fig. 7) showed that the therapeutic targets of crude glyasperin A for atherosclerosis were mainly involved in serotonergic synapse, efferocytosis, arachidonic acid metabolism, chemical carcinogenesis-receptor activation, platelet activation and other signal pathways.
Molecular docking of "active ingredient-target"
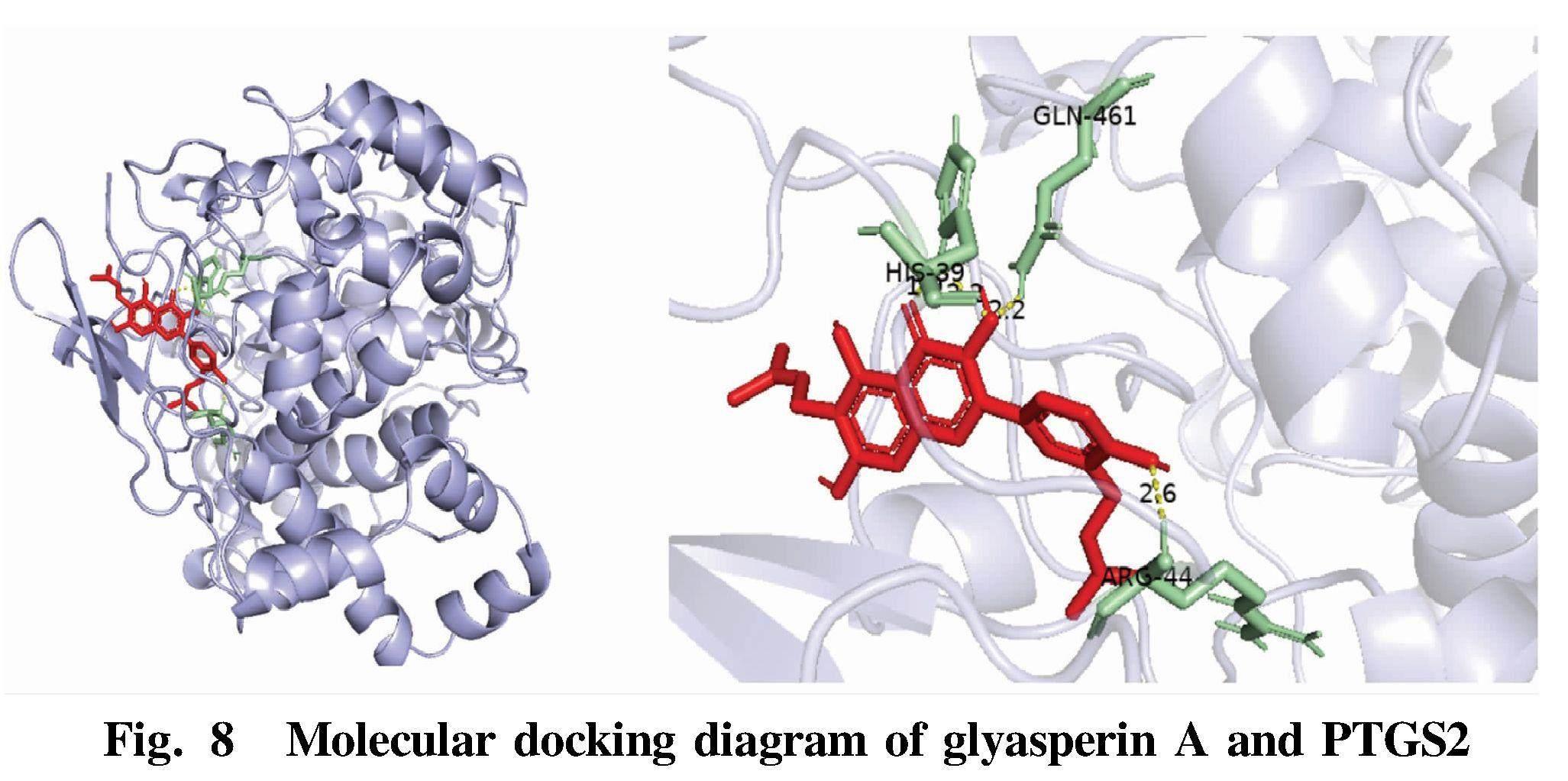
Glyasperin A and potential core targets PTGS2, SRC and MPO were molecularly docked to verify the prediction results. It is generally believed that the lower the binding energy, the more stable the conformation, and less than -7 kcal/mol indicates that the protein can be closely bound to the ligand. The results showed that glyasperin A had strong binding activity with potential core targets PPARG, PTGS2, SRC and MPO, which verified the reliability of the data in this study. Autodockvina was used to visualize the docking results and draw a 3D schematic diagram (Fig. 8-Fig. 10).
Red represents ligand molecules; yellow represents hydrogen bonds; and green represents amino acids in receptor proteins linked to ligands. PTGS2 was mainly connected with glyasperin A through GLN-461, HIS-39 and ARG44. SRC was mainly connected with glyasperin A through GLU-193, THR-191, GLN-192 and ARG-296. MPO and glyasperin A were mainly connected through ARG-46 and GLN-53.
Conclusions and Discussion
With the acceleration of social population aging, cardiovascular diseases are becoming more and more serious, and the incidence and mortality will continue to increase[5]. As a common pathological basis of various cardiovascular diseases (such as hypertension, coronary heart disease and acute myocardial infarction), atherosclerosis has also attracted much attention[6]. The basic feature of atherosclerosis is lipid deposition in the intima of arteries, which will form many uneven atherosclerotic plaques, make the lumen of blood vessels smaller and the elasticity lower, and thicken and harden the walls[7]. Moreover, in the process of plaque formation, internal bleeding, plaque rupture and calcification, thrombosis and atherosclerotic tumor will occur, which will eventually affect the blood supply of arteries and lead to ischemia or necrosis of surrounding tissues or organs[8]. Atherosclerosis is a complex process, and its pathogenesis is still unclear. Clinicopathological examination shows that it is related to the inflammatory reaction in patients with atherosclerosis[9]. Furthermore, the pathogenesis of many cardiovascular risk factors, such as hypertension, diabetes and hypercholesterolemia, is closely related to inflammatory response[10-11].
Glyasperin A can treat atherosclerosis through multiple channels and multiple targets. AKT, also known as PKB (protein kinase B), which is a kind of silk/threonine protein kinase, is an important intracellular signal regulator, and the main factor regulating AKT activity is PI-3K. Therefore, AKT is the main participant in PI-3K-AKT signal transduction pathway, which plays a pivotal role in various physiological and pathological processes of cells by regulating the phosphorylation of various effector molecules downstream. Mammalian AKT includes at least: AKT1, AKT2 and AKT3, which are known to participate in many biological behaviors such as cell survival, proliferation, migration, differentiation and apoptosis[12]. The experiment of Zhang et al.[13] proved that the expression of AKT1 inhibited the proliferation cycle of vascular smooth muscle cells and affected the direction of intracellular signal transduction, which could reduce the degree of intimal hyperplasia of carotid atherosclerosis in damaged rabbits. PPARG is mainly expressed in adipose tissue and immune system, which is closely related to adipocyte differentiation, body immunity and insulin resistance. The key factors affecting PPARG differentiation are preadipocytes and thiazolidinedione targeting molecules[14]. The study of Chen et al.[15] showed that suffering from coronary heart disease might be related to PPARG. Cyclooxygenase-2 (COX-2) is highly expressed under the regulation by many inflammatory cells, and it is expressed by PTGS2. Cyclooxygenase (COX) is the main rate-limiting enzyme in the process of synthesizing prostaglandin (PGs) and thromboxane A2 (TXA2) from arachidonic acid (AA), and it is involved in the pathological changes of atherosclerosis (AS). The increased expression of COX-2 in vascular smooth muscle cells may be induced by vascular injury or stimulation of various inflammatory factors[16]. Overexpression of COX-2 leads to inflammatory reaction, plaque instability and intimal hyperplasia. Studies have shown that the mRNA expression of COX-2 at positions with atherosclerosis is 4.8 times higher than that in normal arteries, and it is regulated by many inflammatory cells, and its metabolites can also promote the occurrence of inflammatory reactions[17]. In the process of AS occurrence and development, it mainly plays a role through activating inflammatory cytokines, increasing vascular permeability and stimulating the migration and proliferation of vascular smooth muscle cells[18].
To sum up, glyasperin A affects atherosclerosis in many ways through AKT1, PPARG, PTGS2 and other targets. In this study, the above results were verified by network pharmacology and molecular docking technology, but there is still a lack of direct experimental verification such as animal experiments. That is to say, this study has certain limitations, and the conclusions need further experimental verification.
References
[1] KONG P, CUI ZY, HUANG XF, et al. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention[J]. Signal Transduction and Targeted Therapy, 2022(5): 007.
[2] LIBBY P. The changing landscape of atherosclerosis[J]. Nature, 592(7855): 524-533.
[3] Centers for Disease Control and Prevention (CDC). Vital signs: Prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol: United States, 1999-2002 and 2005-200[J]. MMWR Morb Mortal Wkly Rep, 2011, 60(4): 109-914.
[4] HU SS. Annual report of cardiovascular health and diseases in China (2019)[M]. Beijing: Science Press, 2020.
[5] LIN X, OUYANG S, ZHI C, et al. Focus on ferroptosis, pyroptosis, apoptosis and autophagy of vascular endothelial cells to the strategic targets for the treatment of atherosclerosis[J]. Archives of Biochemistry and Biophysics, 2022: 715.
[6] WANG Y, ZHAO TR, ZOU Y, et al. Distribution of inflammatory cells in early atherosclerosis[J]. Chinese Pharmacological Bulletin, 2022(7): 038. (in Chinese).
[7] BOFFA MB, MARCOVINA SM, KOSCHINSKY ML. Lipoprotein(a) as a risk factor for atherosclerosis and thrombosis: Mechanistic insights from animal models[J]. Clinical Biochemistry, 2004, 37(5): 333-343.
[8] PARK K, LI Q, LYNES MD, et al. Endothelial cells induced progenitors into brown fat to reduce atherosclerosis[J]. Circulation research, 2022, 131(2): 168-183.
[9] CHU XM, LI B, AN Y, et al. The progression of the relation between inflammation and atherosclerosis[J]. Molecular Cardiology of China, 2010(3): 5. (in Chinese).
[10] SU L, XIAO LY, ZHANG L, et al. Effects of hyperbaric oxygen on cerebral atherosclerotic inflammation and oxidative stress in rats[J]. Journal of Jining Medical University, 2022(3): 045. (in Chinese).
[11] YAN CX, LYU CM, LI XN, et al. Correlation among blood pressure variability, inflammatory markers and carotid atherosclerosis severity in patients with primary hypertension[J]. Chinese Journal of Evidence-Based Cardiovascular Medicine, 2022(001): 014. (in Chinese).
[12] ZENG QH, LIU MN, LI XL, et al. Effects of Zhilong Huoxue Tongyu capsules on diabetic cardiomyopathy rats based on PI3K/AKT1/FoxO3a signaling pathway[J]. Journal of Chinese Medicinal Materials, 2023, 46(1): 197-201. (in Chinese).
[13] ZHANG QQ. Effect of all-trans retinoic acid (atRA) on proliferation of vascular smooth muscle cells and the expression of E2F1 and AKT1 in carotid atherosclerosis in rabbits[D]. Qingdao: Qingdao University, 2011. (in Chinese).
[14] QUINN CE, HAMILTON PK, LOCKHART CJ, et al. Thiazolidinediones: Effects on insulin resistance and the cardiovascular system[J]. British Journal of Pharmacology, 2010, 153(4): 636-645.
[15] CHEN HF, MA RG, SHEN SQ. Association between patients with coronary heart disease and gene variation of PON2 and PPARG[J]. Clinical Education of General Practice, 2020, 18(9): 837-839, 845. (in Chinese).
[16] LI Y, ZHOU L, WANG D, et al. Effects of different doses of blood circulation and traditional Chinese medicine on the expression of PTGS2, PADI4 and ITGAM in aorta of atherosclerotic mice[J]. Chinese Journal of Difficult and Complicated Cases, 2016, 15(11): 1182-1186. (in Chinese).
[17] GUO Z. Expression and significance of COX-2 and MMP-9 in coronary atherosclerosis in patients with acute coronary syndrome[D]. Dalian: Dalian Medical University, 2006. (in Chinese).
[18] LIU HF, LI XH, YANG QD, et al. COX-2 and mPGES-1 expression in carotid atherosclerotic plaques[J]. Chinese Journal of Medical Genetics, 2007. (in Chinese).
杂志排行
农业生物技术(英文版)的其它文章
- Effects of Different Climates and Soil Environments in the Yanshan Production Area on the Growth and Quality of Chestnuts
- Research Progress of QuEChERS Pretreatment Technique in the Detection of Multiple Pesticide Residues
- Determination of Benzo[a]pyrene in Edible Oil by High Performance Liquid Chromatography-Fluorescence Detector (HPLC-FL)
- Exploration on Management Measures of Onion Production in Eastern Henan Province
- Study on Antioxidant Enzyme Activity and Physiology and Biochemistry of Solanum nigrum L. under Glyphosate Stress
- Effects of Slow-release Nitrogen Fertilizer on Yield and Nitrogen Accumulation of Summer Maize in Shajiang Black Soil Area
