Discovery and structure-activity relationship studies of novel tetrahydro-β-carboline derivatives as apoptosis initiators for treating bacterial infections
2024-05-13ShanshanSuHongwuLiuJunrongZhangPuyingQiYueDingLingZhangLinliYangLiweiLiuXiangZhouSongYang
Shanshan Su,Hongwu Liu,Junrong Zhang,Puying Qi,Yue Ding,Ling Zhang,Linli Yang,Liwei Liu,Xiang Zhou ,Song Yang
National Key Laboratory of Green Pesticide/Key Laboratory of Green Pesticide and Agricultural Bioengineering,Ministry of Education/Center for R&D of Fine Chemicals,Guizhou University,Guiyang 550025,China
Abstract Developing and excavating new agrochemicals with highly active and safe is an important tactic for protecting crop health and food safety.In this paper,to discover the new bactericide candidates,we designed,prepared a new type of 1,2,3,4-tetrahydro-β-carboline (THC) derivatives and evaluated the in vitro and in vivo bioactivities against the Xanthomonas oryzae pv.oryzae (Xoo),Xanthomonas axonopodis pv.citri (Xac),and Pseudomonas syringae pv.actinidiae (Psa).The in vitro bioassay results exhibited that most title molecules possessed good activity toward the three plant pathogenic bacteria,the compound A17 showed the most active against Xoo and Xac with EC50 values of 7.27 and 4.89 mg mL-1 respectively,and compound A8 exhibited the best inhibitory activity against Psa with EC50 value of 4.87 mg mL-1.Pot experiments showed that compound A17 exhibited excellent in vivo antibacterial activities to manage rice bacterial leaf blight and citrus bacterial canker,with protective efficiencies of 52.67 and 79.79% at 200 mg mL-1,respectively.Meanwhile,compound A8 showed good control efficiency (84.31%) against kiwifruit bacterial canker at 200 mg mL-1.Antibacterial mechanism suggested that these compounds could interfere with the balance of the redox system,damage the cell membrane,and induce the apoptosis of Xoo cells.Taken together,our study revealed that tetrahydro-β-carboline derivatives could be a promising candidate model for novel broadspectrum bactericides.
Keywords: tetrahydro-β-carboline derivatives,antibacterial activity,ROS,cell apoptosis
1.Introduction
Plant pathogenic microorganisms can destroy crops,cause local or whole plant disease and lead to immense economic losses,which pose a great threat to agricultural production (Rayet al.2013;Kinget al.2017;Panet al.2021).Among them,Xanthomonas oryzaepv.oryzae(Xoo),a Gram-negative bacillus,severely restricts the yield and quality of rice (Singhet al.2018;Zhanget al.2018,2021;Mouet al.2021).Rice leaf blight caused byXoois sudden and mutagenic,spreading infestation very fast,and once the outbreaking is difficult to control (Mollaet al.2020;Fiyazet al.2022).Xanthomonas axonopodispv.citri(Xac) is a crucial plant pathogenic bacteria that occurs in citrus,and causes leaf drop,fruit drop,weakened trees and poorly shaped affected fruits (Hirata 2010;Favaroet al.2017;Xuet al.2022).Kiwifruit bacterial canker incurred byPseudomonas syringaepv.actinidiae(Psa),which is a seriously refractory and epidemic threatening of kiwifruit industry (Floreset al.2020;Wang F Met al.2020).Although some bactericides are being used to control bacteria,these chemicals do not completely protect the crop from bacterial infection due to their unsatisfied field control effect,and emerging pesticidesresistance problems.There is therefore an urgent need for developing new bactericides with new skeleton,ecofriendly,and novel action mechanism.
To date,natural products have been widely explored for drug or pesticides discovery.More importantly,some new molecules originating from natural products by rational structure modification shows greater application potential because of these new molecules circumvent the parent natural products’ serious disadvantages including low drug activity,complex structure,poor physicochemical and pharmacokinetic properties,and significant toxicity (Kambojet al.2021).Thus,natural products can be regarded as the ideal precursor source for the development of agrochemicals (Zhouet al.2018;Sharmaet al.2020).Notably,1,2,3,4-tetrahydroβ-carboline (THC) alkaloids are mostly derived from plants,marine species,etc.(Wang Jet al.2021).As displayed in Fig.1,a great many of them possess remarkable bioactivities,such as anticancer (Harmicine),antioxidant (Eleagnine),antifungal (Venenatine),and antiviral activities,etc.(Youssef 2005;Zhenget al.2014;Sundinet al.2016;Wang and Song 2020;Luo and Song 2021;Wang Qet al.2021).Moreover,THC alkaloids,which consist of a steadfast indole core structure and a versatile piperidine unit,is highly attraction in drug design (Songet al.2014a,b;Spindleret al.2016;Abinayaet al.2022).More interestingly,our previous work discovered that THC derivatives bearing an 1,3-diaminopropan-2-ol pattern firstly showed efficient competence to control intractable plant bacterial diseases (Liuet al.2020).Therefore,THC skeleton showed unprecedented merits for new agrochemical discovery.
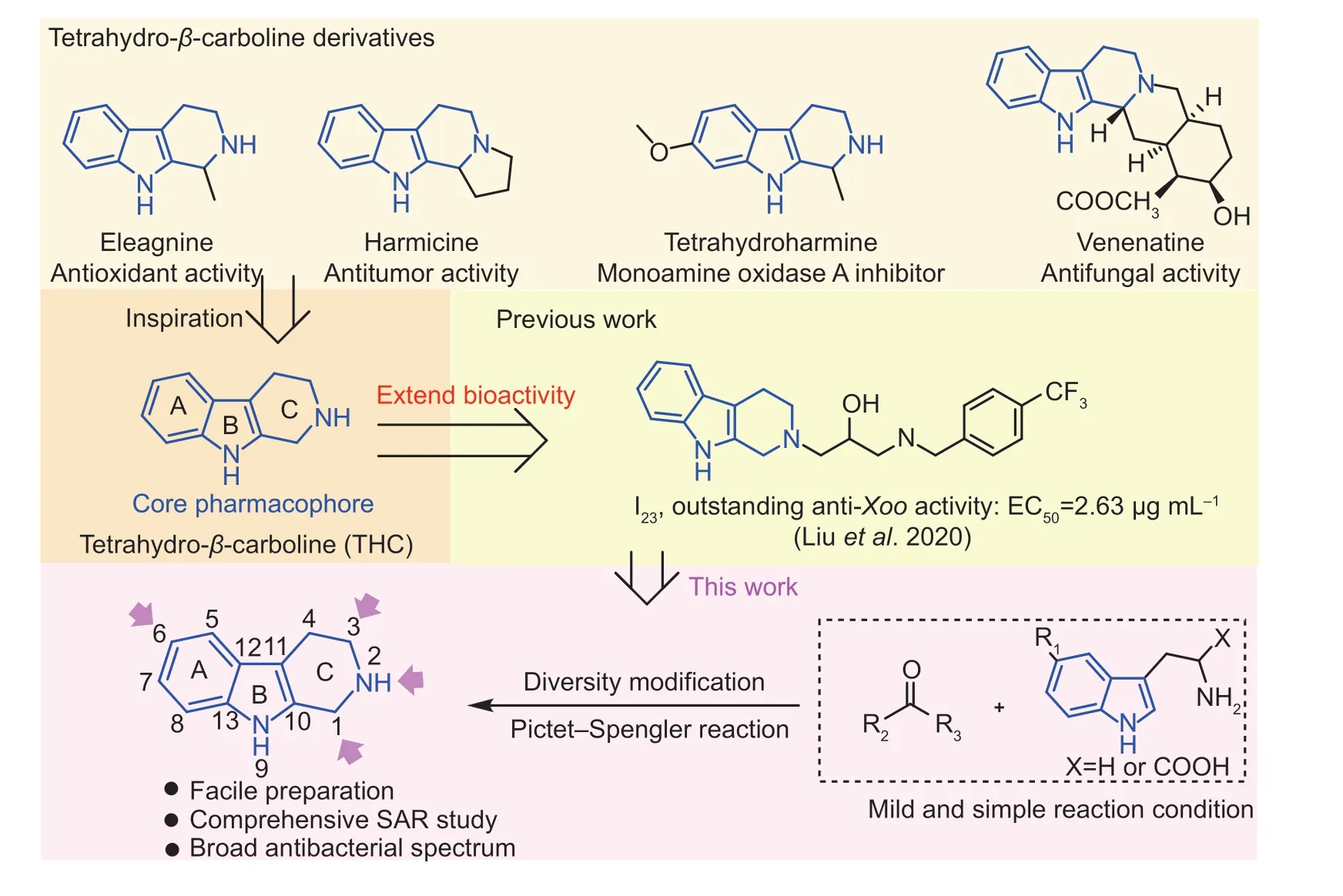
Fig.1 Natural 1,2,3,4-tetrahydro-β-carboline (THC) derivatives and their biological activities,and the conception of this work.Xoo,Xanthomonas oryzae pv.oryzae.
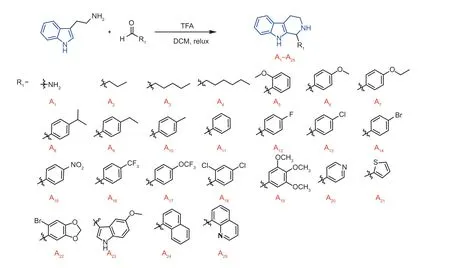
Fig.2 Synthesis route of molecules A1-A25.
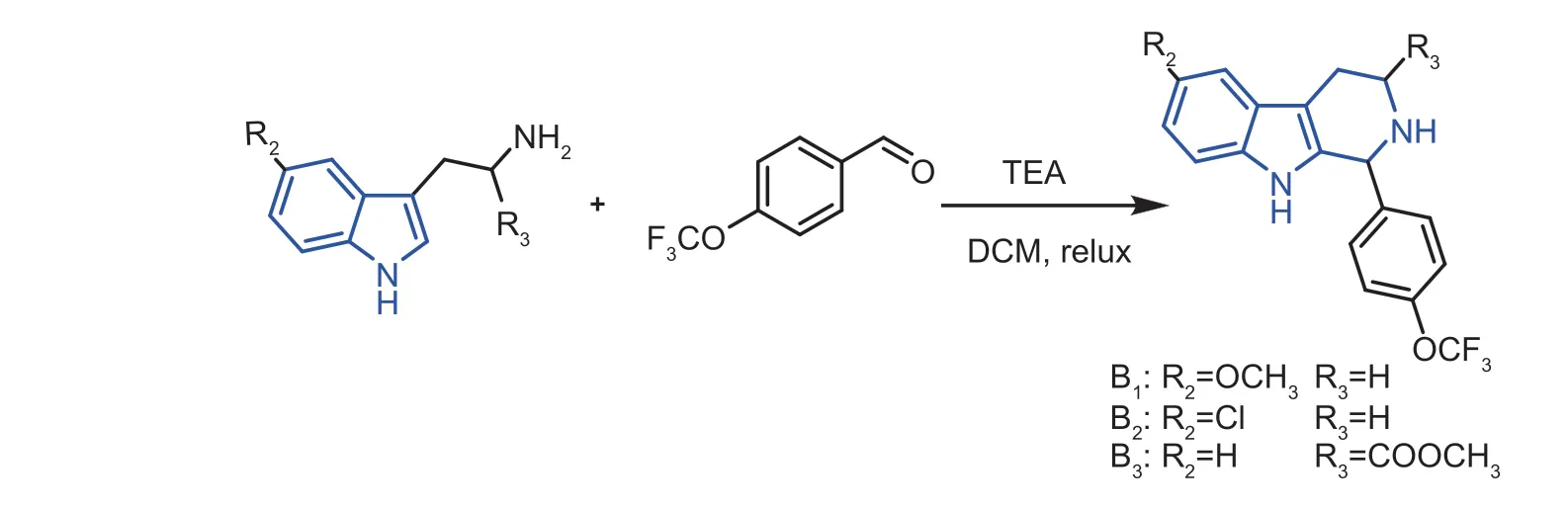
Fig.3 Synthesis route of molecules B1-B3.
To further in-depth understanding of antibacterial behavior and their structure-activity relationship (SAR),a lot of simple and novel THC analogues decorating with differ simple substituents on different position were elaborately prepared and investigated for their anti-phytopathogenic bacterial activity,and the antibacterial mechanisms were discussed systematically.Fluorescence staining assays were used to detect the apoptosis and reactive oxygen species (ROS).
2.Materials and methods
2.1.Instruments and chemicals
1H NMR,13C NMR and19F NMR data were acquired using NMR apparatuses (JEOL-ECX-500 and Bruker Biospin AG-400).High resolution mass spectrum (HRMS) was achieved by a Q-Exactive Orbitrap MS apparatus (ThermoFisher Scientific,USA).OD values were conducted by a Cytation™ 5 multi-mode readers (BioTek Instruments,Inc.,USA).Transmission electron microscopy (TEM) results were obtained on a FEI Talos F200C microscope (Thermo Fisher Scientific Corporation,Massachusetts,USA).Fluorescent images were collected through Olympus BX53 microscope (Olympus,Tokyo,Japan).In addition,all raw materials and reagents were purchased from commercial sources.
2.2.Synthesis of title molecules
All the THC compounds were prepared from tryptamine,and reacted with aldehyde/ketoneviaPictet-Spengler reaction (Raoet al.2017;Shenget al.2021).As shown in Figs.2-5,tryptamine (0.327 g,2.0 mmol) and benzaldehyde (0.250 g,2.4 mmol) were dissolved in 6 mL CH2Cl2in a 15-mL reaction pipe,then TFA (227 mL,3.0 mmol) were subsequently supplemented.After reaction was finished completely,the reaction mixture was quenched by 10% potassium carbonate solution (10 mL),extracted with ethyl acetate (20 mL×2),dried by anhydrous Na2SO4and evaporated in vacuum.Finally,the purified title compounds were obtained by column chromatography.The synthesis procedures are shown in Appendices A-D and the structural identification of compounds is shown in Appendix E.
2.3.Experimental section
The experimental programs,includingin vitroandin vivoantibacterial bioassays,were followed according to our reported methods.Briefly,forin vitroactivity,we screened forin vitroinhibitory activity using the turbidimetric method (Huanget al.2021).The detailed methods are listed in Appendix F.In vivobioactivity assays againstXoo,XacandPsawere carried out by leaf clipping,hole tying and slitting methods,respectively (Wuet al.2019,2020;Wanget al.2022).
2.4.Acridine orange-ethidium bromide (AO/EB) staining
We investigated the mechanism of apoptosis by AO/EB assay (Kumaret al.2017a,b;Tokalaet al.2018;BalaKumaranet al.2020).Xoocells were incubated with various dosages of compound A17(6.25,12.5,25.0,and 50.0 mg mL-1) for 12 h and the control was the same volume of DMSO.They were collected (6,500 r min-1,3 min,4°C),washed twice with PBS (pH 7.2,10 mmol L-1) and then suspended in PBS.The collectedXoocells were mixed with 5 mL of AO (100 mg mL-1) and EB (100 mg mL-1) (v/v=1:1) for 20 min at 28°C in the dark.The cells were then washed again and excited at 488 nm and 520 nm for AO and EB excitation,respectively.
2.5.Morphological study of Xoo cells (SEM)
SEM observation of cell morphology was consistent with previous work (Wuet al.2021).Briefly,after pretreatment with various dosages of molecule A17(0,12.5,25.0,and 50.0 mg mL-1) withXoocells (OD595=0.2) about 12 h.Then,the cells were gently washed with PBS (pH 7.2,10 mmol L-1),fixed with 2.5% glutaraldehyde and gradually dehydrated with different concentrations of ethanol.Finally,the samples were freeze-dried,gold coated and observed by SEM.
2.6.Detection of ROS production stimulated by compound A17
The experimental method of ROS detection was based on the published experimental protocol (Wang P Yet al.2020;Songet al.2022).ROS accumulation was conducted by ROS Assay Kit (Biyuntian Company,Shanghai,China).TheXoocells were incubated with compound A17at various dosages (0,12.5,25.0,and 50.0 mg mL-1),and the control group was DMSO at the same volume for 12 h.Xoocells were collected (6,500 r min-1,3 min,4°C),and washed twice with sterile water,and suspended in sterile water.Xoocells were then treated with 1 mL of DCFA-DA probe for 20 min at 28°C in the dark.Samples were recorded using Fluoromax-4cp fluorescence spectrophotometer (excitation wavelength: 488 nm).
3.Results
3.1.Synthesis
Expecting to obtain some compounds with high activity,simple structure,and short synthetic route,we used the Pictet-Spengler reaction to give the title molecules in one step.
3.2.Antibacterial bioassay
Thein vitroantibacterial efficacy was screened by turbidimetric method,the control agents were thiodiazole copper (TC) and bismerthiazol (BT).The preliminary activity test results of A1-A26(Table 1) demonstrated that most of the molecules possessed good inhibitory activity.Therefore,the EC50values of the title molecules towardsXoo,XacandPsawere displayed in Table 2.
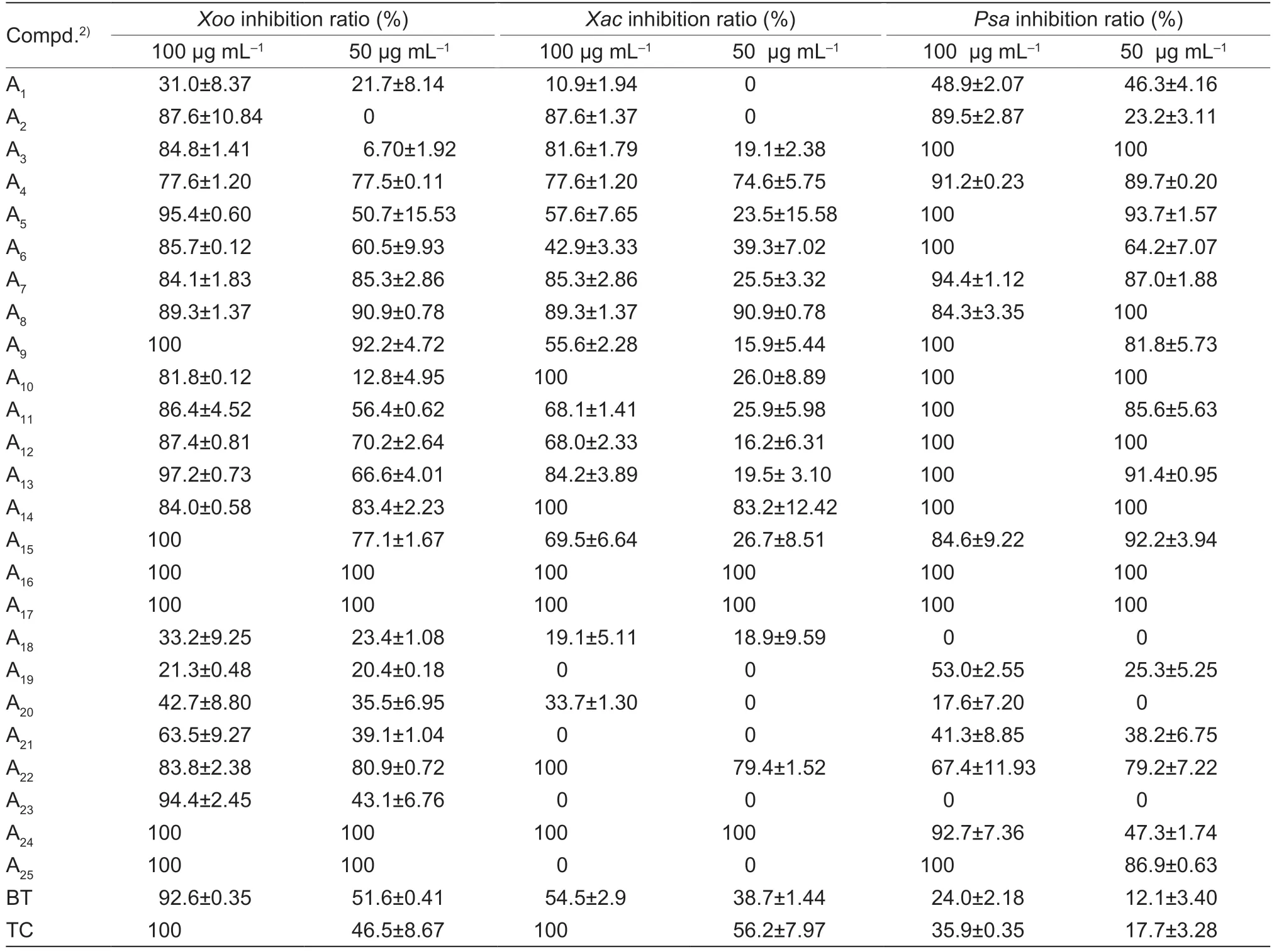
Table 1 Antibacterial activities of title molecules A1-A25 against Xoo,Xac and Psa at 50 and 100 μg mL-11)
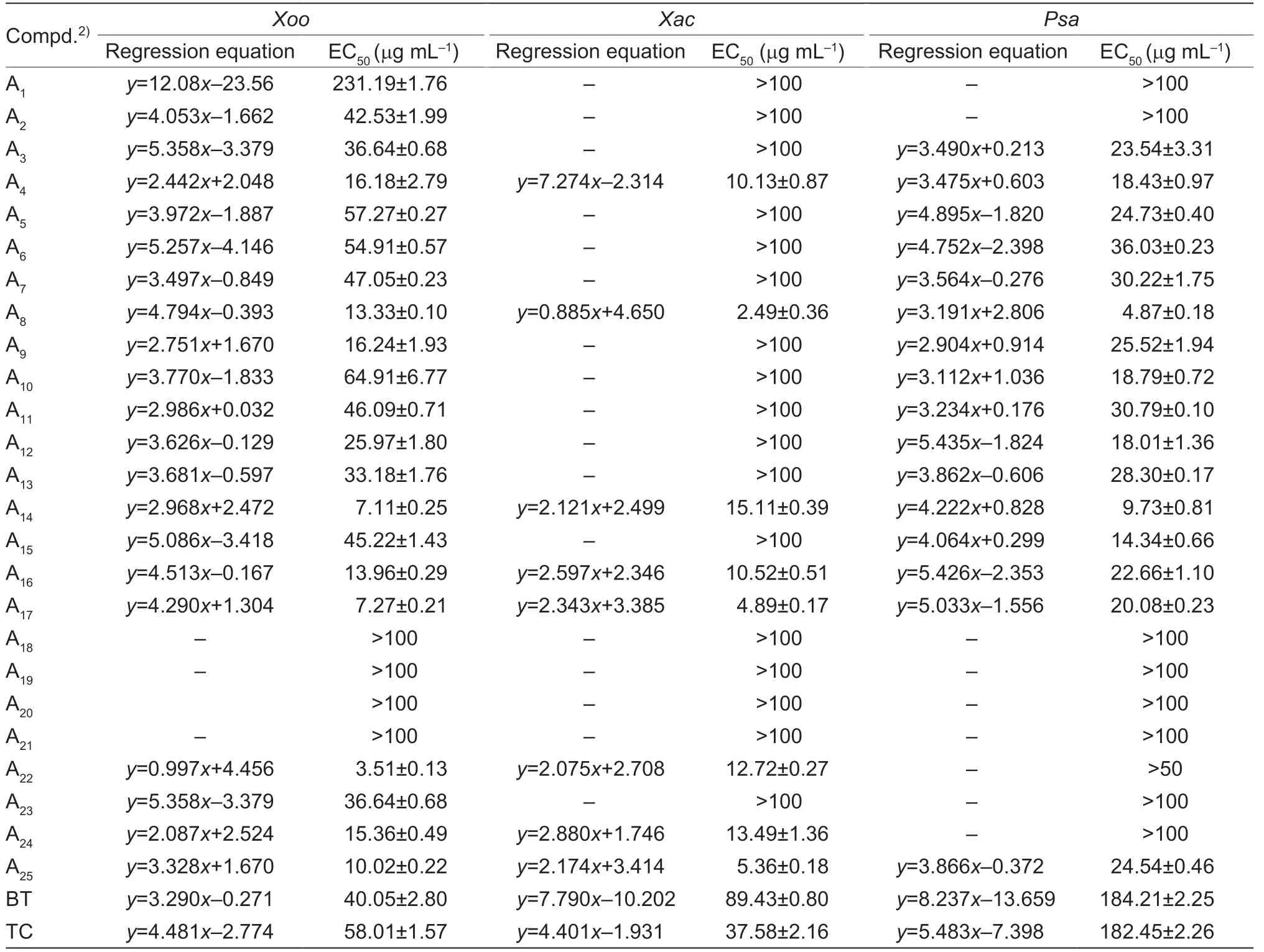
Table 2 In vitro bioassay results of A1-A25 against pathogens1)
3.3.Bioassay results and SAR study of title molecules
As shown in Table 2,most of title molecules possessed moderate to outstanding antibacterial potency.Among them,compound A11(anti-Xoo,EC50=46.09 mg mL-1,anti-Xac,EC50>100 mg mL-1,anti-Psa,EC50=30.79 mg mL-1) was obtained when the benzene ring was attached to the C1 position of the C ring,and this molecule displayed ordinary activity against all three phytopathogenic bacteria.When C4’ of the benzene ring was attached to the weakly electronabsorbing group -F,-Cl,-Br,the activity of compound A14(anti-Xoo,EC50=7.11 mg mL-1,anti-Xac,EC50=15.11 mg mL-1,anti-Psa,EC50=9.73 mg mL-1) was found to be greater than that of A12(anti-Xoo,EC50=25.97 mg mL-1,anti-Xac,EC50>100 mg mL-1,anti-Psa,EC50=18.01 mg mL-1),and also greater than that of A13(anti-Xoo,EC50=33.18 mg mL-1;anti-Xac,EC50>100 mg mL-1;anti-Psa,EC50=28.30 mg mL-1);when C4’ of the benzene ring was attached to the electron-giving group -CH3(compound A10: anti-Xoo,EC50=64.91 mg mL-1;anti-Xac,EC50>100),the compound showed poor activity towardsXooandXac,but when the methyl group was replaced by methoxy (compound A6: anti-Xoo,EC50=54.91 mg mL-1),the anti-Xooactivity of the molecules improved,and the maximum bioactivity was achieved when switching to trifluoromethoxy (compound A17),with EC50values of 4.89 mg mL-1forXacand 7.27 mg mL-1forXoo.The effect of chain length on the C4’ of the benzene ring was also analyzed,and it was found that the anti-Xooactivity of the compounds with ethyl (compound A9: EC50=16.24 mg mL-1) was found to be more active than that with a methyl group (compound A10: EC50=64.91 mg mL-1).The activity of C4’ of the benzene ring with chlorine attached (compound A13: anti-Xoo,EC50=33.18 mg mL-1) was higher than that of C4’ and C6’ (compound A18: anti-Xoo,EC50>100) with chlorine attached,but when the chlorine was replaced with methoxy,the activities of C4’ (compound A6: anti-Xoo,EC50=54.91 mg mL-1;anti-Xac,EC50>100;anti-Psa,EC50=36.03 mg mL-1) and C6’ (compound A5: anti-Xoo,EC50=57.27 mg mL-1;anti-Xac,EC50>100;anti-Psa,EC50=24.73 mg mL-1) were comparable.Secondly,when replacing C1 with tetrahydropyridine (compound A20: anti-Xoo,EC50>100;anti-Xac,EC50>100;anti-Psa,EC50>100),prepared molecule was found to be less inhibitory activity compared to the activity of the benzene ring (compound A11;anti-Xoo,EC50=46.09 mg mL-1;anti-Xac,EC50>100;anti-Psa,EC50=30.79 mg mL-1);when replacing it with quinoline group (compound A25: anti-Xoo,EC50=10.02 mg mL-1;anti-Xac,EC50=5.36 mg mL-1;anti-Psa,EC50=24.54 mg mL-1) and naphthalene group (compound A24: anti-Xoo,EC50=15.36 mg mL-1;anti-Xac,EC50=13.49 mg mL-1;anti-Psa,EC50>100),the activity of the compound was found to be improved.Then,when C1 was replaced with straight chain structures (compound A2: anti-Xoo,EC50=42.53 mg mL-1;anti-Xac,EC50>100;anti-Psa,EC50>100) (compound A3: anti-Xoo,EC50=36.64 mg mL-1;anti-Xac,EC50>100;anti-Psa,EC50=23.54 mg mL-1) (compound A4: anti-Xoo,EC50=16.18 mg mL-1;anti-Xac,EC50=10.13 mg mL-1;anti-Psa,EC50=18.43 mg mL-1),the compounds were found to exhibit poor inhibitory activity againstXacandPsa,and average inhibitory activity againstXoo.The activity of the compounds became even worse as the spatial site resistance increased,and the compounds were almost inactive when C1 (compound A1: anti-Xoo,EC50=231.19 mg mL-1;anti-Xac,EC50>100,anti-Psa,EC50>100) was replaced with amines.
Given the perfect bioactivity of molecule A17(anti-Xooactivity: EC50=7.27 mg mL-1;anti-Xacactivity: EC50=4.89 mg mL-1),we further prepared title molecules based on the parent molecule A17.When reacting with 4-chlorotryptamine or 4-methoxytryptamine and an 4-(trifluoromethoxy)benzaldehyde,the title molecules B1and B2were obtained (Table 3).For anti-Xooactivity,the -OCH3attached (compound B1: EC50=23.89 mg mL-1) activity was lower than that of -Cl (compound B2: EC50=11.18 mg mL-1),but for the anti-Psaactivity,the -OCH3attached (compound B1: EC50=11.02 mg mL-1) activity was higher than that of -Cl (compound B2: EC50>100 mg mL-1).
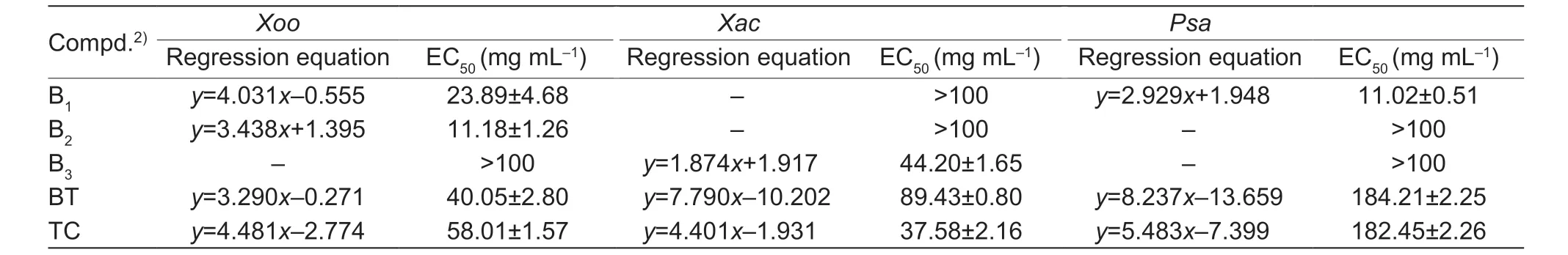
Table 3 In vitro bioassay results of B1-B3 against pathogens1)
Moreover,to probe the synergistic effects of the C-ring NH substitutions,the reaction with compound A17and benzenesulfonyl chloride got the compound C (Fig.4).Interestingly,the compound was failed to kill the three pathogenic bacteria (Table 4),which indicating that the C-ring NH was immense importance for the bacteria.In addition,as displayed in Fig.5,when ketones were used to replace aldehydes in the reaction,the substitution of cyclopentanone did not improve the activity,or even decreased it,and the substitution of straight-chain ketones,showed good activity,with EC50up to 5.31 mg mL-1againstXacand 5.68 mg mL-1againstXoo,however,it showed poor activity anti-Psa(Table 5).Eventually,the SAR study of the C ring focused on the C1,N2 and C6 positions was displayed in Fig.6.
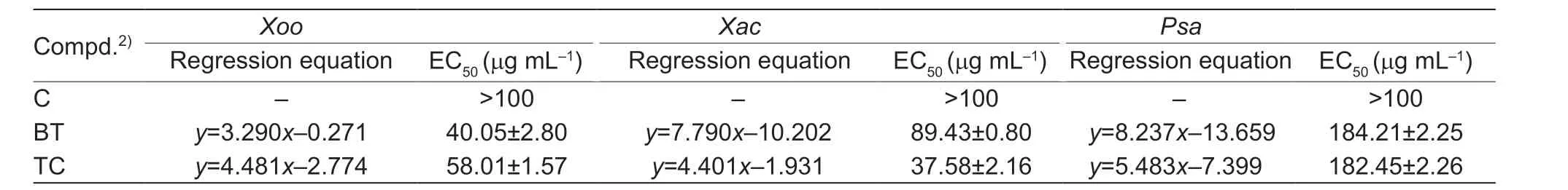
Table 4 In vitro bioassay results of C against pathogens1)
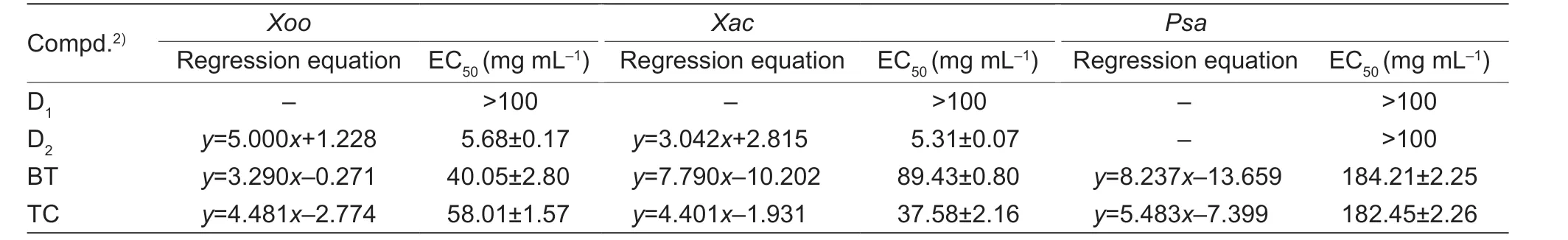
Table 5 In vitro bioassay results of D1 and D2 against pathogens1)
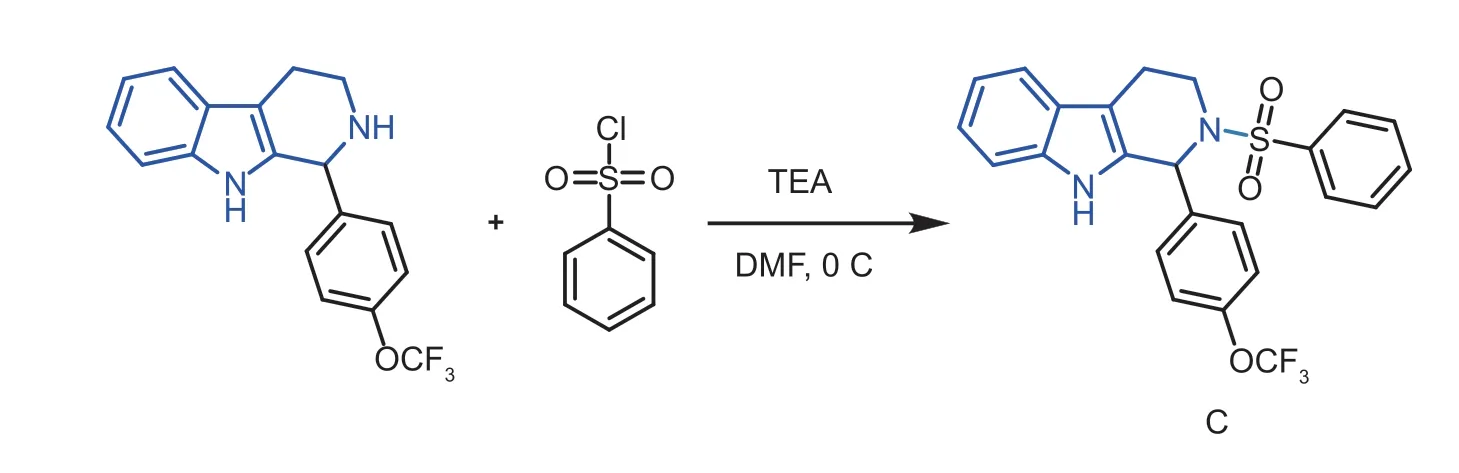
Fig.4 Synthesis route of molecule C.
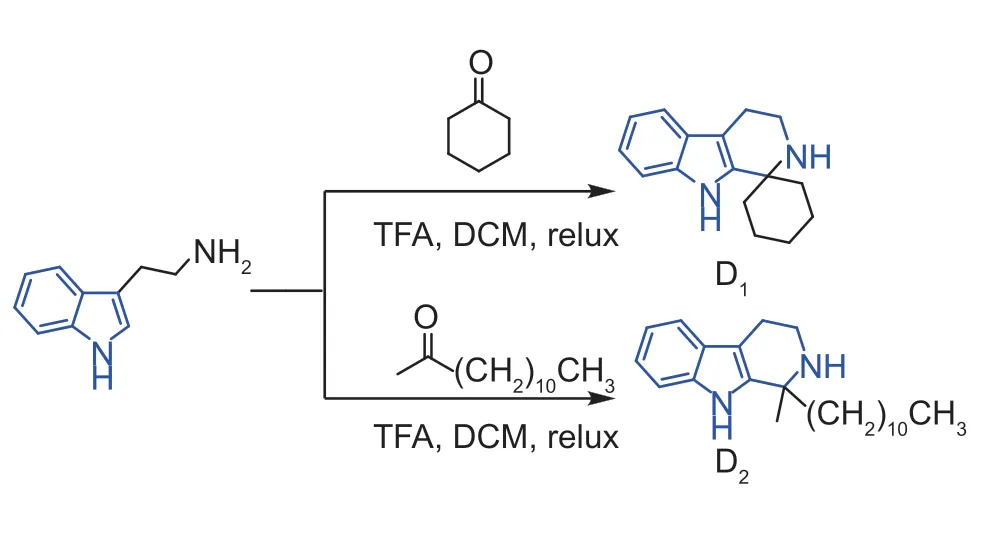
Fig.5 Synthesis route of molecules D1-D2.

Fig.6 Summary of the structure-activity relationship (SAR) of tetrahydro-β-carboline analogues.
3.4.In vivo effectiveness towards rice bacterial blight and citrus bacterial canker and kiwifruit bacterial canker
Given the perfectin vitroanti-Xooactivity of compoundA17(EC50=7.27 μg mL-1) andXac(EC50=4.89 μg mL-1),we carried out thein vivoanti-Xooactivity by pot experiment.The results indicated that (Fig.7;Table 6) the compound A17displayed good protective and curative activity onXoo(52.67 and 43.07%) at 200 μg mL-1,which were superior to the positive control commercial agent TC (38.10 and 35.28%),it is important to note that the addition of orange peel essential oil only slightly increased the control efficiencies of A17against rice bacterial blight.
Table 6 In vivo control efficacies of molecule A17 toward rice bacterial blight at 200 μg mL-1
In terms ofin vivoanti-Xacassay,compound A17showed good preventive effect onXac(Fig.8;Table 7),of which the curative activity (51.50%) was better than that of the commercial TC (26.64%).About kiwifruit bacterial canker,the results showed (Fig.9;Table 8) that the curative and protective efficiencies of compound A8were 64.71 and 84.31% respectively,which was higher than that of TC (62.75 and 80.39%) at 200 μg mL-1.
Harry met every train for the next three or four days. Of course, the railroad5 lines made a routine6 checkup and the police looked into the case. But nobody was any real help. I could see that they all figured that May had simply played a trick on him. But I never believed that, somehow.
Table 7 In vivo control efficiencies of molecule A17 against citrus bacterial canker at 200 μg mL-1

Table 8 In vivo control efficiencies of molecule A8 toward kiwifruit bacterial canker at 200 μg mL-1

Fig.8 Protective and curative activities of compound A17 and thiodiazole copper (TC) of citrus bacterial canker at 200 μg mL-1.
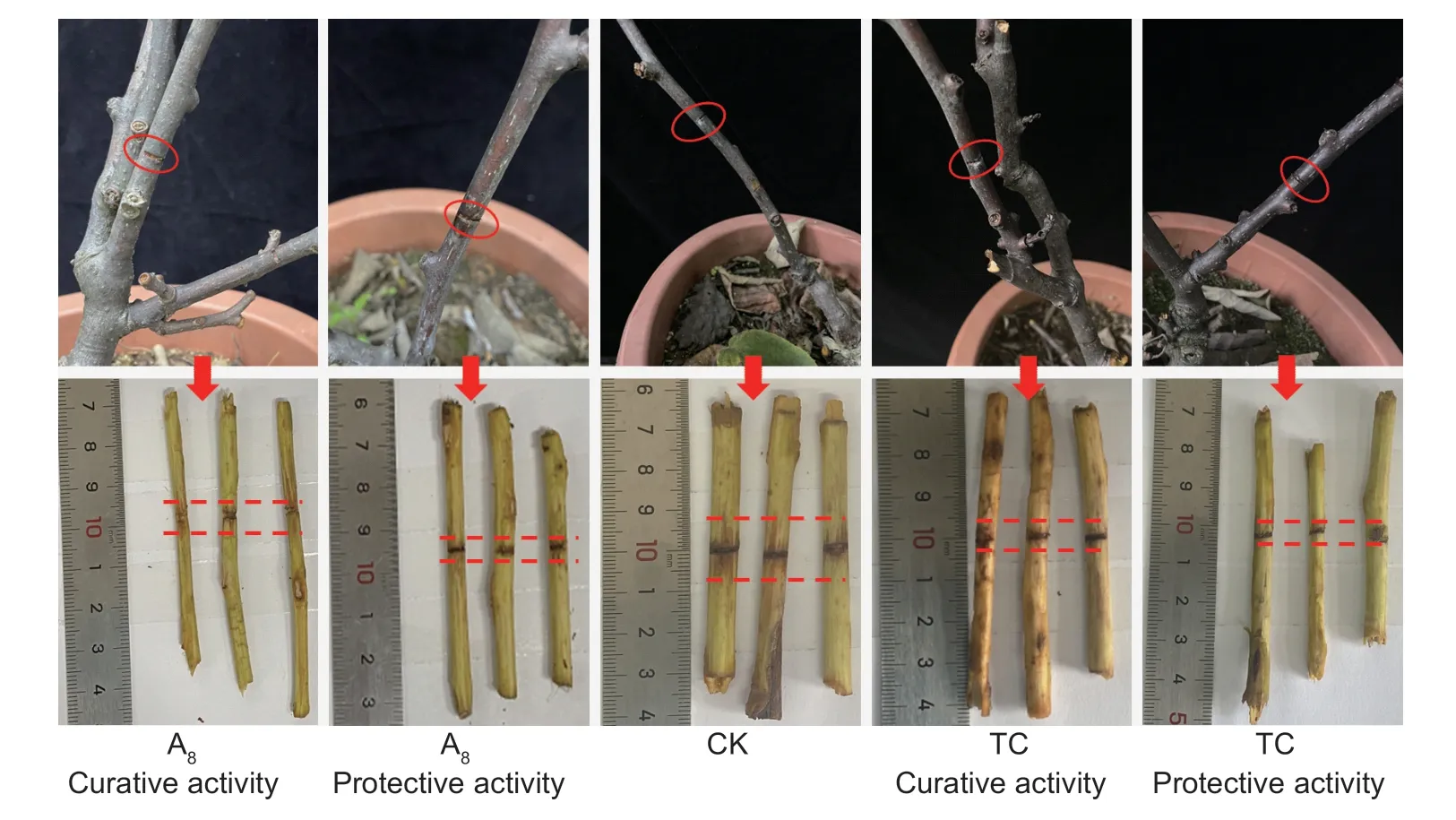
Fig.9 Curative and protective activities of compound A8 and thiodiazole copper (TC) of kiwifruit bacterial canker at 200 μg mL-1.
In addition,when the plants were treated with the molecule at 200 and 500 μg mL-1,it was seen that the title compounds were not toxic to the plants (Fig.10).In conclusion,compound A17and A8have a good antibacterial effect on plant bacterial diseases,suggesting that these THC analogues could be further developed as promising alternatives to combat plant bacterial diseases.
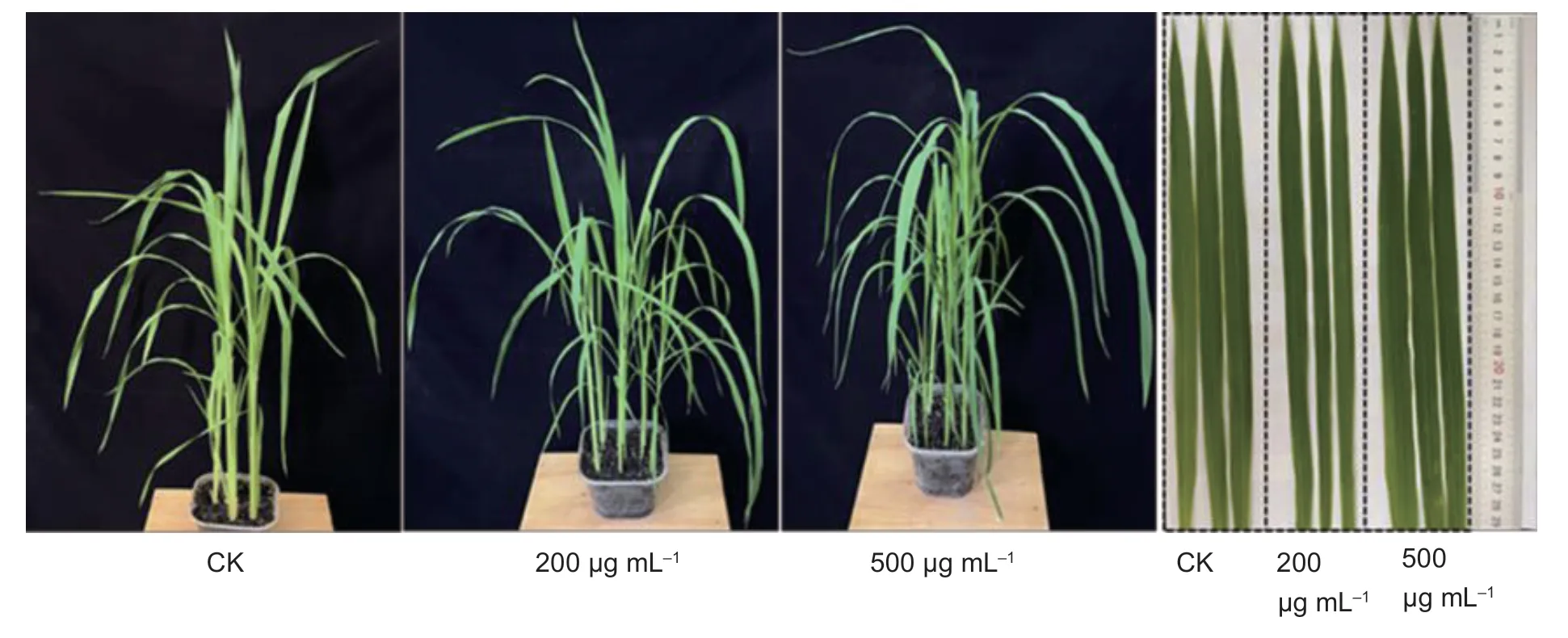
Fig.10 Toxic effects of compound A17 on rice plants at concentrations of 200 and 500 μg mL-1.
3.5.AO/EB double staining experiment
The fluorescent dye acridine orange (AO) has satisfactory membrane permeability,which can penetrate the intact cell membrane of normal living cells,and make nuclear DNA and RNA stain with bright green fluorescence.AO can make apoptotic cells stain with dense yellow-green fluorescence or yellow-green debris particles.The yellow fluorescence of necrotic cells will weaken or even disappear,while the fluorescent dye ethidium bromide (EB) can only make the dead cells with cell membrane damage fluorescence,which can determine whether the bacterial cell membrane damage (BalaKumaranet al.2020).
As Fig.11 displayed,the control cells were morphologically normal and green,the apoptotic cells were orange due to nuclear condensation,nuclear contraction and the necrotic cells were red,and the apoptotic cells in a dose-dependent manner suggesting that molecule A17induced apoptosis inXoocells.The literature studies have shown that the disruption of ROS balance is the key cause of the phenomenon of induced apoptosis (Tokalaet al.2018).
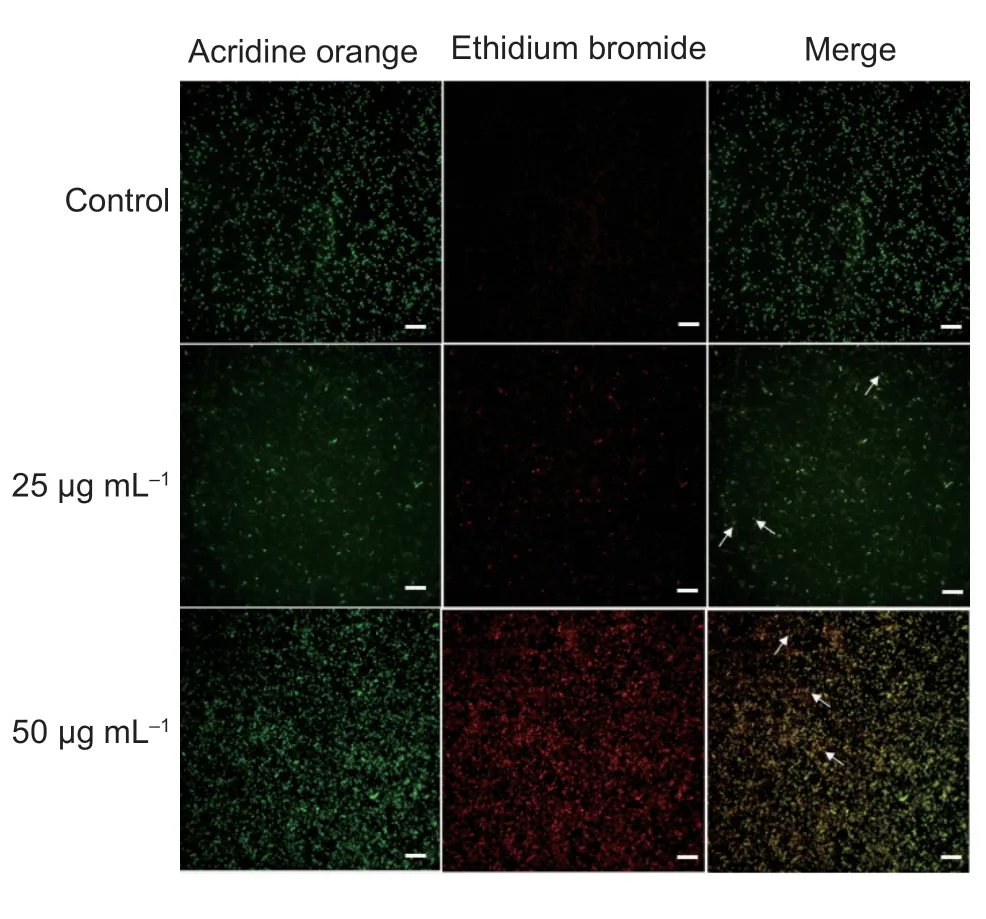
Fig.11 AO/EB double staining of Xoo cells with A17.White arrows indicate apoptotic Xoo cells.Scale bars are 10 μm.
3.6.Determination of ROS content
The cellular ROS levels triggered by molecule A17were certified by using a typical ROS detection kit in which the fluorescent probe DCFH-DA to response the ROS level.Obviously,it could be seen (Fig.12) that after incubation of the bacteria with exogenous compound A17,the fluorescence intensity of the cells gradually increased with increasing of the concentration.Indicating that designed A17can destroy the pathogen redox system,cause the accumulation of ROS,and lead to apoptosis.

Fig.12 Fluorescent image of Xoo stained with the nonfluorescent oxidation reaction dye DCFH-DA (0,12.5,25,and 50 μg mL-1).Scale bars are 10 μm.
3.7.Morphological changes of Xoo cells (SEM)
From these findings,it is preliminarily found that the synthesized compounds have ability to damage the pathogen’s redox system,and induce the ROS outburst,thereby inducing the phenomenon of apoptosis in the pathogen.In-depth cell morphological studies were carried out according to the above apoptosis phenomenon.As could be seen from the Fig.13,after incubation of compound A17withXoocells for 12 h,the cell surface showed different degrees of wrinkling and collapse,and the morphological changes were more severe with increasing dose.The result provides further evidence that the title molecules can strongly induce the cellular ROS outburst,disrupt cell membrane structures,and kill cells.
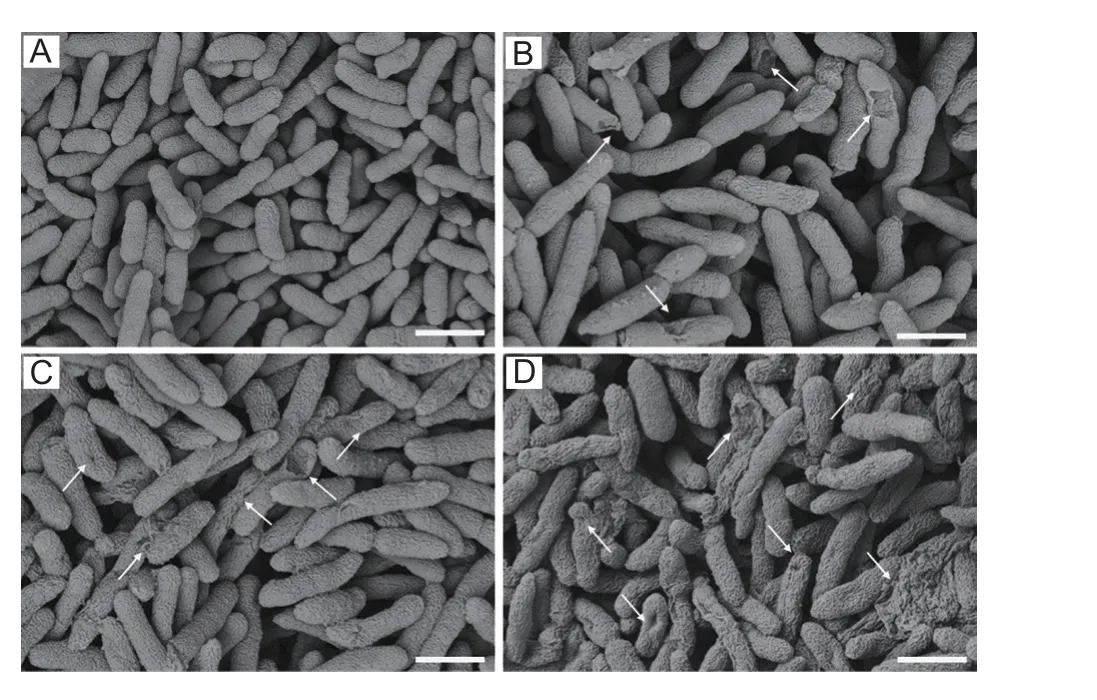
Fig.13 SEM image of Xoo cells after incubation with compound A17 at various concentrations (0,12.5,25,and 50 μg mL-1).Scale bars are 2 μm.
4.Discussion
4.1.In vitro and in vivo bioassay results
Some studies found that THC derivatives have antitumor,antifungal,antiviral,and anti-inflammatory activities (Youssef 2005;Zhenget al.2014;Sundinet al.2016;Wang and Song 2020;Luo and Song 2021;Wang Qet al.2021).However,its antibacterial activity has rarely been investigated.In our previous work,we found that novel THC derivatives containing an 1,3-diaminopropan-2-ol pattern displayed good antibacterial activity (Liuet al.2020).These attractive title compounds contributed new molecular skeleton for treating plant pathogenic bacteria,but the core pharmacophore and the corresponding SAR study still not clear.To extend the in-depth understanding of antibacterial behavior and their SAR,a total of 31 THC derivatives were,herein,successfully generated by using Pictet-Spengler reaction under the mild reaction condition.Of note,in vivoresults (Tables 1-4) showed that these title molecules exhibited remarkable bioactivity towards various bacteria includingXoo,XacandPsa.For instance,molecule A17showed outstanding antibacterial activities,with the EC50values of 7.27 μg mL-1againstXoo,4.88 μg mL-1againstXacand 4.80 μg mL-1againstPsa.Compound A8had the good anti-Xoo(EC50=13.33 μg mL-1),anti-Xac(EC50=2.49 μg mL-1),and anti-Psa(EC50=4.87 μg mL-1) activities.Furthermore,the SAR study was summarized.For the A ring of THC,no substituent at the 6-position was favorable for increasing the antibacterial activity.For the C ring of THC,the NH group at the 2-position was indispensable,and no substituent at the 3-position was beneficial for improving their bioactivity.Furthermore,when 1-position of A ring was substituted phenyl group,4-OCF3group afforded the most active towardXooandXac;the large steric hindrance could slightly increase antibacterial activity;and a long carbon chain was crucial for bioactivity.
The pot experiments indicated that compounds A8and A17showed significant control activities at 200 μg mL-1,which were superior to that of positive control TC.For instance,compound A17had good control efficiencies (200 μg mL-1,protective activity: 52.67%;curative activity: 43.07%) onXoo,which were comparable to results (protective activity: 51.89%;curative activity: 50.53%) of reported compound II9(Liuet al.2020).More interestingly,their control efficiencies could be slightly improved after addition of the auxiliary OPO.Furthermore,compound A17had excellent anti-Psaactivities (200 μg mL-1,protective activity: 84.31%;curative activity: 64.71%),which were superior to previous lead compound II9(protective activity: 65.45%;curative activity: 30.35%).In consideration of these findings,these new engineered compounds showed remarkable and broad-spectrum antibacterial activitiesin vitroandin vivo.
4.2.Investigation of antibacterial mechanisms
In our previous work,THC derivatives have been firstly reported having apoptotic effects toward bacteria,but their apoptotic behavior towards bacteria was not clear (Liuet al.2020).Thus,the in-depth understanding of antibacterial behavior is urgently needed.AO/EB double fluorescence cell staining assays (Huanget al.2010;Venkataramana Reddyet al.2017;Liuet al.2020) are crucial and classical method for verifying the underlying apoptotic behavior.The results thereby showed that changes in the integrity of the cell membrane could be clearly observed,indicating that compound A17induced apoptosis inXoocells.After incubation with molecule A17,ROS fluorescence was enhanced,indicating that the compound may damage the redox system ofXoocells.These interestingly findings were consistent with our previously reported results (Liuet al.2020;Huanget al.2021).Of note,ROS has been revealed to play important roles in cell death among species from bacteria,fungi,plant,and mammals (Shenet al.2016;Dharmaraja 2017;Liu and Zhang 2022).For instance,previous study showed thatβ-carboline derivatives promoted the production of reactive ROS in cancer and fungi cells (Delet al.2005;Shenget al.2021).In comparison of our previously reported anti-quorum sensing strategies (Zenget al.2020,2023;Fenget al.2022,2023;Qiet al.2022;Zhanget al.2023),ROS-mediated apoptosis has quite different characteristics that could be an attractable strategy for controlling plant bacterial diseases.
In addition,THC derivatives have the ability to cause changes in the integrity of cell membranes (Volpatoet al.2013,2015),SEM results demonstrated that the compound A17-induced ROS might cause the damage ofXoocell membrane,thus exhibiting remarkable bactericidal ability.Thus,as the Fig.14 displayed,compound A17could break the redox balance,trigger the production of the ROS,and damage the bacterial membrane,and thereby leading to apoptosis inXoocells.Combining with these interestingly observation results mentioned in previous works (Xianget al.2019,2020;Songet al.2022),the present work provided the forceful impetus to conduct a long-term study of discovering new apoptosis initiators for controlling plant bacterial diseases.
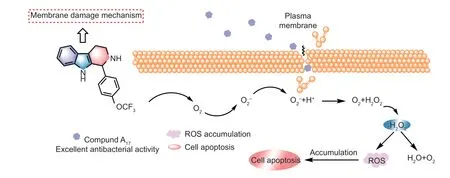
Fig.14 Antibacterial mechanism of compound A17.
5.Conclusion
In this study,a series of tetrahydro-β-carboline analogues were prepared,and their bioactivities against plant pathogenic bacteria were assessed and the underlying mechanism againstXoowas also investigated.The results ofin vitroexperiments showed that the title molecules displayed outstanding inhibitory activity againstXoo,XacandPsa,and the EC50was 7.27 μg mL-1(compound A17),4.89 μg mL-1(compound A17),and 4.87 μg mL-1(compound A8),respectively,better than TC and BT.In vivoexperiments showed that the title molecules possessed good control efficiencies onXoo,Xac,andPsa.The antibacterial mechanism showed that compound A17could causeXoocells apoptosis by destroying the redox system,and inducing the ROS accumulation,and result in killing bacteria.These results suggested that tetrahydro-β-carboline derivatives could be a promising candidate model for novel broad-spectrum bactericides.
Acknowledgements
We acknowledge the supports from National Natural Science Foundation of China (21877021,32160661,and 32202359),the Guizhou Provincial S&T Project,China (2018[4007]),the the Guizhou Province,China [Qianjiaohe KY number (2020)004],the Program of Introducing Talents of Discipline to Universities of China (D20023,111 Program),and the Guizhou University (GZU) Found for Newly Enrolled Talent,China (202229).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Appendicesassociated with this paper are available on https://doi.org/10.1016/j.jia.2023.05.031
杂志排行
Journal of Integrative Agriculture的其它文章
- OsNPF3.1,a nitrate,abscisic acid and gibberellin transporter gene,is essential for rice tillering and nitrogen utilization efficiency
- Fine mapping and cloning of the sterility gene Bra2Ms in nonheading Chinese cabbage (Brassica rapa ssp.chinensis)
- Basal defense is enhanced in a wheat cultivar resistant to Fusarium head blight
- Optimized tillage methods increase mechanically transplanted rice yield and reduce the greenhouse gas emissions
- A phenology-based vegetation index for improving ratoon rice mapping using harmonized Landsat and Sentinel-2 data
- Combined application of organic fertilizer and chemical fertilizer alleviates the kernel position effect in summer maize by promoting post-silking nitrogen uptake and dry matter accumulation
