胆囊癌根治手术成功实施列线图预测模型的建立和验证
2024-05-13郑康鹏唐鑫国徐琦樊钰亭梁博付晓伟方路
郑康鹏 唐鑫国 徐琦 樊钰亭 梁博 付晓伟 方路
基金項目:国家自然科学基金资助项目(82160578);江西省卫生健康委科技计划项目(202210033)
作者单位:南昌大学第二附属医院肝胆外科(邮编330006)
作者简介:郑康鹏(1997),男,住院医师,主要从事肝胆胰腺的临床方面研究。E-mail:zkp2016140243@126.com
△通信作者 E-mail:fanglu@medmail.com.cn
摘要:目的 建立可预测胆囊癌(GBC)患者根治性手术成功实施的列线图并进行初步验证。方法 纳入320例行手术治疗(包括根治性手术、姑息性切除术、腹腔探查术和活检术)的GBC患者。根据纳入时间先后分为训练集(235例)和验证集(85例)。比较实施根治性手术和非根治性手术患者的临床资料,多因素Logistic回归分析影响GBC患者根治性手术成功实施的因素,并绘制列线图预测模型。采用受试者工作特征(ROC)曲线和校准曲线评价预测模型的区分度及校准度,应用临床决策曲线(DCA)评估列线图预测模型的实际效用。结果 单因素分析显示,根治性手术组和非根治性手术组在体质量减轻、黄疸、高血压、淋巴结转移、体质量指数、血红蛋白(HB)、白蛋白(ALB)、糖类抗原(CA)19-9、CA125、总胆红素和直接胆红素差异有统计学意义(P<0.05)。将这11个潜在的预测因素在训练集纳入多因素Logistic回归分析,结果显示无黄疸、高血压、淋巴结转移,HB、ALB升高,CA19-9降低是预测GBC根治手术成功实施的因素。根据Logistic回归得到的6个独立风险因素建立列线图。在训练组和验证组中,列线图的曲线下面积分别为0.901和0.822,模型具有良好的区分度。Hosmer-Lemeshow检验表明模型校准度良好(χ2=5.740,P=0.676)。模型校准曲线均接近理想曲线,表明观察结果与实际结果吻合良好。DCA曲线显示模型对临床使用具有净效益和良好的临床实用性。结论 该列线图可有效筛选适合根治性手术的GBC患者,减少预期根治性手术转为姑息性切除或剖腹探查术的机会,增加患者手术获益的可能性。
关键词:胆囊肿瘤;列线图;黄疸;高血压;CA-19-9抗原;根治性手术;危险因素
中图分类号:R735.8文献标志码:ADOI:10.11958/20231560
Nomogram construction and validation for predicting the possibility successful
implementation of radical surgery in gallbladder cancer patients
ZHENG Kangpeng, TANG Xinguo, XU Qi, FAN Yuting, LIANG Bo, FU Xiaowei, FANG Lu△
Department of Hepatobiliary Surgery, the Second Affiliated Hospital of Nanchang University, Nanchang 330006, China
△Corresponding Author E-mail: fanglu@medmail.com.cn
Abstract: Objective To develop a nomogram for predicting the successful implementation of radical surgery for gallbladder cancer (GBC). Methods A total of 320 patients with GBC who underwent surgical procedures including radical surgery, palliative excision, abdominal exploration, and biopsy were enrolled in this study. Patients were divided into the training set (235 cases) and the verification set (85 cases) according to the time of inclusion. By comparing the clinical data of patients undergoing radical surgery and patients with non-radical surgery, multivariate Logistic regression analysis was conducted to analyze the prediction model affecting the successful implementation of radical surgery in GBC patients, and a column graph was drawn. Receiver operating characteristic (ROC) curve and calibration curve were used to evaluate the differentiation and calibration of the prediction model. Clinical decision curve (DCA) was used to evaluate the practical utility of the nomogram prediction model. Results Univariate analysis showed that there were significant differences in weight loss, jaundice, hypertension, lymph node metastasis, body mass index (BMI), hemoglobin (HB), albumin (ALB), CA19-9, CA125, total bilirubin and direct bilirubin between the radical surgery group and the non-radical surgery group (P<0.05). These 11 potential predictors were included in the multivariate Logistic regression analysis in the training set, and results showed that no jaundice, hypertension, lymph node metastasis, elevated HB and ALB, and decreased CA19-9 were predictive factors for the successful implementation of radical GBC surgery. A nomogram was established based on 6 independent risk factors obtained by Logistic regression. In the training group and the verification group, the area under the curve of the nomogram was 0.901 and 0.822, respectively, and the model has good differentiation. Hosmer-Lemeshow test showed that the model was well calibrated (χ2=5.740, P=0.676). The calibration curve of the model was close to ideal curve, indicating that the observed results were in good agreement with the actual results. The DCA curve showed that the model had a net benefit and good clinical practicability for clinical application. Conclusion The nomogram can effectively screen patients with GBC suitable for radical surgery, thus reducing the chance of conversion of anticipated radical surgery to palliative resection or exploratory laparotomy and increasing the likelihood of surgical benefits for patients.
Key words: gallbladder neoplasms; nomograms; jaundice; hypertension; CA-19-9 antigen; radical surgery; risk factors
胆囊癌(gallbladder cancer,GBC)是一种高度侵袭性的胆道肿瘤,占所有胆道肿瘤的80%~95%,预后较差,5年生存率仅为5%[1]。其发病率具有区域性,东亚和南美洲(如印度和智利)的发病率高于其他地区[2]。GBC发病机制尚不清楚,可能的危险因素包括年龄、急性炎症和结石病史等[3-4]。Glenn等[5]首先提出根治性胆囊切除术,将胆囊和肝十二指肠韧带淋巴结一起切除,用于治疗肝外胆道恶性肿瘤。随后,Pack等[6]提出一种更积极的方法,将全右肝叶切除术与胆囊切除术结合起来治疗GBC。GBC对放化疗均不敏感,晚期GBC患者在接受新辅助化疗后,只有随后进行R0切除的患者才能受益[7]。因此,手术仍然是GBC的首选治疗方法,及时、准确的根治性切除是唯一的治愈方法。尽管GBC容易侵犯邻近器官并發生血液、淋巴及远处转移,但早期GBC患者根治性切除后可完全治愈,晚期患者适当的手术治疗可延长生存期[8-9]。GBC患者是否可以接受根治性手术通常根据影像学检查来评估,但预测准确度往往不理想,患者最终的手术方式大多取决于术中探查和临床经验,导致计划的根治性手术变成姑息性切除、探查或活检,增加了患者的痛苦并浪费医疗资源。目前列线图预测模型已广泛应用于临床研究,本文将列线图预模型与GBC患者根治性手术相结合,旨在为GBC患者设计个性化治疗方案。
1 对象与方法
1.1 研究对象 选取2013年1月—2023年1月南昌大学第二附属医院收治的320例因GBC行手术治疗(包括根治性手术、姑息性切除术、腹腔探查术和活检术)的患者。将2013年1月—2019年12月235例GBC患者作为训练集。2020年1月—2023年1月的85例GBC患者作为验证集。纳入标准:病历完整、经过手术治疗和病理证实的GBC患者。根据美国癌症联合委员会(AJCC)第8版TNM分期,以下情况被视为根治性切除术:(1)Tis和T1a分期接受单纯胆囊切除术。(2)T1b期行胆囊切除+肝楔形切除(距胆囊2 cm以上)及区域淋巴结清扫术。(3)T2期胆囊切除+肝楔形切除(距胆囊2 cm以上)或Ⅳb期+Ⅴ段肝切除+区域淋巴结清扫术。(4)T3、T4期右半肝切除、右三叶切除或肝脏联合其他脏器,实现R0肿瘤切除。非根治性手术:(1)姑息性切除术。标本的大块切除或切缘阳性+胆道内外引流。(2)腹腔探查术。通过腹腔镜观察发现转移,未行姑息性手术且未取活检。(3)活检术。通过腹腔镜观察发现转移,未行姑息性手术且取得活检。
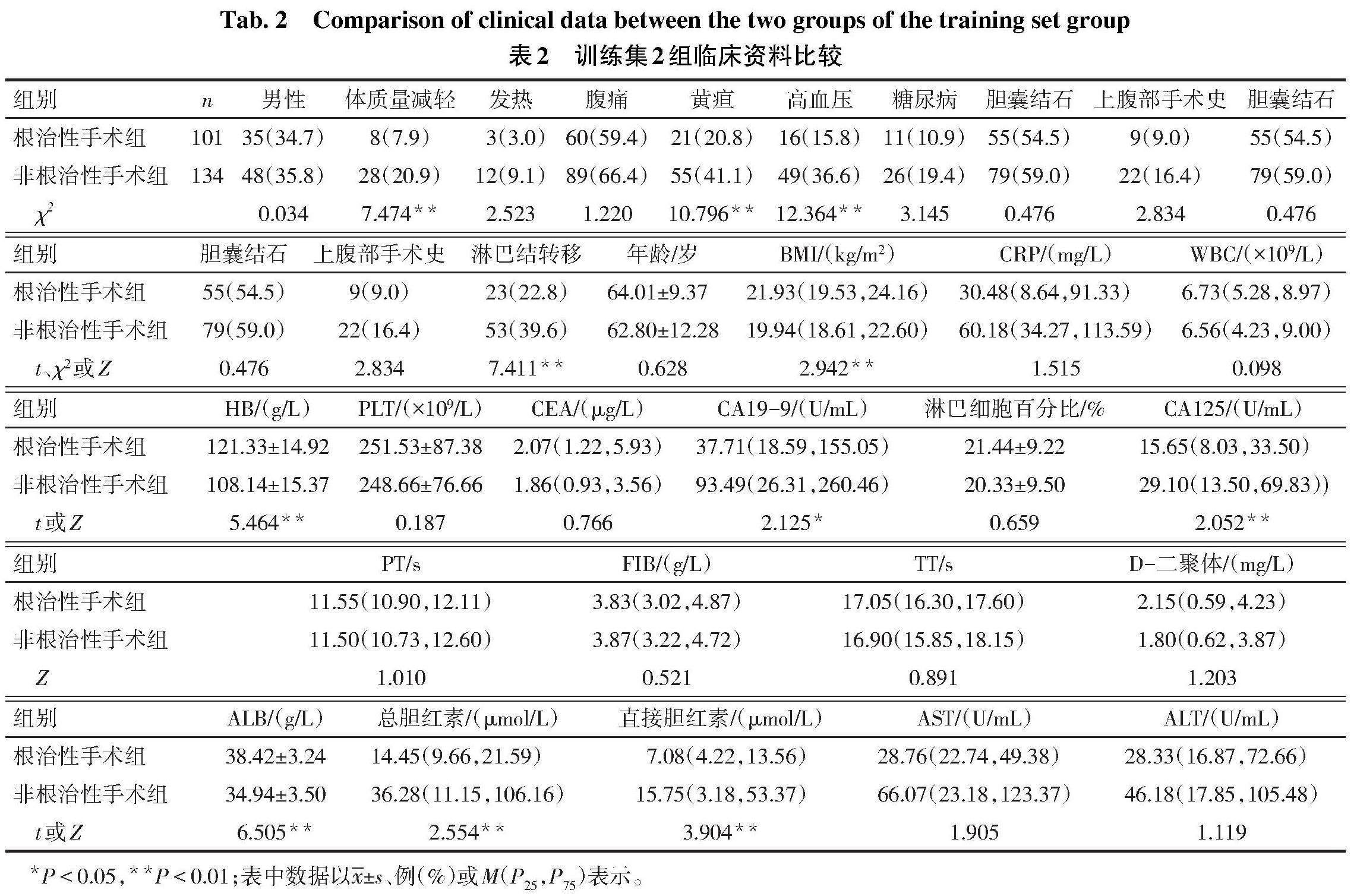
1.2 资料收集 从医院病历系统中提取患者的临床资料:性别、体质量减轻(入院近3个月体质量下降5%以上)、发热、腹痛、黄疸、高血压、糖尿病、胆囊结石、上腹部手术史、淋巴结转移、年龄、体质量指数(BMI)。入院时指标:C反应蛋白(CRP)、白细胞计数(WBC)、血红蛋白(HB)、血小板计数(PLT)、淋巴细胞百分比、癌胚抗原(CEA)、糖类抗原(CA)19-9、CA125、凝血酶原时间(PT)、凝血酶时间(TT)、纤维蛋白原(FIB)、D-二聚体、白蛋白(ALB)、总胆红素、直接胆红素、天冬氨酸转氨酶(AST)、丙氨酸转氨酶(ALT)。
1.3 统计学方法 采用R 4.2.3和SPSS 25.0软件进行数据分析。正态分布的计量资料以[x] ±s表示,组间比较采用t检验;非正态分布的计量资料以M(P25,P75)表示,组间比较采用Wilcoxon检验。计数资料以例或例(%)表示,组间比较采用χ2检验或Fisher确切概率法。采用多因素Logistic回归分析影响GBC根治性手术成功实施的预测因素,构建列线图预测模型。绘制受试者工作特征(ROC)曲线和校准曲线评价预测模型的区分度及校准度,应用临床决策曲线(DCA)评估列线图预测模型的实际效用。P<0.05为差异有统计学意义。
2 结果
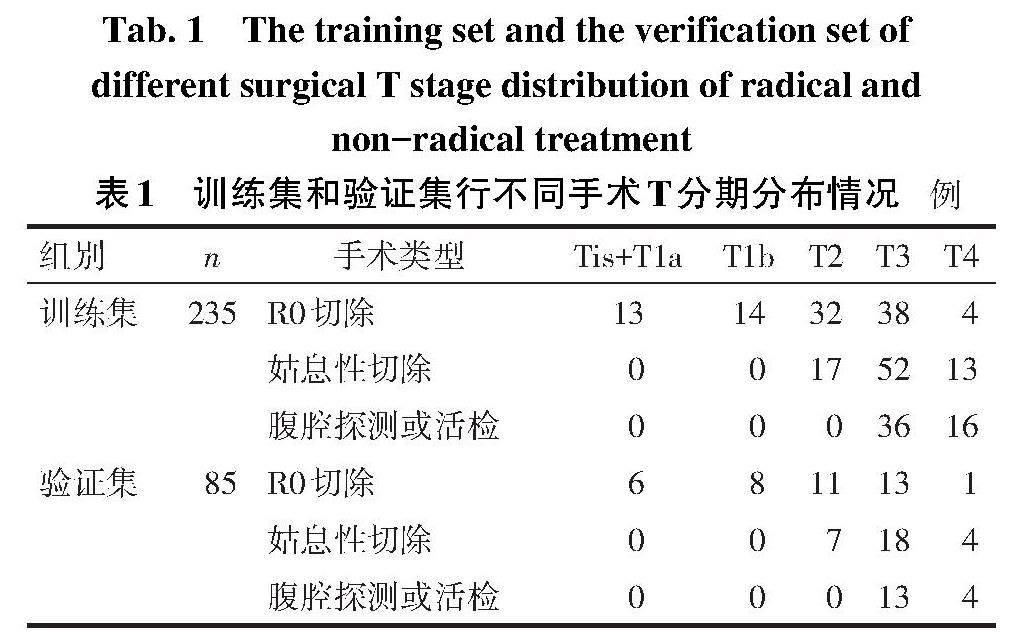
2.1 手术情况 在训练集中,101例(43.0%)行根治性手术,134例(57.0%)行非根治性手术,其中姑息性手术82例,腹部探查或活检52例;在验证集中,39例(45.9%)行根治性手术,46例(54.1%)行非根治性手术,其中姑息性手术29例,腹部探查或活检17例。训练集和验证集行不同手术患者T分期情况见表1。
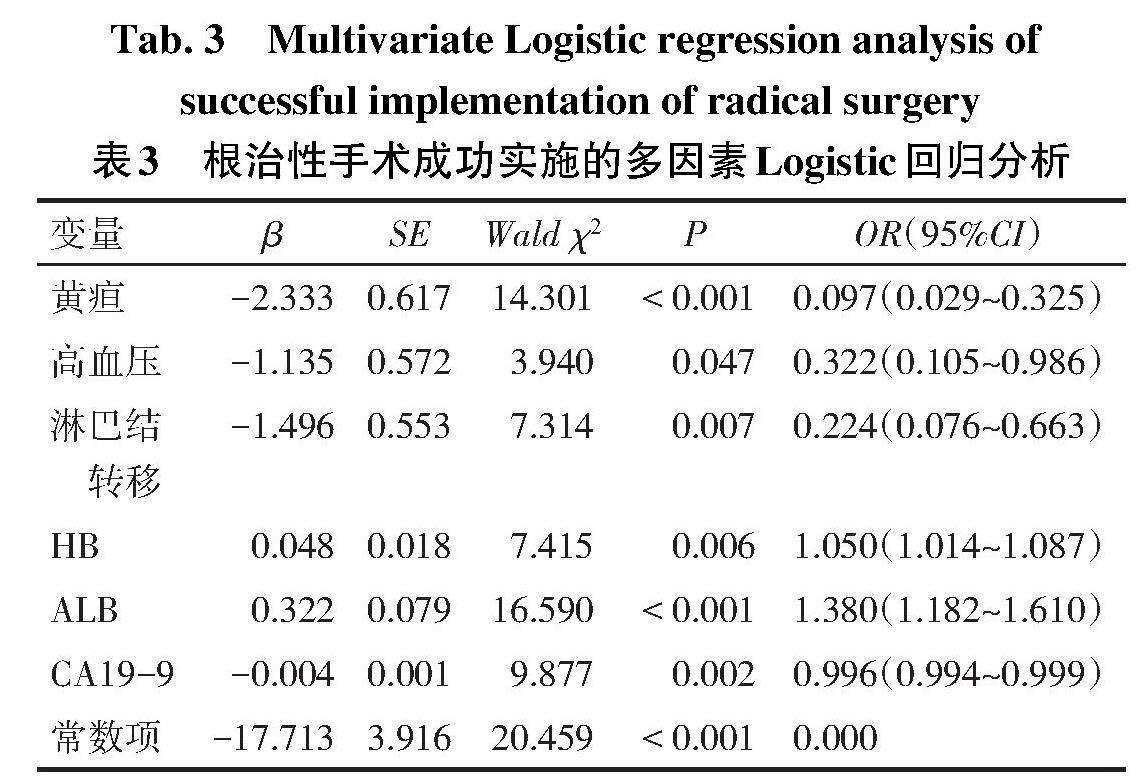
2.2 根治性手术成功实施的因素分析 单因素分析显示,根治性手术组和非根治性手术组在体质量减轻、黄疸、高血压、淋巴结转移、BMI、HB、ALB、CA19-9、CA125、总胆红素和直接胆红素差异有统计学意义(P<0.05),见表2。将这11个潜在的预测因素在训练集纳入多因素Logistic回归分析(因变量:根治性手术=1,非根治性手术=0;自变量:男=1,女=0;余分类变量是=1,否=0),结果显示无黄疸、高血压、淋巴结转移,HB、ALB升高,CA19-9降低是预测GBC根治手术成功实施的因素,见表3。
2.3 列线图的构建和验证 根据Logistic回归得到的6个独立风险因素建立列线图,见图1。分数越高表明成功实施根治性手术的可能性越大。Hosmer-Lemeshow检验表明模型校准度良好(χ2=5.740,P=0.676)。ROC曲线显示,在训练集中,模型曲线下面积(AUC)为0.901,在验证集中AUC为0.822,模型具有良好的区分度,见图2。模型校准曲线均接近理想曲线,表明观察结果与实际结果吻合良好,见图3。DCA曲线显示模型对临床使用具有净收益和良好的临床实用性,见图4。
3 讨论
尽管医疗设备和手术技术在不断改进,然而GBC的预后并未明显改善。早期GBC患者可通过根治性手术彻底治愈;然而,早期患者没有特异的临床症状,缺乏有效的早期诊断指标[10]。多数GBC患者被发现时已处于疾病中晚期,通过术前评估,也难以确定这些患者手术切除的阈值,也给临床医生带来困扰。因此建立一个可筛选GBC患者是否适合进行根治性手术的预测模型尤为重要。本研究表明,ALB、HB、CA19-9、高血压、淋巴结转移和黄疸是影响GBC根治手术的实施的重要预测因素,得到的列线图可有效筛选适合根治性手术的GBC患者。无法接受根治性手术的GBC患者也可通过这些危险因素进行识别,以便及早选择其他治疗方法,使患者接受个性化治疗。
本研究发现,黄疸是GBC根治手术实施的独立预测因素,会降低GBC患者实施根治性手术的概率,这与Tran等[11-12]的研究相似,他们发现虽然黄疸会降低根治性手术的成功率,但并不是手术的禁忌证,部分黄疸患者在进行根治性手术后仍具有较长生存期,特别是当伴有低水平的CA19-9时。此外,高血压也是GBC实施根治手术的独立预测因素。既往研究表明,血压升高与口咽癌、结肠癌、直肠癌、肺癌、膀胱癌、肾癌等癌症的发病率呈正相关[13-14],而使用β受体阻滞剂可能会抑制头颈癌和乳腺癌侵袭[15-16]。但β受体阻滞剂的使用能否降低GBC的侵袭性,从而提高其实施根治性手术的概率尚未可知。
肿瘤标志物在肿瘤组织中表达更加活跃,是早期肿瘤的预测因素。CA19-9水平有助于GBC的诊断和预后评估[17]。本研究中,CA19-9是GBC实施根治性手术的独立预测因子。既往研究表明,术前CA19-9水平与GBC的可切除性相关,当患者血清CA19-9水平在90~450 U/mL和>450 U/mL时,分别有94%和100%的GBC無法手术切除[18]。Liu等[19]还发现能够进行R0手术切除的GBC患者CA19-9水平明显低于不可切除GBC患者,说明CA19-9可作为评估GBC可切除性的辅助指标。
淋巴结转移是GBC常见的转移方式,也是影响GBC预后的独立危险因素之一[20]。GBC主要有3条淋巴转移途径:左侧经胰头后方转移至肝十二指肠韧带,右侧沿胆总管转移至胰十二指肠淋巴结,以及直接向肝门转移[21]。目前影像学检查对于评估GBC淋巴结转移的灵敏度欠佳,PET/CT和增强CT分别仅检测到12%和24%的区域淋巴结转移[22]。而年龄<60岁和CA19-9水平可作为影像学检查的补充,提高淋巴结转移检出率[23]。GBC的侵袭性取决于局部肿瘤扩散和淋巴结转移的程度,而根治性手术的成功实施须保证区域淋巴结的清扫[24]。因此,淋巴结转移可影响GBC根治手术成功实施的概率。
血清ALB水平是评价癌症患者营养状况的基本指标[25]。研究表明,血清ALB可以降低Rb蛋白的磷酸化,增强p21和p57的表达,从而抑制肿瘤细胞的增殖[26]。笔者推测,术前ALB水平较高的GBC患者可能会减缓疾病进展,从而提高手术切除的机会。此外,HB降低也被发现是预测实施GBC根治性手术的独立危险因素。贫血是癌症患者的常见病症,其主要原因可能是恶性肿瘤引起炎症可释放多种炎性因子,导致促红细胞生成素合成减少,从而导致HB降低[27]。患有低HB血症的恶性肿瘤患者往往患有慢性低氧血症,低氧环境的刺激可能会增加肿瘤细胞的生物侵袭性,从而导致肿瘤细胞早期扩散的可能性更高[28-29],影响根治性手术实施。
综上所述,本研究纳入黄疸、高血压、淋巴结转移、HB、ALB和CA19-9这6个预测因素来建立列线图。该模型可用于评估GBC患者成功实施根治性手术的概率,筛选适合根治性手术的GBC患者,辅助临床医生做出判断。然而,本研究有一些局限性。首先,本模型基于单中心回顾性研究,导致了潜在的选择偏倚。其次,本中心是三级GBC转诊中心,会收治大量中晚期患者,而早期患者较少。还需要大样本、多中心研究来证实该模型的有效性。
参考文献
[1] ROA J C,GARCíA P,KAPOOR V K,et al. Gallbladder cancer[J]. Nat Rev Dis Primers,2022,8(1):69. doi:10.1038/s41572-022-00398-y.
[2] HALASEH S A,HALASEH S,SHAKMAN R. A review of the etiology and epidemiology of gallbladder cancer:what you need to know[J]. Cureus,2022,14(8):e28260. doi:10.7759/cureus.28260.
[3] PANG Y,LV J,KARTSONAKI C,et al. Causal effects of gallstone disease on risk of gastrointestinal cancer in Chinese[J]. Br J Cancer,2021,124(11):1864-1872. doi:10.1038/s41416-021-01325-w.
[4] KHAN Z A,KHAN M U,BRAND M. Gallbladder cancer in Africa:a higher than expected rate in a "low-risk" population[J]. Surgery,2022,171(4):855-858. doi:10.1016/j.surg.2021.09.016.
[5] GLENN F,HAYS D M. The scope of radical surgery in the treatment of malignant tumors of the extrahepatic biliary tract[J]. Surg Gynecol Obstet,1954,99(5):529-541.
[6] PACK G T,MILLER T R,BRASFIELD R D. Total right hepatic lobectomy for cancer of the gallbladder;report of three cases[J]. Ann Surg,1955,142(1):6-16. doi:10.1097/00000658-195507000-00002.
[7] HAKEEM A R,PAPOULAS M,MENON K V. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - a systematic review[J]. Eur J Surg Oncol,2019,45(2):83-91. doi:10.1016/j.ejso.2018.08.020.
[8] BALAKRISHNAN A,BARMPOUNAKIS P,DEMIRIS N,et al. Surgical outcomes of gallbladder cancer:the OMEGA retrospective, multicentre, international cohort study[J]. EClinicalMedicine,2023,59:101951. doi:10.1016/j.eclinm.2023.101951.
[9] IMAMURA H,ADACHI T,TANAKA T,et al. Feasibility and safety of laparoscopic gallbladder resection for gallbladder tumours[J]. Anticancer Res,2022,42(2):903-910. doi:10.21873/anticanres.15548.
[10] GIANG T H,NGOC T T,HASSELL L A. Carcinoma involving the gallbladder:a retrospective review of 23 cases - pitfalls in diagnosis of gallbladder carcinoma[J]. Diagn Pathol,2012,7:10. doi:10.1186/1746-1596-7-10.
[11] TRAN T B,NORTON J A,ETHUN C G,et al. Gallbladder cancer presenting with jaundice:uniformly fatal or still potentially curable?[J]. J Gastrointest Surg,2017,21(8):1245-1253. doi:10.1007/s11605-017-3440-z.
[12] YANG X W,YUAN J M,CHEN J Y,et al. The prognostic importance of jaundice in surgical resection with curative intent for gallbladder cancer[J]. BMC Cancer,2014,14:652. doi:10.1186/1471-2407-14-652.
[13] STOCKS T,VAN HEMELRIJCK M,MANJER J,et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project[J]. Hypertension,2012,59(4):802-810. doi:10.1161/HYPERTENSIONAHA.111.189258.
[14] CHRISTAKOUDI S,KAKOUROU A,MARKOZANNES G,et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition[J]. Int J Cancer,2020,146(10):2680-2693. doi:10.1002/ijc.32576.
[15] JIN M,WANG Y,ZHOU T,et al. Norepinephrine/β2-adrenergic receptor pathway promotes the cell proliferation and nerve growth factor production in triple-negative breast cancer[J]. J Breast Cancer,2023,26(3):268-285. doi:10.4048/jbc.2023.26.e25.
[16] CHEN H Y,ZHAO W,NA'ARA S,et al. Beta-blocker use is associated with worse relapse-free survival in patients with head and neck cancer[J]. JCO Precis Oncol,2023,7:e2200490. doi:10.1200/PO.22.00490.
[17] RAWAL N,AWASTHI S,DASH N R,et al. Prognostic relevance of PDL1 and CA19-9 expression in gallbladder cancer vs. inflammatory lesions[J]. Curr Oncol,2023,30(2):1571-1584. doi:10.3390/curroncol30020121.
[18] SHUKLA P J,NEVE R,BARRETO S G,et al. A new scoring system for gallbladder cancer(aiding treatment algorithm):an analysis of 335 patients[J]. Ann Surg Oncol,2008,15(11):3132-3137. doi:10.1245/s10434-008-9917-y.
[19] LIU F,WANG J K,MA W J,et al. Clinical value of preoperative CA19-9 levels in evaluating resectability of gallbladder carcinoma[J]. ANZ J Surg,2019,89(3):E76-E80. doi:10.1111/ans.14893.
[20] 李云超,孙占峰,苏彬,等. miR-7-5p通过靶向抑制成纤维生长因子受体4影响胆囊癌细胞的增殖和迁移[J]. 中国中西医结合外科杂志,2022,28(3):289-294. LI Y C,SUN Z F,SU B,et al. Clinical study on 132 cases of open and laparoscopic inguinal hernia repair[J]. Journal of Surgery of Integrated Traditional and Western Medicine,2022,28(3):289-294. doi:10.3969/j.issn.1007-6948.2022.03.001.
[21] LI Y,SONG Y,ZHANG Y,et al. Progress in gallbladder cancer with lymph node metastasis[J]. Front Oncol,2022,12:966835. doi:10.3389/fonc.2022.966835.
[22] PETROWSKY H,WILDBRETT P,HUSARIK D B,et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma[J]. J Hepatol,2006,45(1):43-50. doi:10.1016/j.jhep.2006.03.009.
[23] YU T N,SHEN B,MENG N,et al. Risk factors of lymphatic metastasis complement poor radiological detection in gallbladder cancer[J]. World J Gastroenterol,2014,20(1):290-295. doi:10.3748/wjg.v20.i1.290.
[24] 钱昌林,刘颖斌. TNM分期在胆囊癌根治性切除术中地位和作用[J]. 中国实用外科杂志,2022,42(9):1046-1050. QIAN C L,LIU Y B. The status and role of TNM staging in radical resection of gallbladder carcinoma[J]. Chinese Journal of Practical Surgery,2022,42(9):1046-1050. doi:10.19538/j.cjps.issn1005-2208.2022.09.21.
[25] VALENZUELA-LANDAETA K,ROJAS P,BASFI-FER K. Nutritional assessment for cancer patient[J]. Nutr Hosp,2012,27(2):516-523. doi:10.1590/S0212-16112012000200025.
[26] NOJIRI S,JOH T. Albumin suppresses human hepatocellular carcinoma proliferation and the cell cycle[J]. Int J Mol Sci,2014,15(3):5163-5174. doi:10.3390/ijms15035163.
[27] MACCI? A,MADEDDU C,GRAMIGNANO G,et al. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study[J]. Haematologica,2015,100(1):124-132. doi:10.3324/haematol.2014.112813.
[28] 袁烁,刘湘云,张家旗,等. 低氧对HTR-8/SVneo细胞增殖及HIF-1α、VEGF、MMP-9、TIMP-1表达的影响[J]. 天津医药,2021,49(12):1240-1244. YUAN S,LIU X Y,ZHANG J Q,et al. Effects of hypoxia on proliferation and the expression of HIF-1α,VEGF,MMP-9 and TIMP-1 in HTR-8/SVneo cells[J]. Tianjin Med J,2021,49(12):1240-1244. doi:10.11958/20211483.
[29] MAMO M,YE I C,DIGIACOMO J W,et al. Hypoxia alters the response to anti-EGFR therapy by regulating EGFR expression and downstream signaling in a DNA methylation-specific and HIF-dependent manner[J]. Cancer Res,2020,80(22):4998-5010. doi:10.1158/0008-5472.CAN-20-1232.
(2023-10-13收稿 2023-11-06修回)
(本文編辑 李志芸)
