Interest of thoracic ultrasound after cardiac surgery or interventional cardiology
2024-05-07MartinBoussugesPhilippeBlancFabienneBregeonAlainBoussuges
Martin Boussuges,Philippe Blanc,Fabienne Bregeon,Alain Boussuges
Abstract Thoracic ultrasound has attracted much interest in detecting pleural effusion or pulmonary consolidation after cardiac surgery.In 2016,Trovato reported,in the World Journal of Cardiology,the interest of using,in addition to echocardiography,thoracic ultrasound.In this editorial,we highlight the value of assessing diaphragm function after cardiac surgery and interventional cardiology procedures.Various factors are able to impair diaphragm function after such interventions.Diaphragm motion may be decreased by chest pain secondary to sternotomy,pleural effusion or impaired muscle function.Hemidiaphragmatic paralysis may be secondary to phrenic nerve damage complicating cardiac surgery or atrial fibrillation ablation.Diagnosis may be delayed.Indeed,respiratory troubles induced by diaphragm dysfunction are frequently attributed to pre-existing heart disease or pulmonary complications secondary to surgery.In addition,elevated hemidiaphragm secondary to diaphragm dysfunction is sometimes not observed on chest X-ray performed in supine position in the intensive care unit.Analysis of diaphragm function by ultrasound during the recovery period appears essential.Both hemidiaphragms can be studied by two complementary ultrasound methods.The mobility of each hemidiaphragms is measured by M-mode ultrasonography.In addition,recording the percentage of inspiratory thickening provides important information about the quality of muscle function.These two approaches make it possible to detect hemidiaphragm paralysis or dysfunction.Such a diagnosis is important because persistent diaphragm dysfunction after cardiac surgery has been shown to be associated with adverse respiratory outcome.Early respiratory physiotherapy is able to improve respiratory function through strengthening of the inspiratory muscles i.e.diaphragm and accessory inspiratory muscles.
Key Words: Ultrasonography;Diaphragm;Phrenic nerve;Hemidiaphragm;Thickening fraction;Physiotherapy
INTRODUCTION
The diaphragm is the main inspiratory muscle and contributes to 60%-70% of the total ventilation at rest.It is a musculotendinous structure (2-4 mm) with a central tendinous portion and a peripheral muscular portion.It includes two hemidiaphragms: The right with a dome positioned higher than the left.Motor innervation of the diaphragm comes from two phrenic nerves formed from the C3-C5 nerve roots.The left and right phrenic nerves cross the neck and thorax between the mediastinal surface of the parietal pleura and the fibrous pericardium to reach the corresponding hemidiaphragm.The left phrenic nerve is close to the subclavian artery and passes in front of the pericardial sac of the left ventricle.The right phrenic nerve runs superficial to the anterior scalene muscle and right subclavian artery and passes over the right atrium and right ventricle.Arterial blood flow to the diaphragm comes from collaterals of the internal mammary artery,collaterals of the abdominal aorta,and vessels originating from intercostal arteries.During contraction,the diaphragm shortens and moves caudally,leading to an expansion of the thoracic cavity.This phenomenon increases abdominal pressure and decreases alveolar pressure below atmospheric pressure resulting in airflow into the lungs[1].Various procedures used in patients with heart diseases can impair diaphragmatic function.Diaphragm dysfunction was exceptionally reported after central vein cannulation and pacemaker battery change[2,3].In contrast,this was regularly observed after cardiac surgery and atrial fibrillation ablation[4,5].We underline in this editorial the interest of assessing diaphragm function after such procedures.
DIAPHRAGM DYSFUNCTION AFTER CARDIAC SURGERY
Impaired diaphragmatic function was reported in a significant percentage of patients after cardiac surgery.Depending on the detection method and the delay from the surgery,diaphragm dysfunction has been variously estimated: 21% for Dimopoulouet al[6],38% for Bruniet al[7],46% for DeVitaet al[8],and 75% for Mouryet al[9].In a recent observational study[10],symptomatic diaphragm dysfunction was found in 272 out of 3577 patients (7.6%).In our experience (unpublished study),the percentage of diaphragm dysfunction in patients admitted in a cardiac rehabilitation center after cardiac surgery was 15% (39 out of 264 patients).Diaphragm ultrasound detected weakness in 10% of cases and hemidiaphragm paralysis in 5%.Various mechanisms may explain diaphragm dysfunction in these patients.Diaphragmatic motion may be reduced by chest pain secondary to sternotomy,pleural effusion or impaired muscle function[11].Furthermore,phrenic nerve damage is a well-known,complication of cardiac surgery.It has been shown that the phrenic nerve can be injured through thermal lesions secondary to topical cardiac cooling with ice-cold solution in the pericardium.To reduce the risk of injury,the use of insulation pads placed between the heart and the left pericardium has been proposed to protect the phrenic nerve from hypothermic surgery[12,13].The use of warm-blood cardioplegia has also demonstrated its interest in reducing the risk of diaphragm paralysis[14].However,other mechanisms may explain phrenic nerve damage during cardiac surgery.During coronary artery bypass grafting,phrenic nerve injury may be secondary to direct surgical trauma during dissection of the internal mammary artery (IMA) or indirect injury due to stretching by the sternal retractor[15].In addition,IMA harvesting may result in decreased blood flow to the phrenic nerve through ligation of some branches such as the pericardiacophrenic artery.These mechanisms could explain the increased risk of phrenic nerve dysfunction in patiens who underwent IMA harvesting compared to the group that did not undergo IMA harvesting[16].Inflammation secondary to cardiopulmonary bypass surgery may also be involved in the development of diaphragm dysfunctionviasignificant production of reactive oxygen species and proinflammatory and pro-apoptotic signaling pathways activation[17].
DIAPHRAGM DYSFUNCTION INDUCED BY ATRIAL FIBRILLATION ABLATION
Minimally invasive treatment of atrial fibrillation appeared in the late 1990s and is now widely used as a safe alternative to antiarrhythmic drugs.Procedures have improved while the number of patients eligible for such treatments has increased.
The most commonly used fibrillation ablation techniques are thermal energy sources with cold (cryoablation around -55°C) or heat (radiofrequency heating around +55°C).Due to the short distance between the ablation site and the phrenic nerve,thermal injury is not uncommon (mainly on the right side) resulting in diaphragmatic dysfunction.Electromyography-guided phrenic nerve monitoring has been proposed to prevent serious phrenic nerve injury during superior vena cava isolation.Detection of reduced contraction of the diaphragm during the procedure leads to the change in the ablation trajectory[18,19].Despite the development of prevention strategies,new sophisticated devices and experienced operators,phrenic nerve injuries remain a possible outcome of up to 15% for early transient paralysis of the diaphragm,which usually disappears at the end of the procedure.Persistent symptomatic diaphragmatic paralysis is rare (reported in less than 1% of cases).A large population study from the "Netherlands Heart Registration" focused on persistent diaphragm dysfunction (> 24 h) among 7433 procedures performed between 2016 and 2017[20].The incidence of persistent diaphragm paralysis was 0.7%,the risk being increased in womens.In a recent prospective,multicenter study conducted in 375 subjects comparing cryoballoon to radiofrequency,data showed that cryoablation has the highest level of phrenic nerve injury with 7.20% transient paralysis compared to 3.20% for radiofrequency[21].Today,a new nonthermal energy modality,called pulse field ablation (PFA) therapy has emerged.PFA therapy involves the application of high voltage levels to tissues in order to induce hyperpermeabilization of cell membranes and cell death through the mechanism of irreversible electroporation.This procedure is believed to be more selective than thermal procedures and may be less damaging to the phrenic nerve[22].
DETECTION OF DIAPHRAGM DYSFUNCTION BY ULTRASOUND
Chest ultrasound has gained much interest in detecting pleural effusion and pulmonary consolidation or edema after cardiac surgery.In 2016,Trovato[23] reported,in theWorld Journal of Cardiology,the interest of using,in addition to echocardiography,thoracic ultrasound for cardiologists.Since the frequency of diaphragm dysfunction is significant after cardiac surgery and atrial fibrillation ablation,the ultrasound analysis of diaphragm function is important.
Ultrasound methods
The two hemidiaphragms can be studied by two complementary ultrasound methods.Diaphragm mobility can be recorded by a sub-costal approach using a cardiac probe[24-26].Excursions of both hemidiaphragms are measured by Mmode ultrasonography during various volitional maneuvers such as quiet breathing (Figure 1) voluntary sniffing and deep inspiration.In addition,it is useful to measure the thickness changes at the zone of apposition during breathing (Figure 2,Video) by a superficial probe using B-mode[27].The percentage of thickening during inspiration provides important information about the quality of the muscle function of the diaphragm[28].These two approaches make it possible to detect paralysis or weakness of hemidiaphragm.
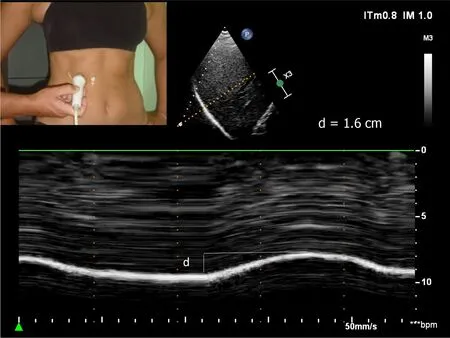
Figure 1 Diaphragmatic motion recorded by M-mode ultrasonography during quiet breathing d: Measurement of diaphragm excursion=1.6 cm.
Diagnosis of hemidiaphragm paralysis
In patients with unilateral diaphragmatic paralysis,hemidiaphragm movement is absent or paradoxical when breathing at rest[29,30].During voluntary sniffing,a paradoxical movement (i.e.cranial) of the hemidiaphragm (Figure 3) is reported using M-mode ultrasonography[29,30].During deep inspiration,a biphasic movement can be recorded with a first paradoxical movement followed by a cranio-caudal excursion[30].The study of inspiratory thickening is important to support the results of the diaphragm excursion analysis.The failure of the paralyzed diaphragm to thicken results in a decrease in the thickening fraction (TF) calculated as the difference between the diaphragm thickness measured at the end of maximal inspiration and the diaphragm thickness at the end of expiration divided by the diaphragm thickness at the end of expiration×100.No significant thickening (TF less than 20%) or thinning of paralyzed hemidiaphragm is observed[31].
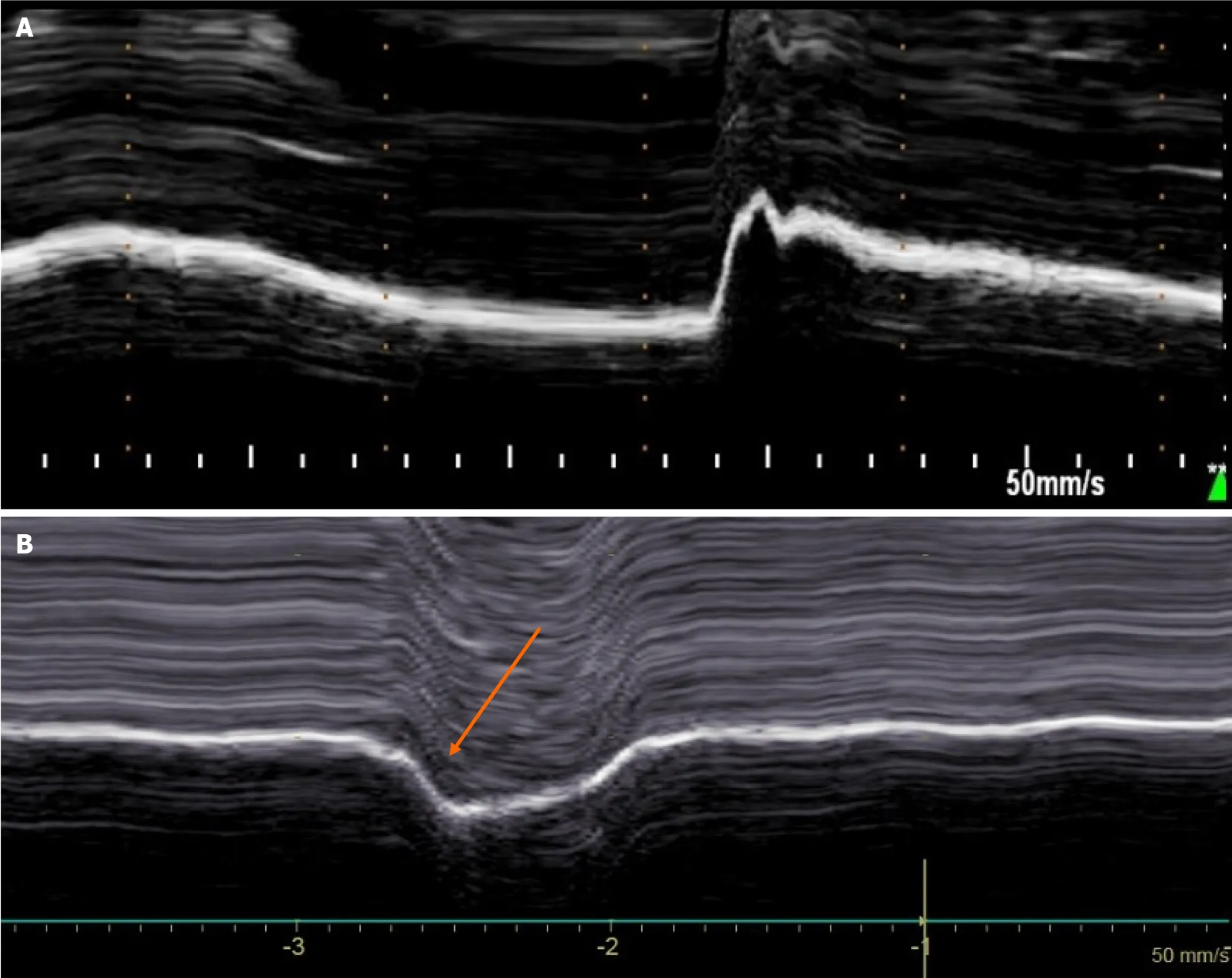
Figure 3 Diaphragmatic motion recorded by M-mode ultrasonography during voluntary sniffing. A: Normal motion;B: Paradoxical movement (arrow) in patient with hemidiaphragm paralysis.
Diagnosis of diaphragm weakness
In some patients,diaphragm dysfunction occurred without complete paralysis i.e.diaphragm weakness.Diaphragm weakness can be detected using normal values of excursions and thickening previously determined from the study of healthy controls[32,33].First,no criteria for complete paralysis should be recorded by ultrasound: No paradoxical movement should be observed during the various maneuvers and the TF should be greater than 20%.Secondly,excursions during deep inspiration should be below the lower limit of normal (LLN) depending on the side and gender according to the reference values[32].
Severity of the weakness may be based on the decrease of the excursion from the lower limit of the normal and the measurement of the thickening fraction[34].
Patients can be classified as follows: (1) Mild hemidiaphragm dysfunction when the excursion is slightly below the lower limit of normal during deep inspiration (excursion > LLN -1 cm) and a normal or slightly decreased thickening fraction (> 40%);(2) Severe hemidiaphragm dysfunction in patients with a marked decrease in hemidiaphragm excursion (< LLN -1 cm) associated with a marked decrease in thickening fraction (< 40%).
CLINICAL CONSEQUENCES OF DIAPHRAGM DYSFUNCTION
The complete loss of function of one hemidiaphragm leads to a restrictive syndrome with a decrease in vital capacity of about 25%.After unilateral diaphragm paralysis,a compensatory increase in neural drive to the functioning hemidiaphragm was demonstrated[35],leading to large excursions to the healthy side[36].The activity of accessory inspiratory muscles is also increased[37].
Disorders induced by diaphragm paralysis can take a wide variety of clinical pictures[38].Bilateral diaphragm paralysis leads to respiratory failure most often requiring ventilatory support.In case of unilateral hemidiaphragm dysfunction,the compensatory mechanism is effective in patients without severe comorbidities and clinical disorders remain weak.Most often,dyspnea is mild and appears during exercise or in supine position.In contrast,in patients with obesity or with severe pre-existing cardiac or respiratory disease,the impairment in respiratory function leads to clinical disorders that can reach respiratory failure.After cardiac surgery,diaphragm dysfunction is associated with a risk of postoperative pneumonia,mechanical ventilation (non-invasive and invasive ventilation) and increased length of stay in the intensive care unit[39].
Diagnosis may be delayed,indeed,respiratory disorders induced by diaphragm dysfunction are frequently attributed to pre-existing heart disease or pulmonary complications secondary to the procedure.Furthermore,elevated hemidiaphragm secondary to diaphragm dysfunction is sometimes not seen on chest X-ray perfomed in supine position in the intensive care unit.Diagnosis is sometimes made later,for exemple when admitted to a cardiac rehabilitation center[40].It remains important because persistent diaphragm dysfunction is associated with late respiratory complications[39].
In addition,high frequency of obstructive sleep apnea (OSA) has been reported in patients with diaphragm dysfunction[41].It is therefore important to seek sleep apnea in these patients because it is recognized that OSA is a risk factor for cardiovascular disease[42].
Less frequently,right-to-left shunt was associated with right hemidiaphragm paralysis[43,44].The mechanism was a redirection of blood flow from the inferior vena cava directly through the patent foramen ovale secondary to a distortion of cardiac anatomy induced by hemidiaphragmatic paralysis.In patients with hypoxemia,closure of the patent foramen ovale may be necessary[45].
TREATMENT
Treatment of diaphragm dysfunction is mainly based on respiratory physiotherapy.In unilateral diaphragm paralysis,inspiratory muscle training improves clinical condition through strengthening of healthy hemidiaphragm and accessory inspiratory muscles[46,47].The long-term prognosis of hemidiaphragm paralysis is usually favorable with a decrease in respiratory disorders due either to the adaptation of healthy inspiratory muscles,or to the spontaneous improvement of diaphragmatic function.
In a population of patients with diaphragm paralysis of various etiologies,Gayan-Ramirezet al[48] reported functional recovery in the first year after diagnosis in 43% of cases and in two years in 52% of cases.After pediatric cardiac surgery complicated by phrenic nerve injury,recovery was documented in about 55% of children over a median follow-up period of 353 d[49].In patients with hemidiaphragm paralysis secondary to atrial fibrillation ablation,after a mean follow-up of 3 years,66% of the study population had complete recovery,17% had partial recovery,and 17% had no recovery[5].The average recovery time was 4 months after injury.In cases of poor tolerance of diaphragm paralysis,mechanical ventilatory support such as non-invasive ventilation may be required[50].In patients with hemidiaphragm paralysis having no recovery and suffering from disabling respiratory disorders,diaphragm plication can be proposed.Surgery is performed through open thoracotomy or video-assisted thoracoscopy[51].Plication of hemidiaphragm reduces dyspnea,and increases both lung function test and exercise capacity[52,53].The improvement in quality of life persists for a long time.It is therefore recommended to consider diaphragm plication in patients with unilateral diaphragm paralysis who have an impairment of quality of life secondary to chronic dyspnea.
CONCLUSION
Cardiac surgery and atrial fibrillation ablation can damage the phrenic nerve causing diaphragm dysfunction.Clinical disorders can be wrongly attributed to pre-existing heart or respiratory diseases,so systematic evaluation of diaphragm function by ultrasound after a procedure at risk of phrenic nerve injury is particularly useful.In such patients,respiratory physiotherapy is able to improve respiratory function through the strengthening of inspiratory muscles.Repeated ultrasound examinations should be performed to monitor potential recovery of diaphragm function.In case of lack of recovery and persistent disabling respiratory disorders,diaphragm plication can be proposed.
FOOTNOTES
Author contributions:The article project was designed by Boussuges A;Boussuges M,Blanc P,Bregeon F,and Boussuges A contributed equally to the article by conducting a literature review,drafting the article,making critical revisions and approving the final version.
Conflict-of-interest statement:All authors have no conflicts of interest to disclose.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:France
ORCID number:Martin Boussuges 0009-0004-6253-4929;Philippe Blanc 0009-0005-2219-7881;Fabienne Bregeon 0000-0002-9244-5474;Alain Boussuges 0000-0001-6176-6200.
S-Editor:Liu JH
L-Editor:A
P-Editor:Zhao S
杂志排行
World Journal of Cardiology的其它文章
- Predictors of permanent pacemaker implantation following transcatheter aortic valve replacement-the search is still on!
- Mechanistic insights into fasting-induced autophagy in the aging heart
- Cardiac arrest,stony heart,and cardiopulmonary resuscitation: An updated revisit
- Sex and racial disparities in non-alcoholic fatty liver disease-related cardiovascular events: National inpatient sample analysis (2019)
- Epicardial adipose tissue in obesity with heart failure with preserved ejection fraction: Cardiovascular magnetic resonance biomarker study
- Severe hypoxemia after radiofrequency ablation for atrial fibrillation in palliatively repaired tetralogy of Fallot: A case report
