Mechanistic insights into fasting-induced autophagy in the aging heart
2024-05-07HannanehParvareshKatarzynaPaczekMdAbdulAlimAlBariNabilEid
Hannaneh Parvaresh,Katarzyna Paczek,Md Abdul Alim Al-Bari,Nabil Eid
Abstract Autophagy is a prosurvival mechanism for the clearance of accumulated abnormal proteins,damaged organelles,and excessive lipids within mammalian cells.A growing body of data indicates that autophagy is reduced in aging cells.This reduction leads to various diseases,such as myocardial hypertrophy,infarction,and atherosclerosis.Recent studies in animal models of an aging heart showed that fasting-induced autophagy improved cardiac function and longevity.This improvement is related to autophagic clearance of damaged cellular components via either bulk or selective autophagy (such as mitophagy).In this editorial,we summarize the mechanisms of autophagy in normal and aging hearts.In addition,the protective effect of fasting-induced autophagy in cardiac aging has been highlighted.
Key Words: Aging;Autophagy;Heart;Fasting;Mitophagy
INTRODUCTION
Cardiac aging
Improvements in treatment procedures have contributed to increased life expectancy and growth in the aged population,especially in industrialized countries[1].Aging is associated with a structural and functional decline in multiple organs,such as the heart.A sedentary lifestyle can also accelerate the incidence of aging-related diseases,including cardiovascular disease (CVD)[2-5].
Cardiovascular aging affects both the heart and the blood circulation system through slow and progressive alterations that can result in the development of left ventricular hypertrophy,diastolic dysfunction,coronary artery disease,stroke,hypertension,atherosclerosis,atrial fibrillation,and heart failure[6-9].Aortic valve sclerosis is a valvulopathy associated with aging and is characterized by myxomatous degeneration,collagen deposition,and progression to aortic stenosis (AS)[10].AS is an indicator of increased CVD risk and is mainly defined as increased leaflet calcification and decreased leaflet mobility[11].Moreover,approximately 13%-16% of elderly people suffer from aortic regurgitation[12],which results in left ventricular dilation and dysfunction over time.Another valvular change related to aging is mitral annular calcification,which usually accompanies aortic valve sclerosis[13].
The free radical theory of aging and the mitochondrial theory have been suggested to explain the cellular deterioration observed in aging and suggest that the age-related decline in mitochondrial function and structure is a major driver of cardiomyocyte senescence,which causes endothelial dysfunction,alteration in the vasculature,and/or vascular injury[14].
Cellular senescence is activated following multiple stressors,including the elevation of reactive oxygen species (ROSs);proinflammatory cytokines;and metabolic,mechanical,and chemical toxicity.Cellular senescence impairs the repair and regeneration of damaged cells in cardiovascular tissues[15-17].Cellular senescence is characterized by genome instability,telomere attrition,and mitochondrial dysfunction[18].
Dysfunctional mitochondria produce less ATP while also generating increased amounts of ROS[19],exposing aged cardiomyocytes to high levels of oxidative stress.Autophagic and proteasomal degradation are the main mechanisms for the removal of damaged mitochondria and abnormal proteins in aged postmitotic cardiomyocytes.However,these mechanisms decline with age[20].Eventually,when these mechanisms are unable to compensate for the accumulated cellular damage,stem-cell exhaustion and altered intercellular communication occur,further contributing to aging[18].
Autophagy in cardiac aging: Reduced autophagy accelerates cardiac aging
Autophagy activity is usually reduced with age[21].A decrease in autophagy in the hearts of aged flies[22] and aged C57BL/6 mice (20-26 months old) has been reported[23,24].
Autophagy is a protective housekeeping mechanism critical for cellular homeostasis and survival.Long-lived,damaged,and dysfunctional organelles;misfolded proteins;and invading pathogens are eliminated through this degradation process,providing building components for cellular renovation to effectively adapt cells to stressful conditions,such as nutrient deprivation,hypoxia,or oxidative stress[25,26].
Autophagy can be selective or nonselective.Under starvation conditions,the protein and any cytoplasmic content can be non-selectively targeted for catabolic recycling to maintain cellular energy production.However,there are also selective forms of autophagy that specifically target damaged organelles.For instance,mitophagy is a type of autophagy that selectively removes damaged mitochondria[27].Mitochondria play a substantial role in cellular functions as well as cellular death.Thus,mitochondrial dysfunction is a crucial determinant of lifespan across species[28,29].
Three types of autophagy have been recognized: Macroautophagy,microautophagy,and chaperone-mediated autophagy,all of which lead to the turnover of intracellular componentsviavarious mechanisms.“Autophagy” is a term that generally refers to macroautophagy,which is the most prevalent form of autophagy[30,31].
Molecular machinery of autophagy
Autophagy is initiated when several autophagy-related gene products (Atg1-Atg12) and other proteins are organized to form a phagophore.These proteins consist of at least five molecular components that mediate fusion between autophagosome (AP) and lysosomes: (1) The Atg1/unc-51-like kinase complex;(2) the Beclin 1/class III phosphatidylinositol 3-kinase (PI3K) complex;(3) Atg9 and vacuole membrane protein 1;(4) two ubiquitin-like proteins (Atg12 and Atg8/LC3) conjugation systems;and (5) proteins that mediate fusion between APs and lysosomes[25,32].
The initial step of AP formation starts with Beclin1 (Atg6) and class III PI3K,which play crucial roles in vesicle isolation.Other Atg proteins are involved in Beclin-1-mediated formation of the Class III PI3K complex.In the next step,the AP undergoes elongationviatwo conjugation systems.First,Atg12 is conjugated to Atg5 with the help of Atg7 and Atg10[33,34],followed by the conjugation of phosphatidylethanolamine to microtubule-associated protein 1 LC3viaAtg4,Atg7 and Atg3.Consequently,the cytoplasmic LC3 (LC3-I) is converted to membranous (LC3-II) form,which is responsible for formation and maturation of the AP[35].In the end,fusion of APs and lysosomes occurs with the formation of autolysosome (AL) for degradation and recycling[36].
The protein kinases mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) are implicated in the regulatory mechanisms of autophagy.Autophagy is inhibited by the mTOR.Phosphorylation of Unc-51 Like autophagy activating kinase-1 (ULK1) by AMPK is involved in autophagy promotion,although mTOR represses this process[37].Figure 1 demonstrates the various mechanisms of autophagy in mammalian cells.
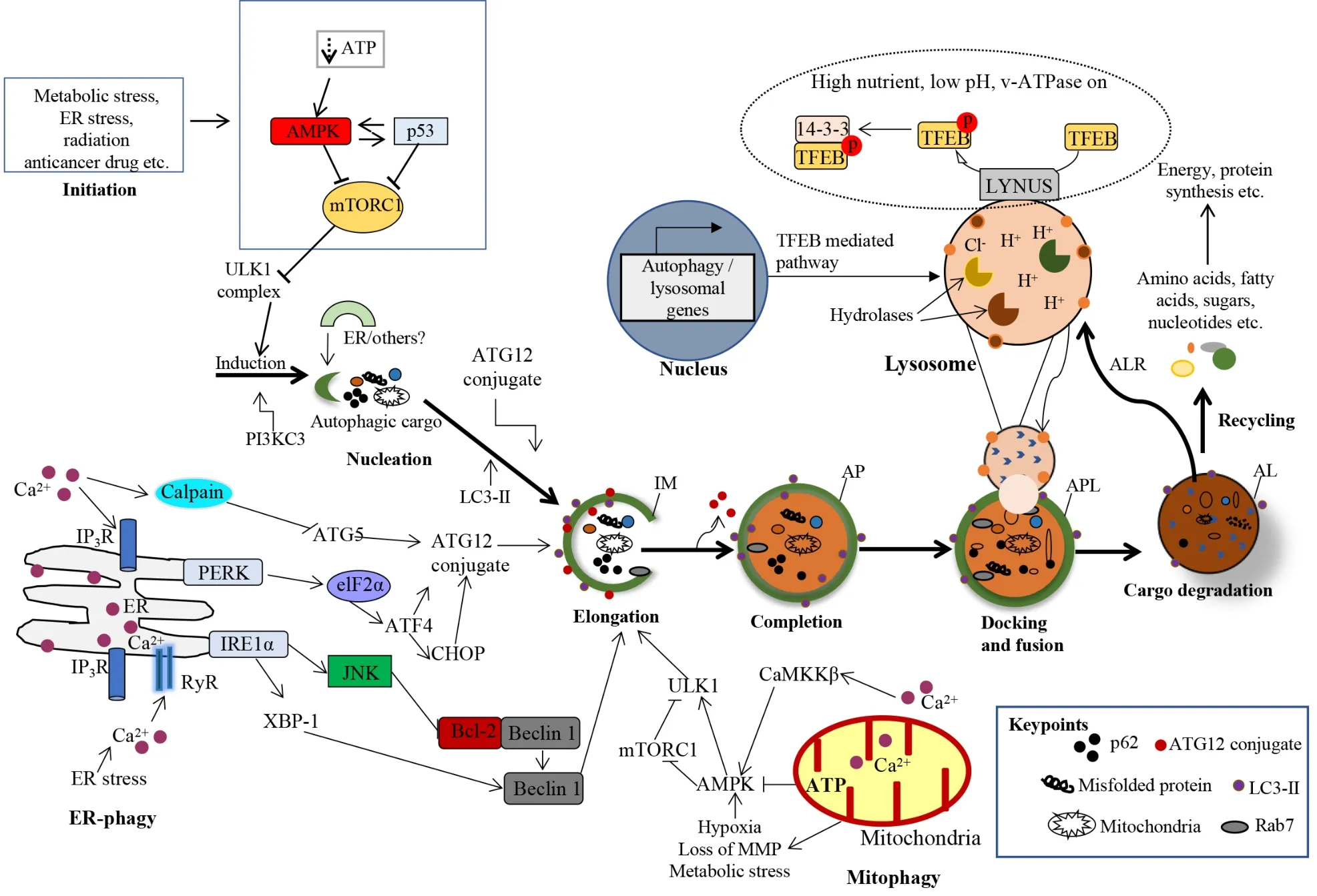
Figure 1 Molecular mechanisms of various stages of autophagy. Autophagy is activated in response to various cellular stresses and is triggered by a decrease in rapamycin complex 1 (mTORC1) activity due to the activation of AMP-activated protein kinase (AMPK) or p53 signaling.mTORC1 suppresses the activity of Unc-51-like autophagy activating kinase 1 (ULK1) complex.Therefore,inhibition of mTORC1 causes the initialization of the ULK1-mediated formation of the isolation (autophagosomal) membrane (IM) in association with the class III phosphatidylinositide 3-kinase complex.The IM expands into an autophagosome (AP) with a double-layer membrane,which can engulf any cellular component,including proteins,damaged organelles,and lipid droplets.The AP merges with the lysosome (via LAMP-1,2),forming autophagolysosome or autolysosome (AL),and resulting in the degradation of the cargo by cathepsins and the autophagic lysosome reformation.The nucleation,elongation and maturation of the IM are dependent on two ubiquitin-like conjugation systems (ATG12 and ATG8),which involve multiple autophagy proteins,including Beclin1,ATG5,ATG16 and MT-associated protein 1 LC3.The AL provides an acidic milieu for hydrolytic enzymes to digest the engulfed components.Nuclear localization of transcription factor EB is critical to the formation of lysosomes and to the enhanced expression of autophagy proteins.Importantly,autophagy could be selective of mitochondria (mitophagy) or ER (ER-phagy).However,the detailed mechanisms of this selected autophagy are beyond the scope of this study[28].AMPK: AMP-activated protein kinase;PI3KC3: Phosphatidylinositide 3-kinase complex;APL: Autophagolysosome;AL: Autolysosome;ALR: Autophagic lysosome reformation;IM: Isolation (autophagosomal) membrane;TFEB: Transcription factor EB;mTORC1: Rapamycin complex 1.Citation: Al-Bari MAA,Ito Y,Ahmed S,Radwan N,Ahmed HS,Eid N.Targeting Autophagy with Natural Products as a Potential Therapeutic Approach for Cancer.Int J Mol Sci 2021;22: 9807.Copyright ©The Author(s) 2021.Published by MDPI.
Autophagy in the heart
Accumulating evidence reveals that autophagy plays essential homeostatic roles in the heart under normal physiological conditions and during the aging process;additionally,it has an essential role in improving the immune response and reducing inflammation[38].Consequently,any perturbations to this process in the cardiovascular system can elicit harmful effects on health.
Autophagy attenuates with age and has serious implications for heart structure and function.A decrease in autophagy causes the development of heart failure,hypertension,atherosclerosis,and ischemic heart disease[39].
Mitophagy is the selective autophagic clearance of damaged mitochondria and is crucial for the bioenergetics of the cardiovascular system;thus,mitophagy dysfunction is generally accompanied by cardiac disorders[27,40,41].In addition,studies have suggested that autophagic degradation of damaged mitochondria decelerates cardiovascular senescence and has a positive effect on the healthy lifespan of animals[42-44].
Age-induced impairment of autophagy
Cardiomyocytes undergo age-related changes in proteostasis pathways,resulting in calcium homeostasis impairment,ROSs induction,hypertrophy and fibrosis,and eventual structural damage and diminished cardiac function.Moreover,with age,the MTOR-1 complex is significantly upregulated,and the AMPK pathway is downregulated.In addition,transcription factors involved in autophagy and lysosomal proteins such as TFEB and Forkhead transcription factor (FOXO) 3 are deactivated with advanced aging,resulting in reduced expression of autophagy genes[28-31].
Any defect in the autophagy process accelerates aging;likewise,aging is suppressed when autophagy is stimulated.Deletion of atg5,a cardiac-specific autophagy-related gene,in adult mice leads to an accelerated aging phenotype,including the development of cardiac hypertrophy,left ventricular dilatation,and contractile dysfunction[20,45].
Mutations in the atg4c gene increase the risk of heart disease in elderly patients and eventually death[46].Cardiomyocyte-specific deletion of glycogen synthase kinase-3 in mice reduced basal autophagy levels and accelerated cardiac aging[47].Dysfunction of autophagy with age slows the turnover of damaged proteasomes and contributes to age-associated CVD and cardiomyocyte senescence[48].Mitophagy is impaired in aged mice,and mitophagy induction improves mitochondrial function and reduces arterial wall stiffness[49].
Acyl-coenzyme A binding protein (ACBP),which is encoded by a diazepambinding inhibitor (DBI),acts as an extracellular feedback inhibitor of autophagy[50].It appears that high ACBP/DBI values correlate with future cardiovascular events (such as heart surgery,myocardial infarction,and stroke),suggesting that ACBP/DBI is indeed a biomarker of biological aging[39].
Mechanisms underlying age-related cardiac remodeling: involvement of autophagy
Although there are many potential causes underlying the decline in cardiovascular function with age,a major determinant of the aging process is likely the progressive loss of quality control due to reduced autophagy.
Hyperactivation of mTOR and reduced AMPK activity[51] in old age can directly inhibit autophagy by inactivating the pro-autophagic ULK1 complex[52],contributing to the downregulation of autophagy activity.
It is conceivable that exposure to excessive ROS during aging promotes the accumulation of oxidized proteins,mitochondrial DNA mutations,and protein misfolding[53].Additionally,several cytosolic and mitochondrion-localized proteins involved in autophagy regulation become dysfunctional,thus contributing to abnormal mitochondrial turnover and the removal of damaged mitochondria[54].This chain of events results in impaired autophagy due to exhaustion of the aged autophagic machinery.
In addition,it has been proposed that a hallmark of aging in postmitotic cells,such as cardiomyocytes,is the aggregation of nondegradable structures inside lysosomes,termed lipofuscin,which impedes lysosomal function and therefore can likely inhibit autophagy[55].
It has been shown that intracellular calcium has a key regulatory effect on cardiomyocyte autophagy.Inositol 1,4,5-trisphosphate (IP3) receptors mediate calcium release and transfer to mitochondria.This process inhibits autophagy by suppressing AMPK activation[56].Since evidence has shown that IP3 receptors are upregulated in the aged,hypertrophied,and failing myocardium of rodents[57] and humans[58],increased IP3 receptor-mediated calcium signaling likely exacerbates autophagy in the aging heart[59].
FOXO and sirtuin proteins are also major metabolic regulators that mediate age-related vascular changes,particularly endothelial dysfunction[9].
Dietary activation of autophagy in the heart via caloric restriction or fasting
Dietary interventions involving caloric restriction (CR) and fasting are among several stress stimuli that can induce autophagy in response to food deprivation[60-62].CR was defined as a reduction in caloric intake using a diet containing adequate amounts of protein,vitamins,and minerals[63].CR is a potent inducer of autophagy in the heart[64],and its positive impacts on health and lifespan in various model organisms,primates and humans have been studied[65-67].CR is the most potent physiological stimulus of autophagy and ameliorates cardiac dysfunction (systolic and diastolic) and attenuates myocardial hypertrophy and fibrosis at the cardiomyocyte level.CR reduces mitochondrial damage,lipid accumulation,oxidative stress,apoptosis,telomere shortening,senescence marker levels,and circulating proinflammatory cytokine levels[68].
Autophagy plays an important role in CR-mediated longevity[69]viaclearance of damaged mitochondria,reduction of oxidative stress,improvement of insulin sensitivity and suppression of inflammatory responses[61,62].
Short-term CR for 10 wk in mice rejuvenated symptoms of the aging heart,such as significant improvement in diastolic function and regression of age-dependent cardiac hypertrophy[70].Moreover,CR reversed age-dependent cardiac proteome remodeling and mitigated oxidative damage and ubiquitination in these mice.
In aged animals,hypertrophy,and fibrosis,as well as systolic and diastolic dysfunctions,improved after CR[68,71].The beneficial effects of CR observed in cardiomyocytes include enhanced mitochondrial fitness and reduced oxidative stress,apoptotic cell death,inflammation,and importantly,senescence[68].In vasculature,CR helps improve endothelial cell function and attenuates collagen deposition,elastin remodeling,and oxidative stress;as a result,CR reduces arterial stiffness[72].Another study revealed improvements in numerous markers of cardiovascular health in humans after shortterm periodic fasting,which is also a pro-autophagic dietary regimen[73].
Intermittent fasting (IF) has attracted the attention of researchers as a dietary intervention associated with better compliance and long-term adherence than CR in recent years[74].IF consists of regular cycles of times with no or minimal caloric intake interrupted by periods of normal food consumption.Alternate day fasting delays cardiac aging in rats,as determined by reduced hypertrophy and fibrosis[75,76] and extended lifespan[77].The advantageous effects of life-long alternate-day fasting were attributed to reduced phosphoinositide 3-kinase signaling,which was associated with reduced myocardial collagen deposition,oxidative stress,inflammatory markers,and B-type natriuretic peptide levels[75,78].
A fasting-mimicking diet (FMD) is considered another form of dietary intervention in which individuals consume low amounts of calories,sugars,and proteins but high amounts of unsaturated fats.Studies of FMD effects in mice have shown improved cognitive function and a rejuvenated immune system,in addition to promoting lifespan and health factors by reducing cancer incidence,obesity,and inflammation[79].FMD was investigated in humans,and the findings showed reduced age-related CVD risk factors,including reduced blood pressure,body mass index,fasting glucose,and inflammation,as well as an improved lipid profile[80].
The efficacy of fasting on autophagy in the heart was assessed in male FBN rats by randomly dividing them into different groups of equal amounts of protein,vitamin,and mineral intake,while the CR groups received 20% less food from a 125% fortified diet for six weeks.Additionally,in addition to one simple CR group,two other CR groups were given 5 or 50 mg/kg/day resveratrol.Compared with AL group,a marked reduction of expression of p62 (autophagy substrate) in the left ventricle was observed in the CR and Resv-50 rats,indicating enhanced cardiac autophagy in the CR group.Similarly,a significant overexpression of Beclin-1 was found in the Resv-50 and CR animals.The CR+Resv-50 group of rats showed dramatically attenuated doxorubicin-induced damage,which can be due to enhanced autophagy[81].Another study investigated the autophagic response of CR on diabetic rat hearts.Diabetic and nondiabetic rats were exposed to a CR diet (30% energy reduction) for 32 wk.Compared with those of diabetic AL rats,diabetic CR rats exhibited an increase in the hepatic and cardiac LC3-II/LC3-I ratio (indicating enhanced autophagy)[82].
A high-fat diet (HFD) (fat 60% kcal/100 kcal fat) was given to the FVBN male mice for 4-20 wk,after which they were subjected to overnight fasting to study the mechanisms of fasting-induced autophagy in the fatty mice heart.After 24 h of fasting,there was a significant conversion of LC3-I conversion to LC3-II in lean mice heart but was not associated with a change in diet-induced obesity (DIO) mice.Furthermore,fasting suppressed mTOR in both lean and DIO mice,as indicated by increased AMPK phosphorylation and enhanced dephosphorylation of S6.Interestingly,mTOR inhibition was greater in obese mice.Taken together,these findings indicate that fasting activates autophagy in the hearts of lean mice[83].
Godaret al[84] investigated the impacts of IF on the autophagy-lysosome machinery in the myocardium.The authors studied the effects of fasting after 24 h,followed by 24 h of refeeding or 24 and 48 h of fasting for six weeks.The AP abundance increased dramatically after 48 h of fasting.Treatment with chloroquine (an autophagy inhibitor) was associated with a significant increase in LC3-II and SQSTM1/p62 after 24 h of fasting but not in fed mice.Thus,fasting induces autophagy in cardiomyocytes;however,autophagy returns to basal levels on gestational days.
The effects of IF on right ventricular (RV) function in a rat model of pulmonary arterial hypertension (characterized by RV mitochondrial dysfunction and resultant lipotoxicity and microbiome dysbiosis) were explored.IF improved RV systolic and diastolic function and decreased RV cardiomyocyte hypertrophy and fibrosis,which was likely mediated by autophagy activation[85].These protective effects could be related to autophagy activation.
Recent findings from studies also show that cardiometabolic parameters (e.g.,adiposity,insulin sensitivity,and cardiac function) can be influenced by the time of day at which food is consumed[86].To test the hypothesis that fasting during the sleep period elicits beneficial adaptation effects on cardiac function,wild-type mice were fasted for 24 h or for either the 12-h light/sleep phase or the 12-h dark/awake phase.Repression of myocardial p-mTOR and protein synthesis occurred during the dark phase;both parameters remained elevated in the hearts of fasted mice during the light phase.In contrast,markers of autophagy (e.g.,LC3-II) exhibited peak responses to fasting during the light phase.Collectively,these data show that the responsiveness of the heart to fasting is temporally partitioned[86].
IF alleviated HFD-induced obesity cardiomyopathy in male C57BL/6J mice by improving cardiac functional and structural impairment and serum lipid metabolic disorders induced by HFD through decreasing lipid deposition,apoptosis and m6A methylation in the heart[87].
Researchers compared the effects of alternate day fasting on elderly (aged 24 months) and young (aged 6 months) male rats.The results of this study indicated that alternate day fasting protected against inflammation and fibrosis in the heart during aging by inhibiting oxidative damage and NF-κB activation[76].Other studies have shown that fasting preconditioning activates AMPK,induces autophagy,decreases ROS levels,and inhibits NF-κB signaling in the cardiac tissues of rats[88].In addition,compared with fasting controls,IF in human subjects resulted in autophagy upregulation and reduced levels of proinflammatory cytokines,indicating the protective effects of fasting on the vascular system.This effect is most likely mediated by the anti-inflammatory effects of autophagy[89].We investigated fasting-induced autophagy among large groups of population in the UAE during Ramadan (the holy Islamic fasting month).The results of this study will be published shortly in specific journals.Furthermore,these results were presented in part at the Sharjah First International Conference on Fasting,February 28-29,2024,at Sharjah University,United Arab Emirates[90].
CONCLUSION
In conclusion,fasting-induced autophagy is beneficial for ensuring cardiac function,preventing disease,and improving longevity.However,additional studiesin vivoin animal models of cardiac aging are needed to determine the specific molecular mechanisms involved in normalizing autophagy by fasting.In addition,large-scale studies on humans are needed.Ramadan fasting,a type of IF (a common religious practice) in Islamic countries,could be investigated in large groups of geriatric people with or without cardiac diseases.Importantly,furtherin vitroresearch should be directed toward human cardiac tissues to better understand the molecular mechanisms of fasting-induced autophagy and its beneficial effects on longevity pathways and prevention of CVDs.
FOOTNOTES
Author contributions:Parvaresh H and Eid N wrote the paper;Al-Bari MAA and Paczek K edited and revised it.
Conflict-of-interest statement:All the authors declare that they have no conflict of interest to disclose.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Malaysia
ORCID number:Md Abdul Alim Al-Bari 0000-0002-1777-3662;Nabil Eid 0000-0002-2938-2618.
S-Editor:Qu XL
L-Editor:A
P-Editor:Guo X
杂志排行
World Journal of Cardiology的其它文章
- Cardiovascular diseases in European ethnic minorities: Beyond the traditional cardiovascular risk factors
- Severe hypoxemia after radiofrequency ablation for atrial fibrillation in palliatively repaired tetralogy of Fallot: A case report
- Epicardial adipose tissue in obesity with heart failure with preserved ejection fraction: Cardiovascular magnetic resonance biomarker study
- Sex and racial disparities in non-alcoholic fatty liver disease-related cardiovascular events: National inpatient sample analysis (2019)
- Cardiac arrest,stony heart,and cardiopulmonary resuscitation: An updated revisit
- Interest of thoracic ultrasound after cardiac surgery or interventional cardiology
