构建一维铜基配位聚合物作为锂离子电池正极材料
2024-04-17姜清艳沙彦勇陈晓娟刘文龙刘洪江
姜清艳 沙彦勇 陈 晨 陈晓娟 刘文龙 黄 浩 刘洪江*, 刘 琦*,
(1常州大学石油化工学院,江苏省精细石油化工重点实验室,常州 213164)
(2上海大学理学院化学系,上海 200444)
(3扬州大学化学化工学院,扬州 225009)
With the further development of science and technology, energy and environmental problems are becoming increasingly prominent, and people′s requirements for secondary batteries are getting higher and higher[1-3].Lithium-ion batteries (LIBs) have become the most widely used energy storage technology in portable electronic products due to their high energy density, wide operating temperature range,and excellent cycle stability. Cathode materials occupy the most important position in the composition of metal-ion batteries[4].An ideal cathode material needs to have the following characteristics: stable structure, high redox potential, excellent kinetic properties, high conductivity, abundant resources, and convenient synthesis[5-7]. At present,commercial cathode materials are mainly inorganic compounds[8], whereas the theoretical specific capacity of the inorganic electrode is below 300 mAh·g-1, and the actual lithium utilization is not more than 60%.Accompanied by the emphasis on environmental pollution and resource consumption, as well as the urgent demand for equipment with high energy density, it is an important task to develop new sustainable cathode materials with high capacity[9-10]. Today, organic materials are considered to be a strong alternative to inorganic electrode materials[11-14]. Among them, organic carbonyl compounds have attracted much attention because of their designable structures, high theoretical specific capacity, and fast kinetics[15-26]. However, quite a few organic materials are small molecules that dissolve easily in the electrolyte, which greatly reduces the utilization rate of active materials and the electrode cycle stability. In response to the above problems,researchers have put forward many methods, such as ingenious molecular design, nanosizing, electrolyte optimization,and surface coating[12,27-31].
The building of metal coordination polymers (CPs)by coordination of organic ligands with metal ions has also been proven to be an effective way of overcoming the dissolution problem[32-34]. This class of CPs exhibits structural diversity, which has different dimensionalities like one-dimensional (1D) chains,two-dimensional(2D) sheets, and three-dimensional (3D) frameworks.3D and 2D CPs with pore structures are usually named metal-organic frameworks (MOFs). By regulating the types of organic ligands and metal ions or metal clusters with redox active sites, CPs/MOFs with multiple redox activity and specific pore size distributions can be produced[35-43]. Theoretically, the multiple redox active sites in CPs will be favored to increase the specific capacity, and the channels in them will be conducive to electrolyte ion transfer. In addition, the flexibility of organic ligands in them can overcome the volume effect of some metal oxide-based electrodes during charging and discharging.Therefore,quite a few CPs as electrode materials have been applied in LIBs so far[32-41,44-59].But,compared to CPs used as anode materials of LIBs[32,36,44-55], there is relatively little research on CPs used as cathode materials[33-35,37-41,56-59]. In 2007,Férey′s group first reported a 3D iron-based MOF(FeⅢ(OH)0.8F0.2[O2C-C6H4-CO2],MIL-53(Fe))that can be used as a cathode material for LIBs, in which Fe centers act as redox-active sites. MIL-53(Fe) only delivered a specific capacity of 70 mAh·g-1and had excellent cycling performance[38].To enhance specific capacity, in 2014, Awaga et al. first proposed the idea of combining redox-active metal ions and redox-active organic ligands into a conjugated structure and synthesized a copper-based 2D CP Cu(2,7-AQDC) (2,7-H2AQDC=2,7-anthraquinonedicarboxylic acid).Cu(2,7-AQDC) as the cathode material of LIBs displayed a specific capacity of 147 mAh·g-1, due to anthraquinone groups and Cu(Ⅱ)ions all taking part in the redox reaction.It is a pity that the capacity of 147 mAh·g-1is still lower than that of LiCoO2[40]. Recently, our group studied the lithium storage properties of a cobalt-based CP ([Co(4-DTBPT)(DMF)2(H2O)2](4-DTBPT)(C10H4O8),Co-DTBPT)and a 2D copper-based CP([Cu(4-DTBPT)2(DMF)2](C10H4O8), Cu-CP) and, in which both Cu (Ⅱ)/Co (Ⅱ)ions and ligands all participated in the redox reaction of Cu-CP/Co-DTBPT electrodes. Although the Cu-CP electrode and Co-DTBPT electrode all exhibited good cycling stability, they only delivered the lower specific capacities of 40.3 and 55 mAh·g-1at 50 mA·g-1, respectively[33,41]. We noted that most of the previous studies have focused on 2D and 3D MOF-based cathode materials while 1D CP-based material received little attention. Because solvent molecules usually occupy the channels of 2D and 3D CPs/MOFs, the diffusion of the electrolyte ion will be restricted. Considering 1D chains in 1D CP can produce a 3D supramolecular structure by weak interactions, such as,π-πstacking and hydrogen bonds, and the flexible space between 1D chains can allow the diffusion of the electrolyte ion, thus, like as 1D organic polymer polyanthaquinone (PAQ)[60], 1D CPs should be also used as the electrode materials for LIBs. 1D CPs as the anode and cathode materials for LIBs have begun to receive attention[33,36-37,46,49-51,55,57-59]. However, until now, as far as we know, only a few 1D CPs used as the cathode materials of LIBs, such as Co-DTBP, [CuL(DMF)2]n, [Cd(NO3)2(DPNDI)] · (DMA)2· (H2O)0.5}n, {[Co(NCS)2(DPNDI)] ·(DMA)3·(H2O)}n, Fe(DHBQ), and DS-Co-MOF, have been reported[33,37,57-59]. Among them, only there is one 1D Cu-based CP, [CuL(DMF)2]n, which can deliver a specific capacity of 268 mAh·g-1at 30 mA·g-1[37].
On the other hand, as a carbonyl compound with redox activity,N,N′-bis(glycinyl)pyromellitic diimide(H2BGPD) has abundant sources,environmental friendliness, and lower cost[61]. For increasing the redox active centers in CPs and obtaining new green and sustainable cathode materials with high performance for LIBs, it may be a feasible way to synthesize new CPs by using the interaction of transition metal ions with redox activity and H2BGPD. Herein, a novel 1D polycarbonyl CP ([Cu(BGPD)(DMA)(H2O)]·DMA, Cu-BD)was synthesized by coordinating interaction of Cu(NO3)2·3H2O with H2BGPD (Fig.1) in the mixed solvent of DMA and ethanol. When Cu-BD was used as the cathode material of LIBs, its discharge-specific capacity still delivered 50 mAh·g-1at 50 mA·g-1after 100 cycles along with a Coulombic efficiency of almost 100%. Besides, it also exhibited better cycle stability after the decay of the initial several cycles. To the best of our knowledge,this is 1D Cu-based CP second being used as the cathode material of LIBs.
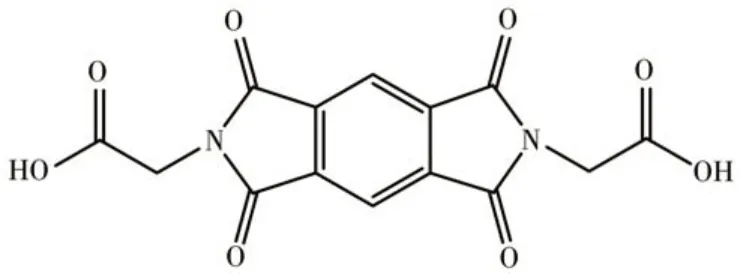
Fig.1 Structural formula of H2BGPD
1 Experimental
1.1 Materials
H2BGPD was synthesized according to the reported method[61]. The synthetic route of H2BGPD was presented in Scheme S1 (Supporting information). Other chemicals with analytical purity were purchased from Shanghai Chemical Reagent Company.
1.2 Synthesis of[Cu(BGPD)(DMA)(H2O)]·DMA
H2BGPD (0.05 mmol, 0.016 6 g) and 4 mL DMA were mixed in a small beaker to dissolve. Cu(NO3)2·3H2O (0.05 mmol, 0.012 g) was added to a small beaker containing 2 mL ethanol, stirred slightly to dissolve, and then transferred to the above beaker to get a solution. After the resulting solution was stranded for two weeks, blue bulk crystals Cu-BD were collected by filtration.Yield based on Cuca.34%.Its molecular formula is C22H26CuN4O11. IR (KBr, cm-1): 3 436(s), 1 718(m), 1 624(m), 1 420(m), 1 384(m), 1 121(w), 963(w),755(w),687(w),634(w),584(w).
1.3 Physical measurements
The Fourier-transform infrared (FTIR) spectrum of the as-prepared sample was tested on a Nicolet 460 spectrometer in the range of 4 000-500 cm-1. Powder X-ray diffraction (XRD) patterns were performed on D/Max 2500PC using CuKαradiation (40 kV, 150 mA,λ=0.154 06 nm) in a range of 2θ=5°-80° to detect the structure of Cu-BD. X-ray photoelectron spectroscopy(XPS) measurements were studied on a Kratos Axis Ultra DLD instrument. The surface structure and morphology of the sample were observed by ZEISS Supra 55-field emission scanning electron microscope(FESEM) in high vacuum mode at an accelerating voltage of 0.1-30 kV. The data of thermogravimetric analysis was collected on a thermal analyzer (STA6000) at a heating rate of 10 ℃·min-1from room temperature to 800 ℃under a nitrogen atmosphere. The ASAP2460 aperture analyzer was applied to measure the nitrogen adsorption and desorption curves. Brunner-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) methods were utilized to calculate the specific surface area,pore size, and pore volume of the Cu-BD sample,respectively.
1.4 X-ray crystallography analysis
For a Cu-BD single crystal, a Bruker diffractometer with a Smart Apex CCD area detector was used to measure its X-ray diffraction. Adopting MoKαradiation (λ=0.071 073 nm), intensity reflections were collected in a range of 2.269°≤θ≤27.560°. With the SHELXTL-97 program and a direct method, the structure of Cu-BD was solved. With anisotropic parameters, all the non-hydrogen atoms were refined. The hydrogen atoms linked to carbon atoms,which were put at the geometric locations,were refined using an isotropic parameter. The crystallographic data of Cu-BD are listed in Table S1.
CCDC:2283641.
1.5 Electrochemical measurements
The CR 2032 coin batteries were assembled in a glove box filled with argon gas (H2O and O2concentrations<0.01 mg·L-1).Cu-BD cathode sheet was prepared by the following method: Cu-BD, acetylene black, and poly(tetrafluoroethylene) (3∶5∶2,w/w) were mixed with an appropriate amount ofN-methylpyrrolidone (NMP),and the resulting slurry was evenly coated on aluminum foil.The coated aluminum foil was dried in a vacuum at 80 ℃for 12 h. There was an average ofca. 1 mg· cm-2of active material Cu-BD on the electrode.Porous polypropylene and pure lithium foil were used as separators and counter electrodes, respectively. 1 mol·L-1LiPF6was used as an electrolyte in dimethyl carbonate (DMC), ethylene carbonate (EC), and diethyl carbonate (DEC) (1∶1∶1,V/V). The cyclic voltammetry curve (CV) was measured in the voltage range of 1.5-4.0 V at different scan rates, such as 0.1, 0.2 mV·s-1,etc.Under the open-circuit voltage,the electrochemical impedance spectroscopy (EIS) was measured in a frequency range of 10-2-105Hz at 5 mV amplitude. Both CV and EIS were tested at room temperature with a CHI600E electrochemical analyzer (Beijing Huake company). The charge-discharge performance tests were carried out on the Neware CT 3008W Battery Test System in the potential range of 1.5 to 4.0 V.
2 Results and discussion
2.1 Crystal structure description of Cu-BD
From Table S1,it can be seen that Cu-BD belongs to the triclinic crystal system, and the space group isP1. Its asymmetric unit structure consists of a Cu (Ⅱ)ion, a BGPD2-anion, a coordination water molecule, a coordination DMA molecule, and a free DMA molecule.As shown in Fig.2a,the Cu(Ⅱ)ion adopts a tetragonal cone coordination geometry, which contains three oxygen atoms (O5, O7A, O7B) from three BGPD2-anions, one oxygen atom (O10) from one DMA molecule and one oxygen atom (O9) from a coordination water molecule. The O7A atom is at the square pyramidal apex positions, while the O5, O7B, O9, and O10 atoms are at the base position. The bond angles of O10—Cu1—O9 and O5—Cu1—O7B are 17.665(6)° and 17.598(5)°, respectively (Table S2). The bond lengths of Cu—O9, Cu—O10, and Cu—O5 are 0.197 78,0.196 07, and 0.193 98 nm, respectively (Table S2),which is close to that of Cu—O in [Cu(BGPD)(DMF)2(H2O)][62], and shorter than that of Cu—O in Cu-CP[41].As shown in Fig.2b, all Cu (Ⅱ)ions are connected by BGPD2-anions, creating a 1D chain structure. These 1D chains and free DMA molecules form a 3D supramolecular structure through hydrogen bonding (O—H…O and C—H…O,Table S3),as shown in Fig.2c.
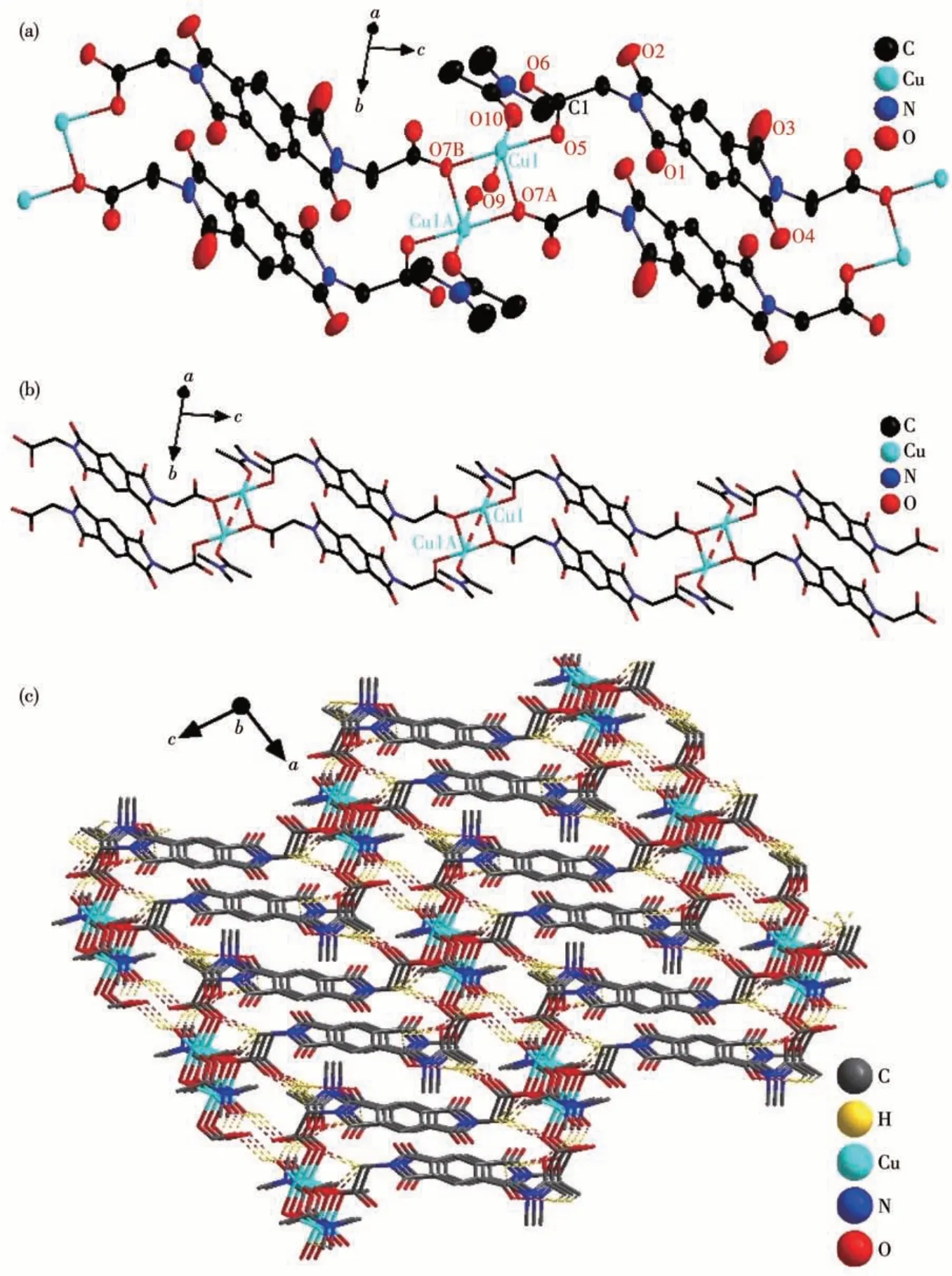
Fig.2 (a)Coordination environment diagram with thermal ellipsoid at 30% probability level(H atoms are omitted for clarity),(b)1D structure diagram of Cu-BD,and(c)3D stacking diagram(hydrogen bond is drawn with the dotted line,and hydrogen atom without hydrogen bond is not drawn)of Cu-BD
2.2 Synthesis and characterization of Cu-BD
A variety of characterization methods have been employed to study the composition and physical characteristics of Cu-BD.The FTIR spectra of the raw material H2BGPD and the product Cu-BD are displayed in Fig.3a. As observed in Fig.3a, There is a strong and wide absorption peak at 3 436 cm-1, belonging to the O—H stretching vibration peak from H2O molecules[41];the existence of stretching vibration peak of C=O at 1 718 cm-1; there are two stretching vibrations peaks appearing at 1 624 and 1 420 cm-1, which belongs toνas(OCO) andνs(OCO) of BGPD2-anions; the tensile vibration peak of C—C on the aromatic ring appears at 1 384 cm-1, while a peak at 584 cm-1is assigned toνs(Cu—O), verifying that BGPD2-anions (C10H4O82-)have been combined with Cu (Ⅱ)ions during the reaction[62]. The infrared analysis results are consistent with the single crystal structure analysis results above mentioned. As shown in Fig.3b, the PXRD peak positions of as-synthesized Cu-BD are the same as that of the simulated diffraction peak originating from Cu-BD crystal data,revealing that the Cu-BD owns a pure phase.
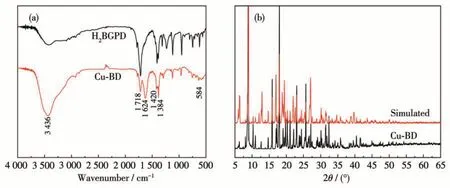
Fig.3 (a)FTIR spectra of Cu-BD and H2BGPD;(b)PXRD patterns of Cu-BD and the simulation based on Cu-BD single-crystal data
The thermogravimetric analysis diagram of Cu-BD is shown in Fig.4a. From the figure, it can be seen that Cu-BD had a mass loss of about 3.4% between 30-121 ℃, which was caused by the loss of water molecules(Calcd.3.1%);Cu-BD had a significant mass loss of 16.1% between 121 and 172 ℃, mainly due to the loss of a free DMA molecule (Calcd.14.9%).Moreover,from 172 to 410 ℃,there was a significant mass loss of 46%, mainly owing to the decomposition of ligand BGPD2-anions and DMA molecule, and then as the temperature gradually increased, the remaining material slowly decomposed, and the residual material at 800 ℃should be CuO(Calcd.13.6%,Obsd.11%).
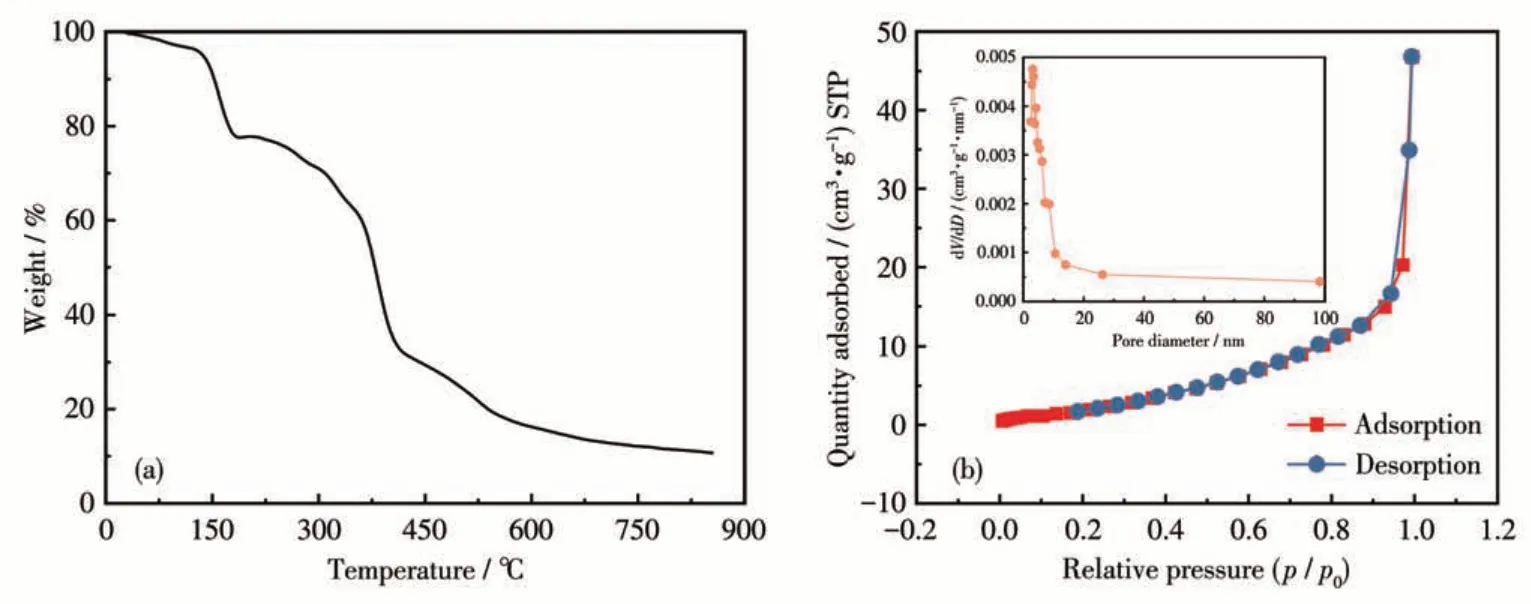
Fig.4 (a)Thermogravimetric curve of Cu-BD;(b)Nitrogen adsorption-desorption isotherm for Cu-BD and pore size distribution curve(Inset)
The adsorption-desorption isotherm for Cu-BD presented in Fig.4b shows this isotherm was between type Ⅲand Ⅳ.The specific surface area was 4 m2·g-1,the total pore volume of a single point adsorption was 0.072 cm3·g-1, and the average pore size reached 5.33 nm based on the BJH method of calculation. The adsorption-desorption isotherm in Fig.4b showed no significant hysteresis effect, and hardly overlapped at low pressure (p/p0<0.2), suggesting that the sample is a mesoporous material, which is attributed to the gap generated by nanoparticle aggregation. The abundant pore structure is conducive to the rapid transfer of lithium ions.
The FESEM images of Cu-BD samples after grinding are shown in Fig.5. Cu-BD was stacked by many irregular nanoparticles with a size of 20-60 nm.
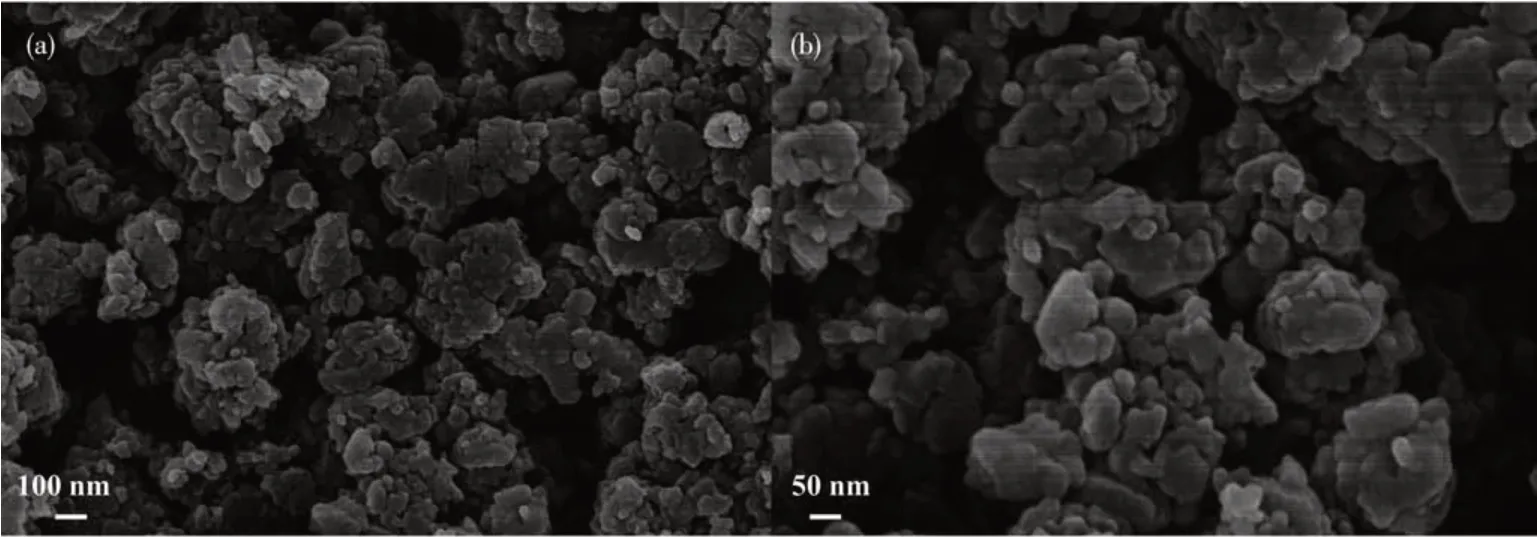
Fig.5 FESEM images of Cu-BD after grinding
2.3 Electrochemical performance of Cu-BD
To explore the lithium storage performance of Cu-BD, we tested the charge-discharge curves of halfcell with Cu-BD as the cathode material in the voltage range of 1.5-4.0 V. At a current density of 0.05 A·g-1,it can be observed from Fig.6a that the Cu-BD electrode could deliver an initial discharging specific capacity of 116.6 mAh·g-1and an initial charge specific capacity of 112.1 mAh·g-1. Based on the chargedischarge curves (Fig.6a), the average discharge voltage was calculated to be 2.1 V. Fig.6b exhibits the cycle performance of the Cu-BD electrode. As seen from Fig.6b,in the first 10 cycles,the discharge-specific capacity declined rapidly, owing to the occurrence of side reactions and the formation of the solid electrolyte interface (SEI) film in the initial charging-discharging process,but after that,the capacity tends to stable, and after 100 cycles, the capacity was stable at 50 mAh·g-1, which is comparable to that of some reported CP/MOF-based cathodes (Table S4). At the same time,Coulombic efficiency was maintained at 100%, as shown in Fig.6b. It can be also found from Fig.6a that the discharge-specific capacity had almost no change in the 50-100 cycles, reflecting a better cycle stability.We further tested the cycling performance of the Cu-BD electrode at a current density of 0.1 A·g-1(Fig.S1)and found that the Cu-BD electrode exhibited good cycling stability at a higher current density.The chargedischarge performance of the Cu-BD electrode at different current densities shown in Fig.6c and 6d reveals that its discharge capacity gradually decreased from 124.4 mAh·g-1to 52.5, 28.1, 23.2, and 17.0 mAh·g-1when the current density increases from 0.05 A·g-1to 0.1, 0.3, 0.5, and 1 A·g-1. When the current density returned to 0.05 A·g-1, its discharge capacity also returned to 78.7 mAh·g-1, indicating that the Cu-BD electrode had better rate performance and stability.
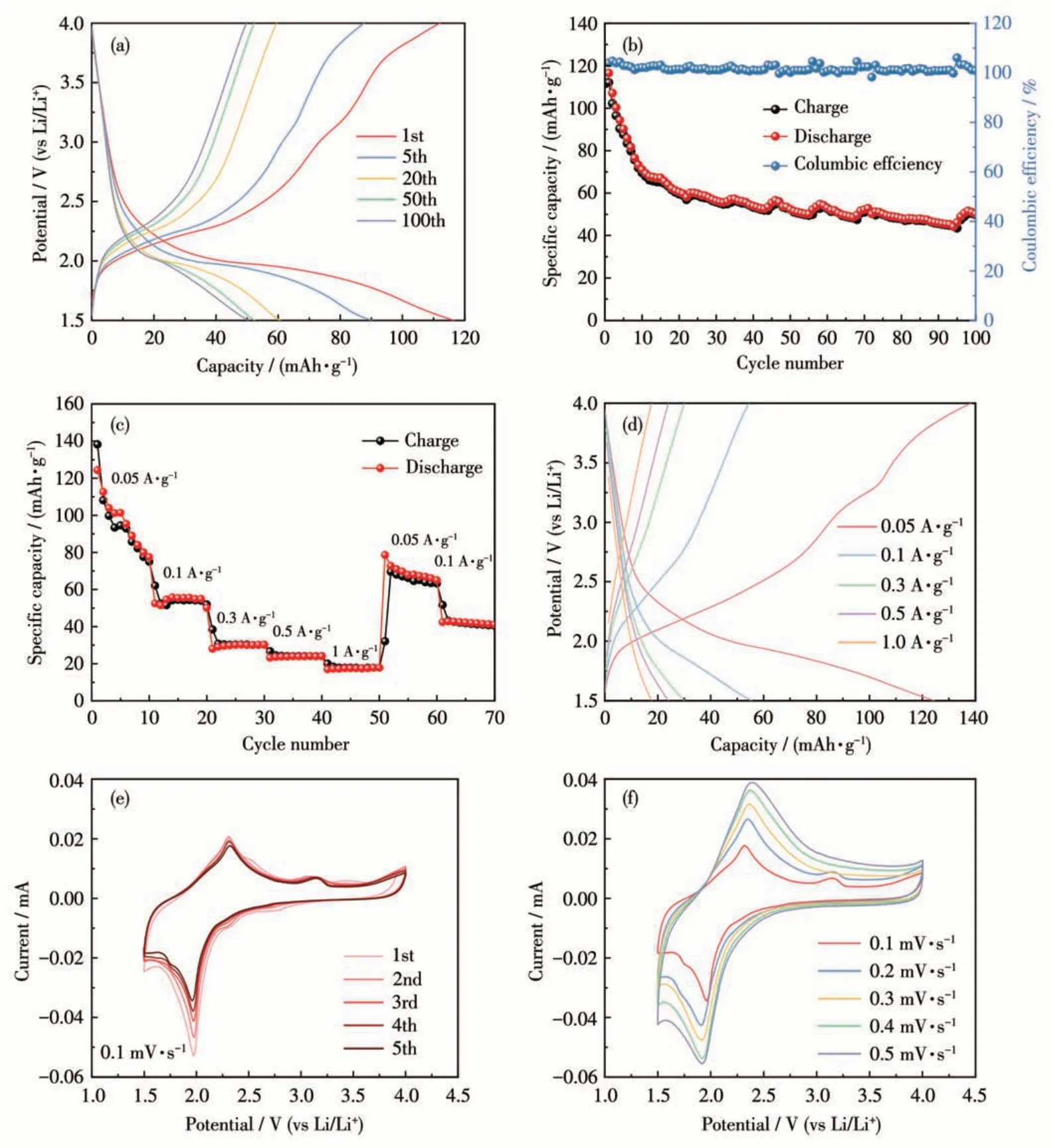
Fig.6 Electrochemical performance of the Cu-BD electrode:(a)charge-discharge plots at 0.05 A·g-1;(b)cycling performance at 0.05 A·g-1;(c)rate performance;(d)charge-discharge plots at different current densities;(e)CV curves at 0.1 mV·s-1;(f)CV curves at different scan rates
To further investigate the redox behavior and the charge transfer resistance of the Cu-BD electrode, we tested its CV and EIS, respectively. The CV curves of the Cu-BD electrode at 0.1 mV·s-1are presented in Fig.6e. As seen in Fig.6e, two weak reductive peaks and one strong reductive peak appeared in the first cycle; the peak at 2.8 V may be ascribed to the reduction from Cu(Ⅱ)to Cu(Ⅰ),the two peaks at 2.3 and 2.0 V may belong to the lithiation of C=O groups from BGPD2-,revealing two Li+ions can insert into one BGPD2-;while the strong oxidation peak at 2.3 V and weak oxidation at 2.5 V may belong to the extraction of Li+ions from the Cu-BD electrode and peak at 3.1 V may be attributed to the oxidation from Cu(Ⅰ)to Cu(Ⅱ)[39-41]. From the second cycle to the fifth cycle, the CV curves were almost identical, demonstrating that the Cu-BD electrode had good stability and reversibility. Fig.6f shows the CV curves of the Cu-BD electrode at different sweep speeds (0.1-0.5 mV·s-1). From Fig.6f, it can be observed that the oxidation peak and reduction peak slightly shift to the higher potential direction and the lower potential direction, respectively, as the scan rate increased, indicating weak polarization of the Cu-BD electrode.
Fig.7 presents the EIS of the Cu-BD electrode after 1, 50, and 100 cycles. It can be found that each Nyquist curve consists of a semicircle in the highfrequency region and a straight line in the low-frequency region. The semicircle that appeared in the high -frequency region was caused by the charge transfer resistanceRct, which is generated during the charge transfer process. Based on the equivalent circuit model(inset in Fig.7), after simulating the EIS data of different cycles with Zview software, the obtained fitting values are summarized in Table S5.Rsstands for the internal resistance of the battery and the resistance of the electrolyte solution. Meanwhile, W and CPE represent Warburg impedance and constant phase elements. It can be seen that the diameter of the semicircle after 100 cycles became smaller, compared with that of the semicircle after 1 and 50 cycles. TheRctvalue after 100 cycles was 146.0 Ω, smaller than theRctvalue after 1 and 50 cycles (345.6 and 186.5 Ω, Table S5),which indicates the Cu-BD electrode has been fully activated after 100 cycles.Rsvalue after 50 cycles(4.232 Ω) was slightly higher than the value after one cycle (3.775 Ω), and almost the same as the value after 100 cycles (4.266 Ω), indicating the Cu-BD electrode had high stability. The fact mentioned above that the Cu-BD electrode presented better cycle stability after the initial several cycles also indirectly supported this change ofRsvalue. This corresponded to the rapid decay of capacity in the initial several cycles. Moreover, the phase angles of the straight lines in the lowfrequency region were all well over 45°, confirming the higher rate of Li+ion diffusion[63].
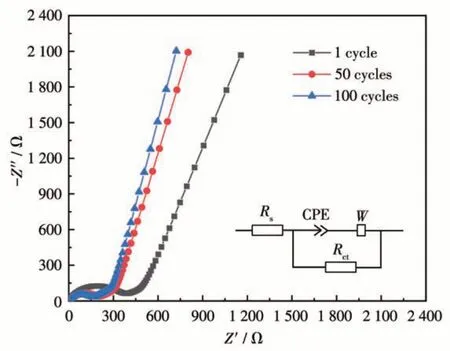
Fig.7 Nyquist plots of the Cu-BD electrode after 1,50,and 100 cycles(Inset:the equivalent circuit.)
The optical photos of Cu-BD crystals exhibited in Fig.S2 showed that they appeared a blue block crystalline morphology. To test the solubility of Cu-BD in the electrolyte, we first grind it into powder and then place it in the electrolyte. As shown in Fig.S3, we can find that after fifteen days of immersion, the Cu-BD sample and the color of the electrolyte were almost unchanged.Dismantling the coin-type battery after 80 cycles, we can observe that the surface of the electrode piece was almost undamaged,and there was no sample residue on the separator. On the contrary, H2BGPD is soluble in organic electrolytes easily (Fig.S4). The facts above mentioned imply that the Cu-BD sample had good insolubility in the electrolyte.
The diffusion coefficient of Li+ions (DLi) can be calculated according to the following formula[64-65]:
whereVmis the molar volume of Cu-BD,Frepresents the Faraday constant,Astands for the surface area of the electrode,dE/dxis the slope of the electrode potential (E) in the function of the composition (x), andσis the Warburg coefficient and is the slope of the lineZ′-ω-1/2(Fig.S5).DLivalue of the Cu-BD was 5.77×10-10cm2·s-1during the first discharge, which was higher than that of Co(Ⅱ)MOF-based electrodes reported[33].
To further investigate the electrochemical reaction mechanism of the Cu - BD electrode, we conducted XRD, FTIR, and XPS tests on the Cu-BD electrodes at different states. The XRD patterns displayed in Fig.8a showed that there was no significant change in the diffraction peak positions of the Cu-BD electrodes under different states, which demonstrates that electrochemical reactions with multiple electrons transfer did not significantly affect the skeleton structure of Cu-BD and the Cu-BD electrode owned better stability. Fig. 8b exhibited that each FTIR spectrum had a peak belonging to C=O stretching vibration at 1 718 cm-1. In the state of complete discharge,the peak intensity of C=O decreased, and when fully charged, the peak intensity of C=O increased, which corresponds to intercalation/deintercalation behavior of Li+ions, and these results revealed that Li+ions performed reversible redox reactions with C=O groups.Fig.9 exhibits the XPS spectra of Cu2pfrom the pristine Cu-BD electrode, the Cu-BD electrode after the first discharge, and after one cycle.The XPS spectrum of Cu2pfrom the pristine Cu-BD electrode showed the presence of Cu(Ⅱ)2p3/2and Cu(Ⅱ)2p1/2(935.5 and 955.2 eV, respectively) and two satellite peaks (943.1 and 963.2 eV, respectively)[41,66]. The obvious shake-up satellite peak indicates that only Cu (Ⅱ)exists in the initial sample. Whereas after the first complete discharge, the peak of Cu(Ⅰ)appeared at 932.9 eV, but a small amount of Cu (Ⅱ)still existed,indicating that partial Cu (Ⅱ)participates in the redox reaction during discharge, and after one cycle (full charged state), the intensity of peaks belong to Cu (Ⅱ)2p3/2and Cu (Ⅱ)2p1/2increased, and the peak of Cu (Ⅰ)still can be observed,meaning some Cu(I)has been oxidized to Cu(Ⅱ). Similar mechanisms have been reported in Cu - CP and Cu3(HHTP)2electrode materials for LIBs[41,67].
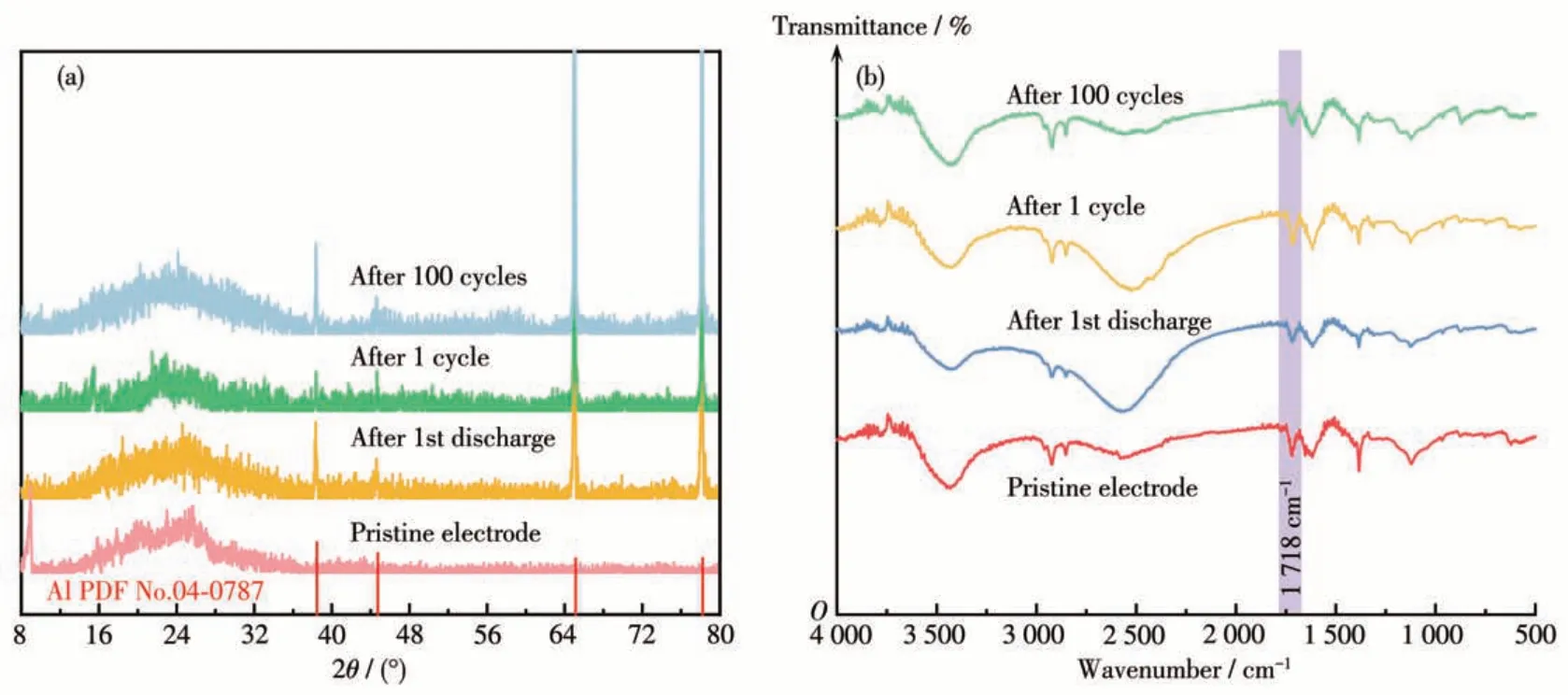
Fig.8 (a)PXRD patterns and(b)FTIR spectra of the Cu-BD electrode
Considering that both carbonyl groups and metal ions are involved in electrochemical reactions, and on the ground of the experimental facts above-mentioned,we speculate the Cu-BD might undergo the following process in the electrochemical reaction:
From Eq.2, it can be found that theoretically, 1 mol Cu-BD can store 3 mol Li+ions,of which 2 mol Li+are intercalated 1 mol BGPD2-anions (Fig.S6), and 1 mol Li+ions from 1 mol Cu (Ⅱ)ions reduced to 1 mol Cu(I) ions, leading to the [Cu (Ⅱ)(BGPD)(DMA)(H2O)](DMA) being changed to [Cu(Ⅰ)Li3(BGPD)(DMA)(H2O)](DMA). Therefore, the theoretical capacity of Cu-BD was 137.2 mAh·g-1(detailed calculation process is presented in Supporting Information), which is higher than the initial discharge capacity (116.6 mAh·g-1). More in-depth studies are needed to accurately understand the electrochemical reaction mechanism of Cu-BD.
3 Conclusions
In conclusion, we successfully synthesized a polycarbonyl 1D copper - based CP [Cu(BGPD) (DMA)(H2O)]·DMA(Cu-BD)by a simple solvent volatilization method, and first used it as cathode electrode for LIBs and investigated its electrochemical performance. The construction of Cu - BD not only introduced double active centers (Cu (Ⅱ)ions and polycarbonate ligands BGPD2-anions) but also effectively solved the dissolution problem for H2BGPD in the electrolyte. Both Cu(Ⅱ)ions and BGPD2-anions ligands take part in the redox reaction of multiple-electron transfer in the chargedischarge processes of the Cu-BD electrode,which was revealed by XPS and FTIR spectra. Although the specific capacity of the Cu-BD electrode was only maintained at 50 mAh·g-1after 100 cycles at 50 mA·g-1, it had better cycle stability after initial several cycles, thus, it will own application potential in the energy storage system of electronics with low-energy density demanding in the future. Our work also provides new insight into the construction of CP-based cathode materials with high performance for LIBs in the future.
