Mitigating the dissolution of V2O5 in aqueous ZnSO4 electrolyte through Ti-doping for zinc storage
2024-04-06ZiheWeiXuehuaWangTingZhuPingHuLiqiangMaiaLiangZhoua
Zihe Wei ,Xuehua Wang ,Ting Zhu ,Ping Hu,d,∗ ,Liqiang Maia,,d ,Liang Zhoua,,d,∗
a Hubei Longzhong Laboratory,Wuhan University of Technology (Xiangyang Demonstration Zone),Xiangyang 441000,China
b State Key Laboratory of Advanced Technology for Materials Synthesis and Processing,Wuhan University of Technology,Wuhan 430070,China
c School of Materials Science and Engineering,Wuhan Institute of Technology,Wuhan 430205,China
d Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory,Xianhu Hydrogen Valley,Foshan 528200,China
Keywords: Aqueous zinc-ion batteries V2O5 cathode materials Aqueous ZnSO4 electrolyte Yolk-shell structure Ti doping
ABSTRACT Aqueous zinc-ion batteries (AZIBs) have become a hotspot for electrochemical energy storage owing to the high safety,low cost,environmental friendliness,and favorable rate performance.However,the serious dissolution of cathode materials in aqueous electrolytes would lead to poor cyclability,which should be addressed before commercialization.Herein,we designed a Ti-doped V2O5 with yolk-shell microspherical structure for AZIBs.The Ti doping stabilizes the crystal structure and relieves the dissolution of V2O5 in aqueous ZnSO4 electrolyte.The optimized sample,Ti0.2V1.8O4.9,delivers a high capacity (355 mAh/g at 0.05 A/g) as well as good capacity retention (89% after 2500 cycles at 1.0 A/g).This work provides an effective strategy to mitigate the dissolution of cathode material in aqueous ZnSO4 electrolyte for cyclability enhancement.
Gradual replacement of fossil fuels with renewable energy is an inevitable trend for alleviating energy depletion and environmental pollution.However,most renewable energy such as solar and wind are intermittent.Developing reliable energy storage technologies is able to overcome the intermittency issue [1,2].Aqueous zinc-ion battery (AZIB) represents a competitive technology for large scale energy storage because of the high theoretical capacity(820 mAh/g) as well as low redox potential (-0.76 Vvs.standard hydrogen electrode) of Zn [3].The mild and near-neutral aqueous electrolytes enable unique advantages of non-flammability,low cost,environmental friendliness,fast charging/discharging capability,and easy assembly of AZIBs [4–7].
A series of cathode materials have been proposed for AZIBs,including manganese oxides [8–12],vanadium oxides [13–18],prussian blue analogs [19,20],and organic compounds [21,22].Among these materials,vanadium oxides attract special attentions due to the high capacity,rich crystal structures,and variable valence states.Take the layered vanadium pentoxide (V2O5) as an example,it possesses an ultrahigh theoretical capacity of 589 mAh/g [23].However,it suffers from poor cycling stability due to the dissolution of vanadium oxide in aqueous ZnSO4electrolytes [24,25].To improve the cyclability,efforts have been devoted to morphology engineering,electrolyte modification,and intercalating foreign ions/molecules into the layered structure,which are able to reduce the dissolution and stabilize the crystal structure [26–28].For example,Yanget al.found that V2O5hollow spheres outperformed commercial V2O5in cyclability and rate performance due to the higher surface area of V2O5hollow spheres [29].Temple-free yolkshell V2O5demonstrated a high capacity and ideal reversibility owing to the volume expansion alleviating ability of yolk-shell structure [30–32].Pre-intercalating various metal ions (Na+,Mg2+,Ni2+,Zn2+,etc.) and crystalline water into layered V2O5could enlarge the interlayer spacings for Zn2+transfer and storage [33–37].For example,with an enlarged interlayer spacing for Zn2+transfer,Ca2+-preintercalated V2O5manifests high capacity,decent rate performance,and long cycling life [38].In most of these studies,the improved electrochemical performances were achieved with high-price Zn(CF3SO3)2or Zn(TFSI)2electrolytes.Improving the electrochemical performance of V2O5in low-cost aqueous ZnSO4electrolyte has been rarely reported [39–41].
Herein,we synthesize Ti-doped V2O5yolk-shell microspheres(TVO) with tunable Ti content by a spray-drying method.3.0 mol/L ZnSO4is used as the electrolyte to explore the effects of Ti doping on electrochemical performance.The yolk-shell TVO mitigates the dissolution of vanadium in aqueous electrolyte and buffers the volume change during Zn2+intercalation/de-intercalation.The yolkshell Ti0.2V1.8O4.9delivers a high capacity of 355 mAh/g at 0.05 A/g.When cycled at 1.0 A/g,89% of the reversible capacity (211 mAh/g)can be retained after 2500 cycles.Thein-situandex-situcharacterizations reveal that the doping of an appropriate amount of Ti into the lattice of vanadium pentoxides stabilizes the crystal structure.Further increasing the Ti amount lowers the specific capacity due to the reduce of active material content.AZIB pouch cells based on the Ti0.2V1.8O4.9cathode and 3.0 mol/L ZnSO4electrolyte demonstrates good cyclability under deep charging and discharging conditions.Our research offered an effective means to extend the service life of AZIBs with low-cost aqueous ZnSO4electrolyte.
The TVO samples with different Ti contents are produced by a simple spray drying method with subsequent annealing in air.The Ti0.2V1.8O4.9displays an X-ray diffraction (XRD) pattern resembled to that of V2O5(Fig.1a).The characteristic peaks at 15.3°,20.2°,21.6°,26.1°,30.9°,32.3°,33.2° and 34.2° are associated with the (200),(010),(110),(101),(400),(011),(111) and (301) diffractions of the orthorhombic phase V2O5(PDF No.96–901–2221).For Ti0.5V1.5O4.75,besides the diffractions from the V2O5phase,additional obvious diffractions at 25.3° and 27.4° associated with the (101) plane of anatase TiO2(PDF No.96–900–9087) and (110)plane of rutile TiO2(PDF No.96–900–4143) can also be detected.The results indicate that doping a low content of Ti (Ti/(Ti+V)≤10%) does not change the crystal structure of V2O5,while doping too much Ti (25%) leads to the formation of residue phases.The diffraction peaks located at 19.5°–21° are enlarged in Fig.1b.For V2O5,the (010) diffraction peak is located at 20.17°.When 10% Ti is doped,the diffraction shifts to 20.29°,while it shifts back to 20.23° when the Ti content is increased to 25%.It should be mentioned that although the Ti4+(61 pm) has a larger ionic radius than the V5+(54 pm),both the Ti0.2V1.8O4.9and Ti0.5V1.5O4.75show a reduced (010) lattice spacing compared to V2O5,indicating the existence of tensile stress in the Ti-doped samples [42,43].According to the Bragg equation (d=nλ/2sinθ),the (010) lattice spacing of V2O5,Ti0.2V1.8O4.9,and Ti0.5V1.5O4.75are calculated to be 4.40,4.37 and 4.38 ˚A,respectively (Table S1 in Supporting information).The grain size of the samples can be determined by Scherrer equation (D=Kλ/βcosθ).The grain sizes for V2O5,Ti0.2V1.8O4.9and Ti0.5V1.5O4.75are determined to be 26.3,17.9 and 22.7 nm,respectively.The XRD Rietveld refinement results of Ti0.2V1.8O4.9and Ti0.5V1.5O4.75are provided in Fig.S1,Tables S2 and S3 (in Supporting information).From the Rietveld refinement results,one can know that the doped-Ti occupies the V sites.
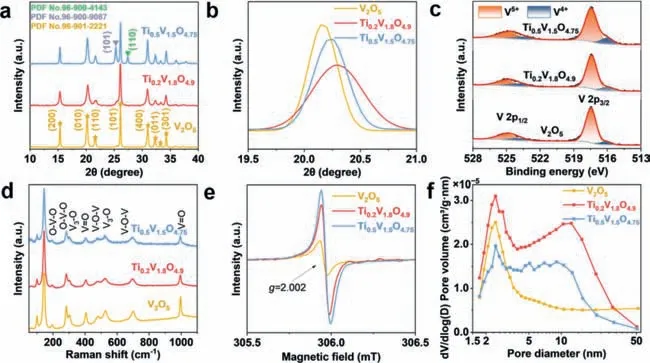
Fig.1.(a,b) XRD patterns,(c) XPS spectra,(d) Raman spectra,(e) EPR spectra,and (f) pore size distributions of V2O5,Ti0.2V1.8O4.9 and Ti0.5V1.5O4.75.
Fig.1c presents the X-ray photoelectron spectroscopy (XPS)spectra of the samples.Both V4+(515.9 and 523.8 eV) and V5+(517.4 and 525.0 eV) exist in the three samples.The V4+content is 12.43% in V2O5and it increases with the increasing of Ti amount.For Ti0.2V1.8O4.9and Ti0.5V1.5O4.75,the V4+contents are 18.14% and 22.69%,respectively.The existence of V4+suggests the formation of oxygen vacancies in the TVO samples to keep charge neutrality[44].The V2O5,Ti0.2V1.8O4.9,and Ti0.5V1.5O4.75show almost identical Raman spectra (Fig.1d).The characteristic peaks at 478 and 697 cm-1are corresponded to the bending vibration and stretching vibration of V-O-V bond.And the peaks at 405 and 994 cm-1are associated with the V=O bonds [42,45].To further study the effects of Ti doping,electron paramagnetic resonance (EPR) has been conducted.All three samples present a peak atg=2.002 (Fig.1e),suggesting the presence of oxygen vacancies.The signal increases obviously with Ti doping amount,demonstrating that the substitution of V5+with Ti4+is accompanied by the introduction of oxygen vacancies for charge compensation [46].
N2adsorption-desorption isotherms of the three samples (Fig.S2 in Supporting information) all match with IV-type isotherm with H3 hysteresis loop [47].The V2O5shows a pore size centered at ∼2.4 nm,while the Ti0.2V1.8O4.9and Ti0.5V1.5O4.75show a bimodal pore size distribution (Fig.1f).Table S4 shows the BET surface areas of the samples.The Ti0.2V1.8O4.9possesses the highest surface area (27.05 m2/g),while the V2O5and Ti0.5V1.5O4.75show surface areas of 18.54 and 15.85 m2/g,respectively.As for the pore volume,the V2O5,Ti0.2V1.8O4.9and Ti0.5V1.5O4.75show pore volumes of 0.10,0.08 and 0.04 cm3/g,respectively.
Scanning electron microscope (SEM) images show that the obtained V2O5(Fig.2a and Fig.S3a in Supporting information)has a porous spherical structure built up with interconnected nanoparticles with sizes of 50–100 nm.Between the interconnected nanoparticles,there are rich large pores with sizes of tens of nanometres.From a broken microsphere,a porous yolk can be observed below the porous shell,demonstrating the yolk-shell structure.Transmission electron microscopy (TEM,Fig.2b) further verifies the yolk-shell structure of V2O5.High-resolution TEM(HRTEM,Fig.2c) image shows clear lattice fringes from the (010)planes of orthorhombic V2O5.High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image and the corresponding elemental mappings show that the yolk of yolkshell V2O5contains a hollow cavity in the center.Energy dispersive X-ray spectroscopy (EDS) elemental mappings (Fig.2d) show that the V and O elements distribute homogeneously in the yolk-shell microspheres.
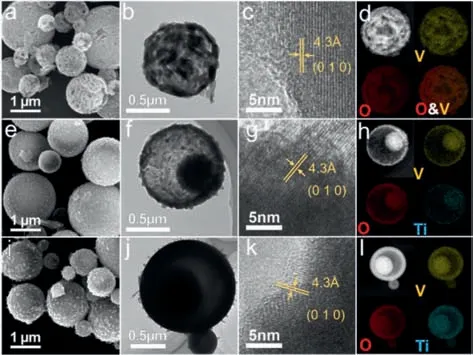
Fig.2.(a) SEM image,(b) TEM image,(c) HRTEM image,and (d) EDS mappings of V2O5.(e) SEM image,(f) TEM image,(g) HRTEM image,and (h) EDS mappings of Ti0.2V1.8O4.9.(i) SEM image,(j) TEM image,(k) HRTEM image,and (l) EDS mappings of Ti0.5V1.5O4.75.
With the introduction of 10%–25% of Ti,the yolk-shell structure of the samples can be well maintained (Figs.2e,f,i and j),while the large mesopores on the surface disappear.With the introduction of 10% Ti,the grain size of the sample decreases noticeably (Fig.S3b in Supporting information),agreeing well with the XRD results.Further increasing the Ti content from 10% to 25%,the grain size increases obviously (Fig.S3c in Supporting information),which is responsible for the decrease of surface area.Different from the yolk-shell V2O5which possesses a hollow cavity in the yolk (Fig.2b),both the Ti0.2V1.8O4.9and Ti0.5V1.5O4.75possess a solid spherical yolk (Figs.2f and j).HRTEM confirms the high crystallinity of both Ti0.2V1.8O4.9(Fig.2g) and Ti0.5V1.5O4.75(Fig.2k).EDS elemental mappings (Figs.2h and l) show that the Ti distribute homogeneously in the yolk-shell microspheres,demonstrating the successful doping.
To study the effect of Ti doping on the electrochemical performance,charge-discharge tests were performed in CR-2025 type coin cell with mild 3.0 mol/L ZnSO4electrolyte.Representative cyclic voltammetry (CV) curves of the samples are presented in Fig.S4 (Supporting information).The non-overlapping feature of CV profiles and gradual increasing of enclosed CV area indicate that all three samples experience a similar activation process.The transformations in peak position and peak intensity can be ascribed to the structure evolution of V2O5to ZnxV2O5∙nH2O during the cycling,which is common for vanadium oxide based cathode materials [48–50].After five CV cycles,all three samples show two pairs of redox peaks at ∼0.58/0.78 V and 0.87/1.15 V (Fig.3a).
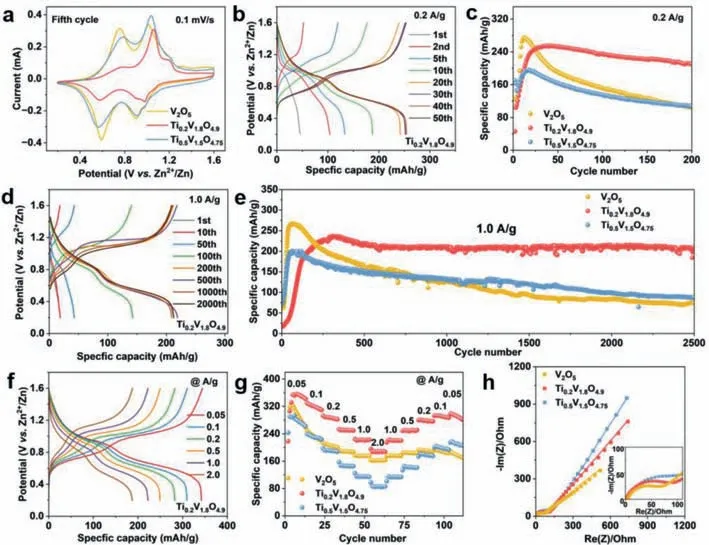
Fig.3.(a) The 5th cycle CV curves of V2O5,Ti0.2V1.8O4.9 and Ti0.5V1.5O4.75 at 0.1 mV/s.(b) GCD profiles of Ti0.2V1.8O4.9 at 0.2 A/g.(c) Cycling performance of V2O5,Ti0.2V1.8O4.9 and Ti0.5V1.5O4.75 at 0.2 A/g.(d) GCD curves of Ti0.2V1.8O4.9 at 1.0 A/g.(e) Cycling performances of V2O5,Ti0.2V1.8O4.9 and Ti0.5V1.5O4.75 at 1.0 A/g.(f) GCD curves of Ti0.2V1.8O4.9 at different current densities.(g) Rate performances of V2O5,Ti0.2V1.8O4.9 and Ti0.5V1.5O4.75.(h) EIS plots of V2O5,Ti0.2V1.8O4.9 and Ti0.5V1.5O4.75.
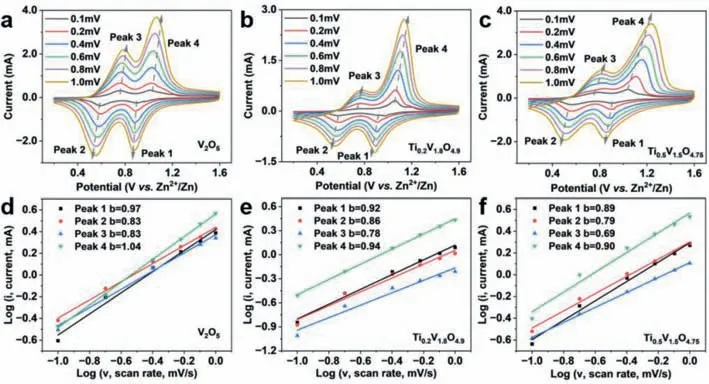
Fig.4.The CV curves of (a) V2O5,(b) Ti0.2V1.8O4.9 and (c) Ti0.5V1.5O4.75 at different scan rates.log(i) vs. log(v) plots of (d) V2O5,(e) Ti0.2V1.8O4.9,(f) Ti0.5V1.5O4.75.
The galvanostatic charge discharge (GCD) curves of V2O5,Ti0.2V1.8O4.9and Ti0.5V1.5O4.75at 0.2 A/g are presented in Fig.3b and Fig.S5 (Supporting information),and the cyclic performances of V2O5,Ti0.2V1.8O4.9,and Ti0.5V1.5O4.75are presented in Fig.3c.Agreeing with the CV profiles,all three samples experience an activation process during cycling (Fig.3c).The activation process is caused by the gradual structural evolution from layered V2O5to layered ZnxV2O5·H2O with expanded interlayer spacings [51–53].After the activation process,the Ti0.2V1.8O4.9displays two discharge plateaus at ∼0.6 and 0.9 V,corresponding to the Zn2+intercalation.Upon cycling,the capacity of Ti0.2V1.8O4.9increases gradually until it reaches at ∼250 mAh/g after ∼25 cycles,after which the capacity decreases slightly.After 200 cycles at 0.2 A/g,the capacity retains at 211 mAh/g.Both the Ti0.5V1.5O4.75and V2O5show quicker capacity fading after the activation process.When cycled at 1.0 A/g for 2500 cycles,the capacity retention of Ti0.2V1.8O4.9reaches 89.4% against the highest capacity,which is much higher than those of V2O5(27.9%) and Ti0.5V1.5O4.75(43.9%) (Figs.3d and e,Fig.S6 in Supporting information).
The GCD curves of V2O5,Ti0.2V1.8O4.9and Ti0.5V1.5O4.75under a serious of current densities (0.05–2.0 A/g) are shown in Fig.3f and Fig.S7 (Supporting information).The discharge capacities of Ti0.2V1.8O4.9reach 341,309,282,249,222 and 187 mAh/g at 0.05,0.1,0.2,0.5,1.0 and 2.0 A/g,respectively.When the current density returns to 0.05 A/g,the capacity can be recovered to 298 mAh/g(Fig.3g).Compared to the Ti0.2V1.8O4.9,the V2O5and Ti0.5V1.5O4.75exhibit lower capacities at the same current densities.The above results suggest that a proper amount of Ti doping can boost the cyclability as well as rate performance of V2O5.
Fig.3h presents the electrochemical impedance spectroscopy(EIS) plots and the equivalent circuit is shown in Fig.S8 (Supporting information).TheRsandRctstands for the solution resistance and the charge transfer resistance between the electrode/electrolyte interface,respectively.TheRctvalues of V2O5,Ti0.2V1.8O4.9and Ti0.5V1.5O4.75are 54,85 and 119Ω,respectively.
Vanadium oxide based cathode materials generally suffer from serious dissolution in aqueous electrolytes,which causes rapid capacity decay upon cycling.To study the dissolution of active materials in electrolyte,the cathode slices are immersed in 3.0 mol/L ZnSO4aqueous solution,where the dissolution of vanadium based oxides can be told from the color change of the electrolyte (Fig.S9 in Supporting information).After 14 days,the solution with V2O5change into light yellow,suggesting the dissolution of V2O5in aqueous ZnSO4.However,the solution with Ti0.2V1.8O4.9shows a lighter color,suggesting its mitigated dissolution.
Figs.4a-c show the CV curves of V2O5,Ti0.2V1.8O4.9,and Ti0.5V1.5O4.75at different scan rates.According to power law equation about the relationship between the current (i) and the scanning rate (v) [54–57]:
whereaandbare adjustable parameters.When the value ofbis close to 0.5,the electrochemical reaction is diffusion-controlled,while ifbis close to 1.0,it indicates a capacitive-controlled process [58,59].Thebvalues of the four redox peaks for V2O5are in the range of 0.83–1.04,while thebvalues for Ti0.2V1.8O4.9and Ti0.5V1.5O4.75are in the ranges of 0.78–0.94 and 0.69–0.90,respectively (Figs.4d-f).The highbvalues (closed to 1) of all three samples suggest the capacitive-controlled electrochemical processes.With the introduction of Ti,thebvalue displays a decreasing trend,demonstrating that the Ti doping enhances the diffusion contribution.
To further study the structure transformation of Ti0.2V1.8O4.9during the charge and discharge process,ex-situXPS,in-situXRD,andin-situRaman are conducted (Fig.5).In discharged state,the high-resolution V 2p spectrum present three components from V(V),V(IV) and V(III) [60,61],with percentages of 23.68%,61.91%,and 14.36%,respectively.In charged state,only V(V) and V(IV) exist in the sample and their percentages are 49.01% and 50.91%,respectively (Fig.5a).For the high-resolution Zn 2p spectra,the sample in discharged state show much stronger peaks than that in charged state (Fig.5b).The results indicate that the Zn2+is inserted into the Ti0.2V1.8O4.9accompanied by the reduction of V species during discharge and the Zn2+is extracted from the material accompanied by the oxidation of V species during charge process.
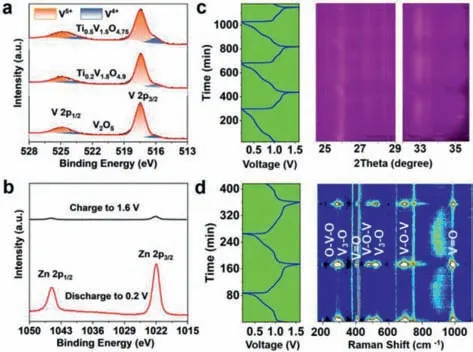
Fig.5.(a) V 2p and (b) Zn 2p ex-situ XPS spectra of the Ti0.2V1.8O4.9 recorded at different electrochemical states.(c) In-situ XRD pattern and (d) in-situ Raman spectra of the Ti0.2V1.8O4.9 recorded at 0.1 A/g.
Fig.5c presents thein-situXRD pattern,from where the (101),(011) and (301) diffractions are observed at 25.9°,32.9° and 34.9°.During the discharge/charge processes,the diffraction peaks vary in intensity periodically,while the peak position shows negligible change.Thein-situXRD results demonstrate that the Zn2+is intercalated into and extracted from the Ti0.2V1.8O4.9highly reversibly.Fig.5d presents thein-situRaman spectra.The O-V-O (193,283 cm-1),V3-O (303,524 cm-1) and V-O-V (478,697 cm-1) bands weaken gradually during discharge and almost disappear when the potential reaches ∼0.87 V and below.During charge,the V3-O and V-O-V bands reappear when the voltage reach over ∼1.2 V.For the V=O bands (994 cm-1),it shows a same regular intensity change and shift towards lower wavenumber slightly during discharge and moves back during charge.The periodic change in thein-situRaman spectra demonstrates the excellent reversibility of Ti0.2V1.8O4.9upon Zn2+intercalation/extraction.
To verify the application potential,a pouch cell was assembled with Ti0.2V1.8O4.9cathode and 3.0 mol/L ZnSO4electrolyte.It shows a capacity of 236 mAh/g and 95.7% capacity retention after 100 cycles at 0.1 A/g (Fig.S10 in Supporting information).These results demonstrate that doping an appropriate amount of Ti effectively enhances the cycling stability of V2O5cathode materials in aqueous ZnSO4electrolyte.Considering the good zinc storage performances in both coin cells and pouch cells,the Ti0.2V1.8O4.9cathode material shows a bright application prospect in AZIBs.
In conclusion,we synthesized a series of yolk-shell structured titanium vanadium oxides with different Ti content by a simple spray drying method.Doping 10% of Ti reduces the lattice spacing of V2O5but does not alter the crystal structure.Introducing 25% of Ti leads to the formation of anatase/rutile residue phases.With the doping of an appropriate amount of Ti,the dissolution of V2O5in aqueous ZnSO4electrolyte can be alleviated,leading to improved cyclability.When employed as the cathode material for ZIBs,the optimized material,Ti0.2V1.8O4.9,demonstrates not only high capacity (355 mAh/g) but also ideal cyclability (89% after 2500 cycles,1.0 A/g) in aqueous ZnSO4electrolyte.Ex-situXPS,in-situXRD,andin-situRaman characterizations demonstrate the Zn2+intercalation/de-intercalation is highly reversible in the Ti0.2V1.8O4.9.This work provides an effective strategy to mitigate the dissolution issue of cathode material in aqueous electrolytes by transition metal doping.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.52102299),the Independent Innovation Project of Hubei Longzhong Laboratory (No.2022ZZ-18),the Guangdong Basic and Applied Basic Research Foundation (No.2021A1515110059),and the Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory (No.XHT2020-003).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108421.
杂志排行
Chinese Chemical Letters的其它文章
- Rocking-chair ammonium ion battery with high rate and long-cycle life
- Graphdiyne scaffold anchored highly dispersed ruthenium nanoparticles as an efficient cathode catalyst for rechargeable Li–CO2 battery
- Cost-effective natural graphite reengineering technology for lithium ion batteries
- Alkyl-thiophene-alkyl linkers to construct double-cable conjugated polymers for single-component organic solar cells
- Modulating the p-band center of carbon nanofibers derived from Co spin state as anode for high-power sodium storage
- The compatibly large nonlinear optical effect and high laser-induced damage threshold in a thiophosphate CsInP2S7 constructed with[P2S7]4- and [InS6]9-
