Cost-effective natural graphite reengineering technology for lithium ion batteries
2024-04-06PeiLiuHonginWngToHungLiewuLiWeiXiongSholunHungXingzhongRenXiopingOuyngJingtoHuQinlingZhngJinhongLiu
Pei Liu ,Hongin Wng ,To Hung ,Liewu Li ,Wei Xiong ,Sholun Hung ,Xingzhong Ren,Xioping Ouyng,c,Jingto Hu,∗,Qinling Zhng,∗,Jinhong Liu,,∗
a Graphene Composite Research Center,College of Chemistry and Environmental Engineering,Shenzhen University,Shenzhen 518060,China
b Shenzhen Eigen–Equation Graphene Technology Co.,Ltd.,Shenzhen 518000,China
c School of Materials Science and Engineering,Xiangtan University,Xiangtan 411105,China
Keywords: Natural graphite Reengineering technology Liquid-polyacrylonitrile Lithium ion batteries High performance
ABSTRACT Graphite tailings produced by natural graphite is usually regarded as garbage to be buried underground,which would result in a certain waste of resources.Here,in order to explore the utilization of natural graphite tailings (NGT),a liquid-polyacrylonitrile (LPAN) is used to modify the NGT fragments and aggregate them together to form secondary graphite particles with low surface area and high tap density.Moreover,the modified NGT show much better electrochemical performances than those of original one.When tested in full cells coupled with NMC532 cathode,the material achieves a high rate capability and cycle stability at the cutoff voltage of 4.25 V as well as 4.45 V,which maintains 84.32% capacity retention after 500 cycles at 1 C rate (4.25 V),higher than that of the pristine one (73.65%).The enhanced performances can be attributed to the use of LPAN to create a unique carbon layer upon graphite tailings to reconstruct surface and repair defects,and also to granulate an isotropic structure of secondary graphite particles,which can help to weaken the anisotropy of Li+ diffusion pathway and form a uniform,complete and stable solid-electrolyte-interface (SEI) on the surface of primary NGT fragments to promote a fast Li+ diffusion and suppress lithium metal dendrites upon charge and discharge.
Strategies have been made to whittle down the adoption of fossil energy and promote the development of global low-carbon economy to step into a new energy era as early as possible [1].Lithium ion batteries (LIBs) as one of the promising techniques with high capacity,high energy-density,less memory effect and slower self-discharge [2],have experienced swift and violent development in the past decades,but this is just the beginning,and the social demands would be more staggering in view of the everincreasing needs for electric vehicles and renewable grid-level energy storage [3–5].Graphite remains the major choice of anode in commercial LIBs by means of its high capacity (372 mAh/g),low working voltage (∼0.1 Vvs.Li/Li+),and long cycle life [6–8],and the demand would increase substantially with further development of LIBs,leading to a high-cost proportion when considering its limited resource and/or sluggish production capacity[9].In view of the rapid increasing in the number of LIBs and scrapped LIBs,waste graphite recycling has attracted much attention in both academia and industries [10–13],which can make the waste graphite re-adopted as adequate anode materials for LIBs and even processed into high-performance graphene [14].
Graphite can be normally divided into two types,artificial graphite (AG) and natural graphite (NG).AG is a kind of graphite produced under a high temperature calcination condition,which is quite different from NG formed by the extreme power of nature with long-term high temperature and high pressure geological environments [15].By means of the outstanding structural stability,AG performs better than NG in function batteries,which can be mainly attributed to the easy surface exfoliation and particle cracking of NG during the repeat cycling [16,17].However,when taking material cost,specific capacity and material manufacture in practical applications into consideration,NG is superior to AG.It is noticed that the market occupancies of both AG and NG experience rapid increase in the past decade.For instance,the proportion of NG is increasing year by year (Fig.1a),which accounts for 39%of the whole anode material market compared with 58% of AG in 2020,and it will further increase up to 49% in 2030 higher than that of AG (41%) expectedly [15].The NG mined in the Earth’s crust is defective in structure and massive in volume,so it cannot be used directly,and the manufacturing procedures including crushing,granulation,washing,graphitization,particle screening,etc.are required.However,during these processes,especially for the granulation,there produce a lot of tailings wastes,which are usually thrown away,resulting in resource waste and environmental burden [18].Thus the reutilization of nature graphite tailings (NGT)can contribute to the pressure relief of graphite market and the material cost reduction of LIBs [19].
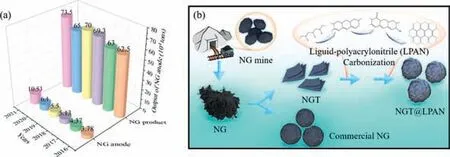
Fig.1.(a) Total production of natural graphite and its market occupancies in anode materials in recent years.(b) Schematic illustration of NGT production and synthesis diagram of NGT@LPAN.
Surface carbon coating is an effective way to improve the electrochemical performance of graphite anode,which can suppress the parasitic reactions with organic electrolyte and promote the formation of dense solid electrolyte interface (SEI) on the surface,thus achieving superior cyclability and rate performance [20,21].However,as to NGT,it is hard to produce a high tap-density graphite anode by traditional carbon coating treatment due to the ultra-high surface area.The large contact area with electrolyte would increase the first irreversible capacity,and the processing of slurry fabrication is also full of difficulties and challenges,making it stumbling to scale up.Hence,it would be significant to develop a unique method to realize carbon coating on primary particle,defect repairing,gap filling and secondary granulation of NGT fragments at one time to improve the processability and electrochemical performances.
In this work,a unique and polyfunctional liquidpolyacrylonitrile (LPAN) was exploited as carbon source for the recycle of NGT.Benefiting from polyfunctional structure of LPAN polymer,a tight coating layer was formed on the primary NGT particle to modify surface and repair defects after carbonization,and a secondary graphite particle with low surface area and high tap density was constructed.The material shows superior electrochemical performances than the origin one in the aspects of first discharge capacity (400.33 mAh/g) and Coulombic efficiency (92.07%) in half cells.When coupled with NMC532(Ni:Mn:Co=5:3:2) cathode,the graphite||NMC532 full cell exhibits stable cycling performance with a capacity retention of 84.32% after 500 cycles at 1 C rate.
Graphite is a common anode material and widely used in commercial LIBs,in which NG has attracted more attention recently due to the price advantage as compared to artificial one.NG exploited from Earth’s crust is defective in structure and massive in volume,which has to undergo steps of crushing,granulation and particle screening before used as commercial NG anode in LIBs.During these processes,large amounts of NGT are generated,which are always considered as garbage to be buried underground.In view of the ever-increasing demands for LIBs,NGT resource needs to be utilized to avoid waste of resources.Based on this,NGT with large surface area is reengineered using a unique liquidpolyacrylonitrile (LPAN) as carbon source (Fig.1b).LPAN is purchased from Shenzhen Eigen-Equation Graphene Technology Co.,Ltd.,which is polyfunctional (nitrile,oxygen-contained groups,etc.)and can be well compatible with NGT for close contact,thus capable of modifying the surface and repairing the defects of NGT after carbonization.Apart from this,LPAN is unique and easy to be graphited after pre-oxidation at 220 °C in muffle furnace,which tends to undergo denitrification reaction to bridge chainlike neighbor molecules and form a 2D-structured carbon plane with high conductivity.
NGT@LPAN was synthesized by mixing NGT with LPAN followed by spray drying and carbonization.Three samples with different LPAN addition amounts of 7%,10% and 15% were compared here with NGT,named as NGT@LPAN-7%,NGT@LPAN-10% and NGT@LPAN-15%,respectively.The specific surface area of the sample decreases with the rise of LPAN amount (Fig.2a,Fig.S1 and Table S1 in Supporting information),accompanied by the increase of tap density (Fig.2a),illustrating the addition of LPAN can help to optimize the microstructure features of NGT,which would be beneficial to the processability and electrochemical performances substantially.According to the first charge/discharge curves,NGT@LPAN-10% shows both high discharge capacity (400.33 mAh/g) and Coulomb efficiency (CE,92.07%),which is the best choice as compared to other three samples and will be used for the following studies.The capacities and CEs of NGT,NGT@LPAN-7% and NGT@LPAN-15% are 403.17,394.94,388.93 mAh/g and 89.05%,91.63%,92.16%,respectively (Fig.2b).Although origin NGT exhibits the highest first discharge capacity of 403.17 mAh/g,corresponding first CE is the lowest (89.05%) of all,indicating a more irreversible capacity for NGT caused by more side reaction with electrolyte due to the large surface area and defective structure.From the scanning electron microscopy (SEM) images shown in Figs.2c and d and Fig.S2 (Supporting information),it can be seen that origin NGT presents a monodisperse sheet-like morphology with sizes of several micrometers,while NGT@LPAN mainly presents a compact spherical particle morphology (∼5 μm) with some graphite fragments.Small amounts of graphite fragments may be conducive to the rise of compact density for electrode.Further TEM images show that there exists lots of defect in NGT structure (Fig.2e),which almost disappears after carbon coating (Fig.2f).Moreover,a thin carbon layer with thickness of 1–2 nm emerges on the graphite surface of NGT@LPAN as compared to NGT (Figs.2e and f).The surface carbon layer can passivate the active surface(outer surface and internal defect) of graphite with organic electrolyte to prevent graphite layers from Li+ions co-embedding with organic solvents during the discharging process,and reduce the electrolyte decomposition to form SEI film more effectively,thus exhibiting higher first CE and gram capacity,and also improved cycling performances.X-ray diffraction (XRD) and Raman results(Figs.S3 and S4 in Supporting information) show that layered graphite structure (PDF#41–1487),crystallinity and graphitization degree are almost unchanged after carbon coating treatment.Overall,large numbers of structure gaps and defects (between flake layers and even through some of them) exist in the NGT,making larger active graphite surface exposed to the electrolyte for more side reaction.After carbon coating modification,these gaps and defects would disappear,as can be reflected by the reduced surface area of NGT@LPAN,indicating that LPAN could not only modify the surface of graphite,but also penetrate into the interior and fill up the gaps and defects during carbonization process.
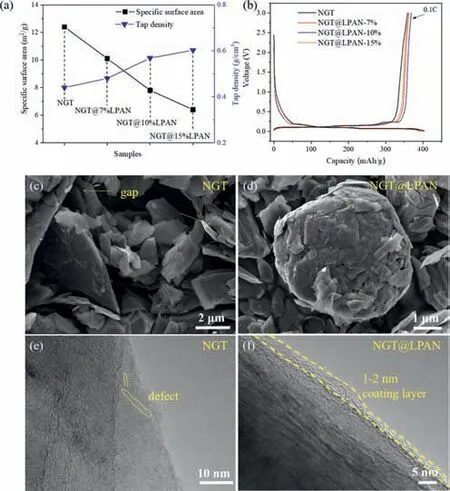
Fig.2.(a) Specific surface area and tap density of NGT with different LPAN coating contents.(b) Charge/discharge curves of NGT with different LPAN coating contents.SEM images of (c) NGT and (d) NGT@LPAN.TEM images of (e) NGT and (f)NGT@LPAN.
In order to make clear the carbon coating modification on the affection of electrochemical performances,full cells coupled with NMC532 were assembled and tested under the voltage range of 2.7–4.25 V (Fig.3).The cells were activated at 0.1 C and then operated at 0.33 C for 100 cycles.It is clear that NGT@LPAN cell shows a good cycle life with no capacity loss with top capacity of 150.1 mAh/g,which is much better than that of NGT with capacity loss from 144.9 mAh/g to 125.8 mAh/g (capacity retention of 86.82%) (Fig.3a).Further increasing the rate to 1 C for 500 cycles,the cycle life of NGT@LPAN is also superior to that of NGT,with capacity retention of 84.32% (from 129.5 mAh/g to 109.2 mAh/g)vs.73.65% (from 124.5 mAh/g to 91.7 mAh/g) (Fig.3b).The result can also be confirmed by comparing the charge-discharge curves of 25th,50th,100th,250thand 500th,showing that the improvement of charge/discharge capacities is remarkable (Figs.3c and d).The improved cycling performance for NGT@LPAN as relative to NGT is closely in connection with the carbon modification on the graphite surface and the defect repairing inside,which make the material compatible well with the electrolyte to form a uniform and dense SEI film on the active surface,preventing from the coembedding of solventized lithium ions and the solvent reduction to strip the structure layer and gas generation.Moreover,the complete and compact carbon layer formed on the surface and filled in the gaps can play a key role in buffering the expansion and contraction of graphite layer structure caused by Li+(de)intercalation,which makes the structure of primary graphite particles more stable,thus improving the cycling performance accordingly.
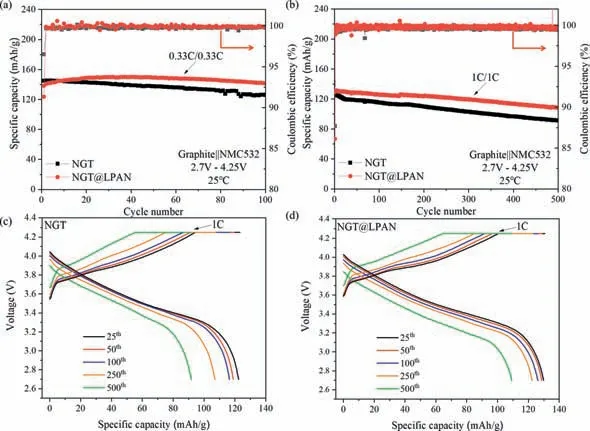
Fig.3.Cycle performances of NGT and NGT@LPAN at the voltage range of 2.7–4.25 V,and the corresponding rates are (a) 0.33 C and (b) 1 C.Before cycling,the prepared cells were firstly tested at C/10 for the initial formation cycles.Charge/discharge curves of (c) NGT and (d) NGT@LPAN.
To further study the deeper charge and discharge ability of the materials,full cells test with higher cutoff voltage of 2.7–4.45 V was also carried out (on condition that others are same with 2.7–4.25 V) (Fig.4a).It turned out as expected that NGT@LPAN is better than NGT in both capacity and cycle stability.To clarify the reason of difference in performance,full cells with NGT and NGT@LPAN cycled for 60 cycles were disassembled and analyzed.It can be seen that there presents a gray substance with large area on the surface of NGT electrode,while such substance is undetected on the electrode of NGT@LPAN (Figs.4b-d).The gray substance can be explained by the lithium metal deposition,which has a great impact on capacity decay and even on the safety issues of cells [22].It can be concluded that NGT modified with a carbon layer can help to suppress the generation of lithium metal dendrites.There may be two reasons for the result: One is the anisotropy weakening after granulation,the other is the SEI film enhancement.Further comparative characterization on SEI film is also done.Clearly,SEI film formed on NGT@LPAN is dense,uniform and continuous with typical thickness of 5 nm,while it is not continuous in NGT particle (part of the graphite is almost exposed to the electrolyte)(Fig.4e).A complete coating of SEI film on NGT surface can passivate the active surface of graphite to prevent sustained electrolyte decomposition for side reaction during cycling,which contributes to a higher capacity retention (Fig.4f and Fig.S5 in Supporting information).Besides the micro morphologies,the chemical components of SEI film are also important to the electrochemical performances,so X-ray photoelectron spectroscopy (XPS) test is performed here (Figs.4g-k,Figs.S6 and S7 in Supporting information).In general,the more the decomposition of organic electrolyte is,the higher the content of Li2CO3will be [23–25].According to the XPS analysis (Fig.4h),the content of Li2CO3is 2.70% in NGT as fitted from C 1s spectrum,which is higher than that in NGT@LPAN (1.07%),and a similar trend of Li2CO3content is also observed in O 1s spectrum,confirming the side reaction between graphite and electrolyte is significantly inhibited after carbon modification.LiF is another important component for SEI film,and the SEI film rich in LiF has the function of stabilizing structure and improve the electrochemical performances [26–28].In contradiction with the trend of Li2CO3content,LiF content in NGT (18.15%)is less than that in NGT@LPAN (25.98%),indicating a more stable SEI film formed on the surface of NGT@LPAN.The P 2p spectrum shows that the lithium hexafluorophosphate (LiPFxand LixPOyFz)decomposited mainly from LiPF6are almost consistent in content.The reason for the better SEI film component and amount of NGT@LPAN can be explained by the reaction activity inhibition of NGT surface and defect with the organic electrolyte by interface reconstruction and defect repairing after carbon modification.It can be concluded that the post-generated carbon modifier on NGT surface formed from unique LPAN cannot only aggregate the graphite fragments together for secondary granulation to ameliorate surface area and tap density and enhance the isotropy of Li+ion diffusion,but also modify the NGT surface and repair the defects to optimize the electrolyte decomposition and form a complete SEI film with high stability for superior electrochemical performances[29,30],thus capable of recycling NGT for waste reuse to lower the material cost of commercial LIBs.
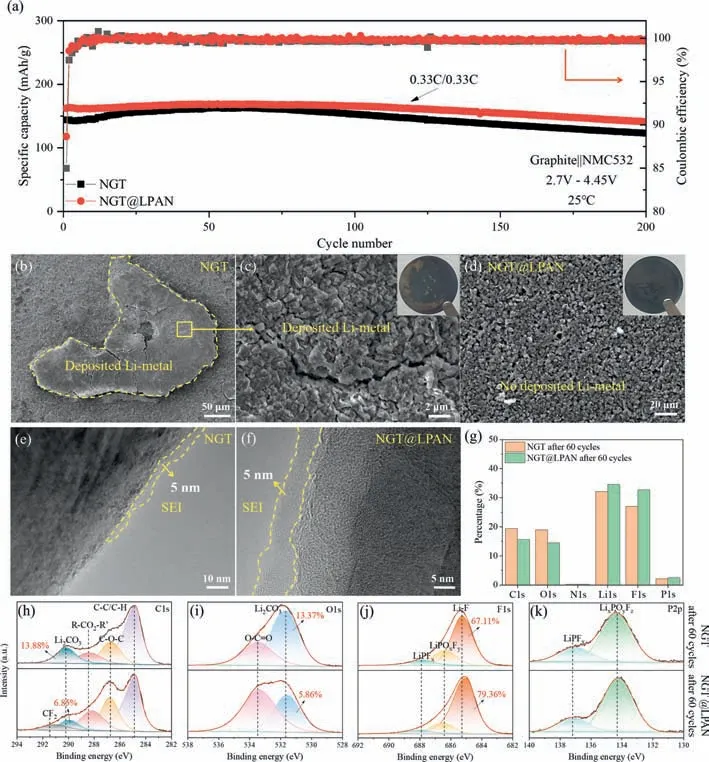
Fig.4.(a) Cycle performances of NGT and NGT@LPAN at 0.33 C under 2.7–4.45 V.Before cycling,the prepared cells were firstly tested at C/10 for the initial formation cycles.SEM images of (b,c) NGT and (d) NGT@LPAN after 60 cycles with the cutoff voltage of 4.45 V.TEM images of (e) NGT and (f) NGT@LPAN.(g) The surface element percentages for the cycled NGT and NGT@LPAN electrodes collected from XPS data.XPS spectra of (h) C 1s,(i) O 1s,(j) F 1s and (k) P 2p for the cycled NGT and NGT@LPAN electrodes.
In summary,a unique LPAN with polyfunctional groups is used to reengineer NGT to form secondary graphite particles with low surface area and high tap density.NGT@LPAN shows much better electrochemical performances than the origin one in first discharge capacity (400.33 mAh/g) and coulombic efficiency (92.07%) in half cells.When coupled with a NMC532 cathode for a full cell,the graphite||NMC532 exhibits higher rate capability and more stable capacity retention (84.32%,tested for 500 cycles at 1 C rate).The superior performances of NGT@LPAN can be explained by the reason that the carbon layer reconstruct the NGT surface and repair the structure defect,and then optimize the side reactions with electrolyte to form a uniform,complete and stable SEI film to promote a fast Li+diffusion and suppress lithium metal dendrites.The goal of this work is to shed new light on a promising way to reengineer wasted graphite resources including but not limited to NGT,such as the graphite wastes from scrapped LIBs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the financial support of National Key Research and Development Program of China (No.2020YFC1909604),National Natural Science Foundation (NNSF)of China (Nos.52202269,52002248),Shenzhen Key Projects of Technological Research (No.JSGG20200925145800001),and Shenzhen Basic Research Project (Nos.JCYJ20190808145203535,JCYJ20190808163005631) for providing financial support for this work.We are also grateful to the Instrumental Analysis Center of Shenzhen University (Xili Campus) for providing the facilities for our material analyzes.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108330.
杂志排行
Chinese Chemical Letters的其它文章
- Rocking-chair ammonium ion battery with high rate and long-cycle life
- Mitigating the dissolution of V2O5 in aqueous ZnSO4 electrolyte through Ti-doping for zinc storage
- Graphdiyne scaffold anchored highly dispersed ruthenium nanoparticles as an efficient cathode catalyst for rechargeable Li–CO2 battery
- Alkyl-thiophene-alkyl linkers to construct double-cable conjugated polymers for single-component organic solar cells
- Modulating the p-band center of carbon nanofibers derived from Co spin state as anode for high-power sodium storage
- The compatibly large nonlinear optical effect and high laser-induced damage threshold in a thiophosphate CsInP2S7 constructed with[P2S7]4- and [InS6]9-
