How ligand coordination and superatomic-states accommodate the structure and property of a metal cluster: Cu4(dppy)4Cl2 vs.Cu21(dppy)10 with altered photoluminescence
2024-04-06HimingWuGyAndrewRjiniAnumulZhixunLuo
Himing Wu ,Gy N.Andrew ,Rjini Anumul ,Zhixun Luo,b,∗
a Beijing National Laboratory for Molecular Sciences (BNLMS),State Key Laboratory for Structural Chemistry of Unstable and Stable Species,Institute of Chemistry,Chinese Academy of Sciences,Beijing 100190,China
b School of Chemistry,University of Chinese Academy of Sciences,Beijing 100049,China
Keywords: Copper nanocluster Yellow emission Dual emission Superatomic states Ligand dppy
ABSTRACT We have synthesized two copper nanoclusters (NCs) with a protection of the same ligand diphenylphosphino-2-pyridine (C17H14NP,dppy for short),formulated as Cu4(dppy)4Cl2 and Cu21(dppy)10,respectively.The former one bears a distorted tetrahedron Cu4 core with its six edges fully protected by chlorine and dppy ligands,while the latter presents a symmetric Cu21 core on which ten dppy molecules function as monolayer protection via well-organized monodentate or bidentate coordination.Interestingly,the Cu4(dppy)4Cl2 cluster exhibits a strong yellow emission at ∼577 nm,while Cu21(dppy)10 displays dual emissions in purple (∼368 nm) and green (∼516 nm) regions respectively.In combination with TD-DFT calculations,we demonstrate the origin of altered emissions and unique stability of the two copper nanoclusters pertaining to the ligand coordination and metallic superatomic states.
Ligand-protected metal nanoclusters (NCs for short) allow for tuneable charge distribution and energy transfer between the metallic core and organic ligands,giving rise to a diversity of structures,compositions and crystalline forms [1–4].Due to the unique electron configuration (n-1)d10ns1[5],coinage metal NCs have shown their advantages of facile synthesis and distinctive optical,electrical and chemical properties with promising applications in chemo-sensing,bio-labelling and catalysis [6–9].The Cu NCs are of special interest because they usually exhibit earth-abundant,costefficient and luminesce properties due to weak spin-orbit coupling interaction and high reorganization energy under photoexcitation[10–12].However,the single crystal synthesis of Cu NCs is challenging due to the lower MI/M0half-cell potential of Cu (0.52 V)compared with that of Ag (0.80 V) and Au (1.68 V) [13–15].Difficult as was the work,significant advances have been made to synthesize the ligand-protected Cu NCs,such as those of thiolate[16,17],phosphine [18],selenate [19],alkynyl [20] and halogen ligands [21–23],shedding light on the powerful ligand engineering which causes a diversity of cluster structures.
Along with the significant advances in synthesizing atomically precise metal NCs,it is recognized that superatomic electron configuration profits to thermal stability of metal NCs [24,25];also,both ligand accommodation and superatomic states are sensitive to cluster sizes [26–29].Some stable metal NCs are rationalized with a magic number of valence electrons simply by considering that the highly electrophilic ligands capture electrons of the metallic core.This has been applied for 2e-superatomic [Cu13(S2CNnBu2)6(acetylide)4]+[30],8e-superatomic [Ag21{S2P(OiPr)2}12]+[31],18e-superatomic Au44(DMBT)26[32],20e-superatomic Pd55(PiPr3)12(CO)20[33],34esuperatomic [Ag78(iPrPhS)30(dppm)10Cl10]4+[34],58e-superatomic[Au69(PR3)20Cl12]–and Au102(p-MBA)44NCs [35,36].Apart from the successful synthesis of diverse metal NCs,ongoing efforts have been devoted to understanding how ligand coordination and metal superatomic-states accommodate the structure and property of a metal cluster,thus beneficial to rational design of cluster-based functional materials.
A versatile ligand,diphenylphosphino-2-pyridine (dppy for short),has been widely applied in the synthesis of luminescent metal NCs [37–39],and the flexibility of its bidentate or monodentate coordination enables for tuneable accommodation [40–43].Here we report a comparative study on the contributions of both superatomic stability and ligand coordination to the copper NCs by utilizing the dppy as ligand.With reduction of [Cu(OAc)2]in the presence of NaBH4,we have synthesized the single-crystals of two luminescent clusters,Cu4(dppy)4Cl2and Cu21(dppy)10NCs.Amongst them,the Cu21(dppy)10cluster shows a superatomic metallic core but allow for well-organized monodentate and bidentate coordination of the dppy ligands.Interestingly,the Cu21NCs exhibit dual emissions in purple and green regions;in comparison,the Cu4(dppy)4Cl2cluster has a tetrahedron Cu4coreviafull protection by bridged sulfur of the monodentate dppy ligands,giving rise to a broad band of yellow emission at ∼577 nm.
Fig.1a displays the single-crystal structure of the Cu4(dppy)4Cl2NCs (Figs.S1 and S2,Table S1 in Supporting information).Singlecrystal parsing results at 110 K show that the Cu4(dppy)4Cl2NCs are crystallized in a monoclinic crystal system with a space group of I2/a.The single-crystal composition is determined to be Cu4P4N4C70H60Cl9,with two additional non-coordination moieties CH2Cl2and Cl3being involved in the single crystals [44–50].Interestingly,the metallic core displays a slightly distorted tetrahedron Cu4of which each Cu atom is coordinated with its adjacent three Cu atoms with average Cu-Cu distance at 2.821 ˚A (2.651–2.956 ˚A).Meanwhile,four dppy ligands form bidentate coordination on four edges of the tetrahedron Cu4core of which the other two edges are linked with chlorine bridges,giving rise to full protection of the six edges of the Cu4core.Also,each Cu atom binds to its adjacent three Cu atoms,and is simultaneously coordinated with N,P and Cl atoms.Natural population analysis (NPA) shows that the charge distribution on each Cu and Cl atom is 0.20e and -0.60e respectively (Fig.S15 and Table S2 in Supporting information),indicative of electron attraction of chlorine rendering a 2e-superatomic Cu4core (4e -0.6e×2 -0.2e×4=2e).The saturated hexa-coordinate of copper,and the well-organized charge transfer interactions account for the prominent stability of such a 2e-superatomic copper cluster [51–54].
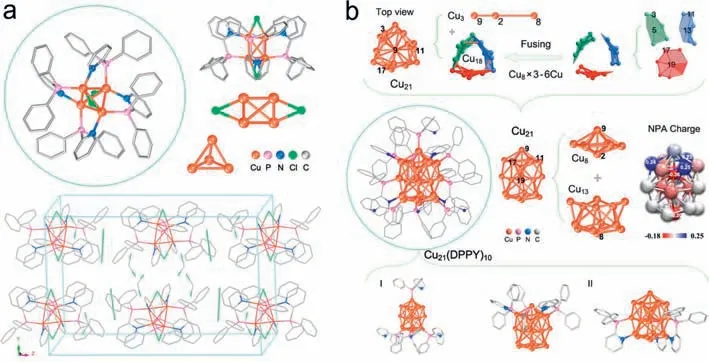
Fig.1.Single-crystal structures of (a) Cu4(dppy)4Cl2,with a CH2Cl2 and a Cl3 involved and (b) Cu21(dppy)10.The insets include the natural population analysis (NPA) of charge distribution of Cu21 nanocluster.Cu in orange,P pink,N blue,Cl green,and C grey.For clarity,H atoms are omitted.Capped sticks and ball-stick styles (instead of thermal ellipsoids) are displayed for the two kinds of Cu clusters.
We have synthesized a larger copper cluster Cu21(dppy)10with the same ligand and similar procedure,but find there is a different case of the structure and coordination modes.Fig.1b dissects the single crystal structure of the as-prepared Cu21NCs (Figs.S3–S5 and Table S1 in Supporting information).The single-crystal parsing reveals that the Cu21(dppy)10is crystallized in a trigonal space group with a precise composition of Cu21C170H140N10P10.Thequasi C 3-symmetric Cu21core can be viewed as the connection of a gyroscopic-like Cu8and a bottom bowl-like Cu13along the line through the atoms of Cu9,Cu2 and Cu8.In the Cu13moiety,the Cu-Cu average distance is 2.652 ˚A (2.477–2.994 ˚A),which is almost equal to the Cu-Cu bond length (averaged at 2.651 ˚A) in the Cu8moiety (Fig.S16 and Table S3 in Supporting information),but smaller than the average Cu-Cu bond length in Cu4(dppy)4Cl2.The varied Cu-Cu bond lengths are also consistent with the previously reported Cu NCs [55–58],due to flexible Cu coordination.Different from Cu4(dppy)4Cl2,the Cu21core is stabilized by ten dppy ligands which form two types of coordination bonds.Four of the ten dppy ligands bind to four Cu atoms through Cu-P bonds,while the other six ligands bind to twelve Cu atoms by six Cu-P bonds and six Cu-N bonds,and additional five Cu atoms do not link to the ligand(including the central atom Cu8).This is different from the other Cu NCs protected by the dppy ligands [59].The average Cu-P bond length in Cu21(dppy)10is similar to that of the Cu(I) phosphine complex [60] and the [Cu25H22(PPh3)12]Cl cluster [61].The calculated NPA charge distribution (based on the single crystal structure) reveals that there are different types of Cu atoms involved in the Cu21(dppy)10.Specifically,the body-centred Cu atom (Cu2)and bottom-centred Cu atom (Cu8) are largely negative (-0.36 and-0.20|e|);the three vertex atoms of the gyroscopic-like Cu8moiety (i.e.,Cu1,Cu10,Cu19) are positively charged (0.24,0.23 and 0.25|e|);the three face-centred (Cu5,Cu13,Cu19) and three vertex Cu atoms (Cu3,Cu11,Cu17) of the three heptagons are slightly negative,while the other Cu atoms are slightly positive or close to zero.Note that,the total NPA charge of the capping gyroscopic-like Cu8region is 0.29|e|,while the total NPA of bottom bowl-like Cu13is -0.19|e|.Although a diversity of the NPA charge distribution on the coper atoms,the total NPA charge on the Cu21core is close to zero (Fig.S17 and Tables S4–S6 in Supporting information),which is in sharp contrast to that of the Cu4core at 0.80|e|,showing a different mechanism of stability.
Fig.2 presents the typical mass spectra of the two copper NCs,collected in a positive modeviaan electrospray ionization mass spectrometer (ESI-MS).Notably,there are two prominent peaks for the Cu4(dppy)4Cl2NCs atm/z1379.01 and 1422.96,corresponding to [Cu4(dppy)4Cl2+H]+and [Cu4(dppy)4Cl2+2Na]+.Besides,the peak atm/z1466.93 could be assigned to[Cu4(dppy)4Cl2·CH2Cl2+5H]+.The absence of other strong abundance peaks suggests high chemical purity and stability of the Cu4(dppy)4Cl2NCs.Note that the experimental isotopic pattern and simulated mass distribution match well with each other(insets in Fig.2a).Similarly,Fig.2b shows the ESI-MS spectrum of Cu21NCs,where a small peak atm/z1991.76 is assigned to[Cu21(DPPY)10+17H]2+,of which the isotopic patterns match with the simulated mass distribution (insets in Fig.2b).A few fragment peaks are also seen atm/z1707.64,1838.28 and 1903.24,corresponding to dissociation and hydrogenation of the copper core as well as the loss of a few dppy ligands.This also agrees with the phosphine-protected Ag clusters due to weak bonding interactions[62].

Fig.2.ESI-MS of (a) Cu4(dppy)4Cl2 and (b) Cu21(dppy)10 NCs in the positive ion mode,respectively.Insets display the experimental spectrum in a comparison with the simulated isotopic patterns.
We have studied the absorption and photoluminescence properties of the two copper NCs.Fig.3a shows the absorption spectrum of Cu4(dppy)4Cl2NCs in DCM,where a characteristic peak at 260 nm and a weak broad band at 472 nm are observed.For this small cluster,we have conducted TD-DFT calculations and check out all the likely electronic excitation transitions at the optimized S0minima of Cu4(dppy)4Cl2(Table S7 in Supporting information).As a result,the 260 nm peak is primarily caused by somequasi-degenerate electronic transitions (e.g.,HOMO →LUMO+30/31).Considering that the HOMO is mainly located on the Cu4metal core,while the LUMO+30/31 are contributed by the ligands,the electronic transition at ∼260 nm corresponds to metal-to-ligand charge transfer (MLCT) transition.This is consistent with the previous reported study [63].Besides,the TD-DFT calculations also find electronic transitions at 436,518 and 546 nm,associated with the frontier molecular orbitals,which interprets the experimental observation of a broad weak band at 400–600 nm.Fig.3b shows the photoluminescence spectrum of the Cu4(dppy)4Cl2NCs in DCM,where a remarkable yellow emission band at 577 nm is displayed.The quantum yield of the copper NC at room temperature is estimated to be ∼1.83%.This is consistent with the previously reported halogen-protected copper NCs which also exhibit yellow emission at ∼600 nm [64,65].Timeresolved decay measurements were also carried out for the Cu4NC in DCM (Fig.3c),where the yellow emission is associated with a relatively long lifetime of 121.87 ns.Furthermore,we measured the emission spectra of the Cu4(dppy)4Cl2NCs in altered low temperatures (Fig.3d),and found that the luminescence shows enhanced intensity with decreasing temperature from 298 K to 150 K,while minor attenuation of intensity from 125 K to 78 K.Notably,the emission at 78 K shows a much longer lifetime up to microsecond (with fitted values at 46.03 μs and 95.50 μs).This is different from the monotonic increase tendency of low temperature phosphorus emission of small organic molecules.It is inferred that the dramatic changes of emission intensity and lifetime of such copper NCs could be associated with both electronic transitions between singlet and triplet states,as well as vibrational relaxation of the structure.

Fig.3.(a,b) The absorption and photoluminescence spectra of the Cu4(dppy)4Cl2 NCs in DCM.(c) Time-resolved emission decay at 298 K (λex=330 nm).(d) Temperaturedependant emission spectra at 78–298 K in DCM.(e,f) The absorption and photoluminescence spectra of the Cu21(dppy)10 NCs in DCM.(g,h) Time-resolved emission decay at 298 K (λex=270 nm).
Similarly,Fig.3e shows a typical UV–vis absorption spectrum of the Cu21(dppy)10NCs in DCM,where a characteristic peak appears at 258 nm.This is consistent with the excitation spectrum(Fig.S12 in Supporting information),and similar to the absorption spectrum of the aforementioned Cu4(dppy)4Cl2NCs because their frontier orbitals are mainly contributed by copper.Further,we examined the luminescent property (Fig.3f),where dual emissions at two bands are observed,namely,the purple at 368 nm and green at 516 nm regions.By changing the excitation wavelength to 290 nm (Fig.S13 in Supporting information),the two-band emissions retain and display minor redshifts.Notably,the green emission at 516 nm is much stronger than the purple emission,contributed by the dppy ligand which has an emission at 500 nm (Fig.S11 in Supporting information).It is worth mentioning that the FWHM of the 516 nm emission of Cu21(dppy)10NCs is relatively narrower than that of the Cu4(dppy)4Cl2NCs (143 nmvs.160 nm).The fluorescence variation of the two copper NCs could be related to the symmetry of the protective ligands and the different copper core.Time-resolved fluorescence decay measurements were also carried out for the Cu21(dppy)10NCs.As shown in Figs.3g and h,the purple and green emissions are associated with short lifetime,with fitted values for purple emission at 3.37 ns and green emission at 0.08 ns and 2.81 ns.This short lifetime of copper NCs is consistent with previous studies [66].For the purple and green emissions,we have also measured the quantum yield in DCM but found very small values (ca.0.2% and 2%,respectively).
To elucidate the origin of the stability and optical properties of the two copper NCs,by DFT-calculations we have performed an analysis on the canonical molecular orbitals (CMOs),Kohn-Sham molecular orbital energy levels,total and partial density of states(DOSs) of both Cu4(dppy)4Cl2and Cu21(dppy)10NCs (Fig.4).It is noteworthy that the Cu atoms significantly contribute to the orbitals ranging from HOMO to HOMO-19 (>50%) at optimized S0minima of Cu4(dppy)4Cl2,with a large HOMO-LUMO energy gap up to 2.34 eV,which is consistent with the DOS patterns shown in Fig.4a (Table S8 in Supporting information).Notably,the HOMO of Cu4(dppy)4Cl2exhibits superatomic S orbital pattern,shedding light on the 2e-superatomic feature.In comparison,the CMOs of Cu21(dppy)10reflect more superatomic states,as shown in Fig.4b (Figs.S18–S21 and Table S9 in Supporting information),where the superatomic 1S,1P and 1D orbitals can be recognized,indicative of 18e-superatomic stability.As a comparison,we have also conducted a calculation on the cationic [Cu21(dppy)10]+and find similar superatomic orbitals (1S|1P|1D||) along with an enlarged HOMO-LUMO gap.The inherent superatomic states in such metal NCs,embodied by partly itinerant (/delocalized) electrons,enable to balance the nuclear-electron interactions and thus optimal accommodation on the metal-metal bonding and metal-ligand interactions [67,68].

Fig.4.Total and partial density of states (DOSs) and orbital energy levels of (a) Cu4(dppy)4Cl2 based on the single-crystal structure and DFT-optimized S0 minima,and (b)Cu21(dppy)10 based on the single crystal structure.Each orbital is drawn with colour labels to indicate the relative contributions of the atomic orbitals.The isosurface value of molecular orbitals is ±0.03 au H atoms are omitted for clarity.
Considering the moderate luminescence and relatively stable emission of the Cu4(dppy)4Cl2NCs,we have evaluated its chemo sensing for ions detection.As shown in Fig.5,a few common ions including Cu2+,Fe3+,K+,Mg2+,HCO3-,CO32-,I-,Cl-(1.0 mmol/L for all) have been tested.It is found that the presence of Clions results in apparent increase of the emission intensity,which contrasts with all the other tested cationic and anionic ions.The chemo sensing toward Cl-ions is likely associated with the distinct coordination interactions between Cl-and the Cu4(dppy)4Cl2NCs.
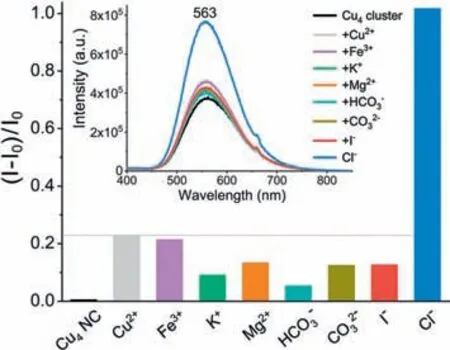
Fig.5.The varied relative intensities of Cu4(dppy)4Cl2 NCs (1 mg/mL,λex=330 nm)in the presence of typical testing ions (1.0 mmol/L).All the anions are sodium salts,while the cations are sulphates.The insets display the emission spectra of the Cu4(dppy)4Cl2 nanoclusters in DMSO.
In summary,we report here a comparative study of two phosphine-protected Cu NCs,Cu4(dppy)4Cl2and Cu21(dppy)10.The former has a tetrahedral Cu4core of which the six edges are fully protected by bridged chlorine and bidentate dppy ligands,while the latter has a Cu21core pertaining to vivid superatomic states.The full passivation of the 2e-superatomic Cu4core accounts for its enhanced stability;in comparison,the stability of the larger cluster Cu21(dppy)10is associated with its superatomic states of the Cu21core,as well as well-organized ligand coordination and surface charge distribution.The ten dppy ligands form two types of coordination on the symetric Cu21core,with four monodentate dppy ligands on the top one and bottom three Cu atoms through PCu bonds,while the other six dppy molecules as bidentate ligands to link 12 outside Cu atoms through both P-Cu bonds and N-Cu bonds.Interestingly,the Cu4(dppy)4Cl2NCs find an interesting yellow emission,while the Cu21(dppy)10NCs exhibit dual emissions in the purple and green regions.We have conducted a chemo-sensing experiment by utilizing the red emission of Cu4(dppy)4Cl2NCs and find distinctive response to the chlorine anions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Prof.Chunxi Zhang for friendly discussion.This work was financially supported by the National Natural Science Foundation of China (Nos.22003072 and 21722308),the Ministry of Science and Technology of the People’s Republic of China(No.2020YFA0714602).
杂志排行
Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
